Review Article Open Access
Utilization of Transgenic Silkworms for Recombinant Protein Production
Ken-Ichiro Tatemastu, Hideki Sezutsu and Toshiki Tamura*National Institute of Agrobiological Sciences, Tsukuba, Ibaraki 305-8602, Japan
- Corresponding Author:
- Dr. Tamura T
National Institute of Agrobiological Sciences
Tsukuba, Ibaraki 305-8602, Japan
E-mail: ttamura@affrc.go.jp
Received date: February 21, 2012; Accepted date: March 15, 2012; Published date: March 17, 2012
Citation: Tatemastu K, Sezutsu H, Tamura T (2012) Utilization of Transgenic Silkworms for Recombinant Protein Production. J Biotechnol Biomaterial S9:004. doi:10.4172/2155-952X.S9-004
Copyright: © 2012 Tatemastu K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Keywords
Silkworm; Bombyx; Silk gland; Transgenic; Recombinant protein
Introduction
The production of useful recombinant proteins is a very important field of study. Thus far, methods for the production of recombinant proteins have been established in bacteria, yeast, mammalian cells, insect nuclear polyhedrosis virus (NPV), and transgenic organisms, and have been applied to the production of pharmaceutical proteins [1,2]. These methods have several advantages and disadvantages depending on the proteins and the purpose of their utilization. Notably, the cost and posttranslational modification of proteins differ between different organisms. Recently, a recombinant protein production method using a transgenic silkworm (Bombyx mori) was developed and was found to be useful in the production of different types of recombinant proteins.
The procedure for creating the transgenic silkworm was established using the DNA transposon piggyBac as a vector to insert the target gene into the silkworm chromosome [3]. Since piggyBac is a very active transposon and works in many organisms, the transgenic silkworm can be generated very easily by injecting helper and vector plasmid DNA into silkworm eggs. Moreover, the silkworm possesses a very efficient silk protein production system, suggesting that it can be used as a bioreactor for the synthesis of recombinant proteins. The organ that synthesizes silk proteins is called the silk gland. In commercial silkworms reared by farmers, it has the ability to produce more than 0.5 g of silk proteins. In addition, the silkworm has been fully domesticated over the course of more than 5,000 years and can only survive in the care of humans. Silkworms cannot survive outside if they are accidentally released. These facts indicate that the silkworm poses no risk to the natural environment. In fact, the larvae do not escape from the rearing bed and the moths cannot fly. In addition, mass-rearing of silkworms for the production of large amounts of recombinant proteins is possible, and the posttranslational modification and folding of proteins produced in the silkworm is more similar to that in mammals than that in bacteria and plants. Thus, the production of recombinant proteins in the silkworm is thought to have many advantages when compared with other production systems.
In this report, we introduce a procedure for the construction of transgenic silkworms using the transposon piggyBac, silk protein synthesis processes in the silk gland, and the schemes developed for producing recombinant proteins in the silkworm.
Construction of a transgenic silkworm using the transposon piggyBac
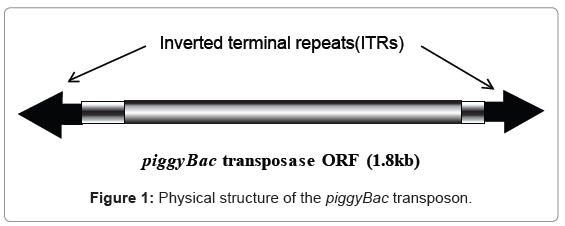
Transgenic silkworms can be generated by injecting vector and helper plasmid DNAs into preblastoderm eggs [3]. The use of the transposon is required because injection of an ordinal plasmid or linear DNA into the eggs does not yield transgenic silkworms. Although many different types of transposons have been described, only piggyBac and minos are known to be suitable for use as vectors in creating transgenic silkworms [3,4]. As shown in figure 1, the transposon piggyBac is a 2.4-kb DNA with two inverted terminal repeats (ITRs) at both ends and a transposase open reading frame [5]. The transposase functions to insert the DNA sequence between the two ITRs in the TTAA site of chromosomal DNA. The vector plasmid generally possesses the DNA sequences for a marker gene to detect the transgenic insect, cloning sites for insertion of the target gene, and two ITRs at both ends.
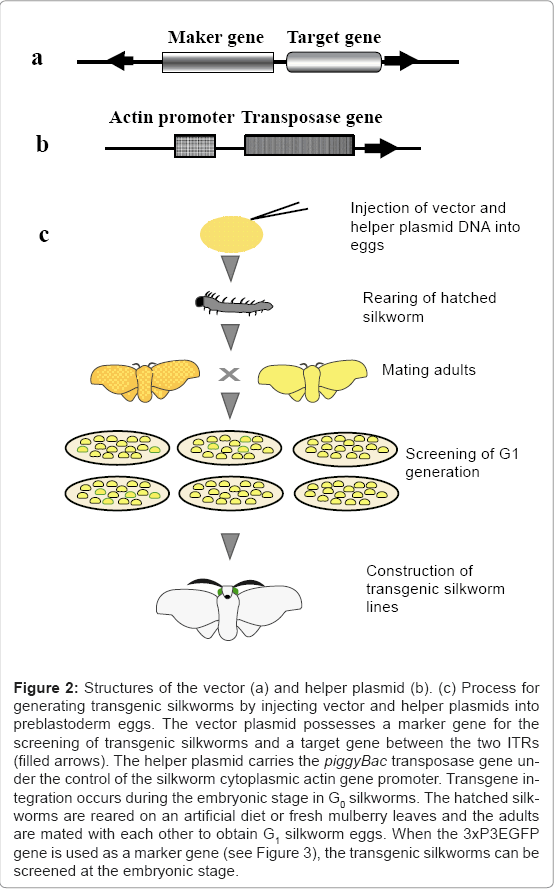
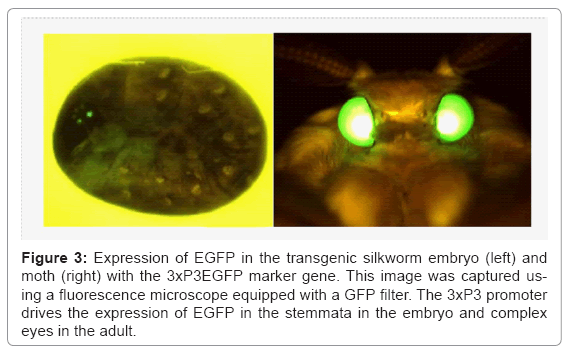
A representative vector plasmid, pBac [3xP3EGFP] [6], is shown in figure 2a. The vector possesses 3xP3EGFP as a marker gene, a cloning site for insertion of the target gene, and two ITRs. Transgenic silkworms produced using this vector can be easily detected by the expression of EGFP in the stemmata of embryos and larvae, and in the complex eyes of pupae and adults (Figure 3). The structure of the helper plasmid [3] is shown in figure 2b. In the helper plasmid, the piggyBac transposase gene is under the control of the silkworm cytoplasmic actin gene (A3) promoter and possesses only one ITR. Since transposition of the transgene requires two ITRs, the transposase gene in the helper plasmid does not insert into the chromosome of the silkworm. The procedure for creating the transgenic silkworm is summarized in figure 2c. A solution containing a mixture of vector and helper plasmid DNAs is injected into the silkworm eggs at 2-8 h after oviposition and the hatched larvae are reared until they reach adulthood. The adults are then mated with each other or the host strain to obtain the G1 generation.
Figure 2: Structures of the vector (a) and helper plasmid (b). (c) Process for generating transgenic silkworms by injecting vector and helper plasmids into preblastoderm eggs. The vector plasmid possesses a marker gene for the screening of transgenic silkworms and a target gene between the two ITRs (filled arrows). The helper plasmid carries the piggyBac transposase gene under the control of the silkworm cytoplasmic actin gene promoter. Transgene integration occurs during the embryonic stage in G0 silkworms. The hatched silkworms are reared on an artificial diet or fresh mulberry leaves and the adults are mated with each other to obtain G1 silkworm eggs. When the 3xP3EGFP gene is used as a marker gene (see Figure 3), the transgenic silkworms can be screened at the embryonic stage.
Figure 3: Expression of EGFP in the transgenic silkworm embryo (left) and moth (right) with the 3xP3EGFP marker gene. This image was captured using a fluorescence microscope equipped with a GFP filter. The 3xP3 promoter drives the expression of EGFP in the stemmata in the embryo and complex eyes in the adult.
The silkworms can be raised on an artificial diet (Nihon Nosan, Yokohama, Japan) to allow experiments to be performed throughout the year. Transposition of the target gene in the vector to the chromosome of germ-line cells occurs in G0 and the screening of transgenic silkworms is performed in G1. The procedure is simple and easy to perform. To achieve high transformation efficiency, injecting the DNA into the eggs at the correct position is important. The injection methods developed in other organisms, such as Drosophila and mouse, do not work well in the silkworm. The silkworm egg chorion is hard and strong, and the tip of a thin glass capillary cannot penetrate the eggs. To inject the helper and vector DNA into the part of the egg that generates germ-line cells required the development of injection procedures and machines. The use of our newly developed method allows the precise injection of DNA into the site where the germ-line cells are generated.
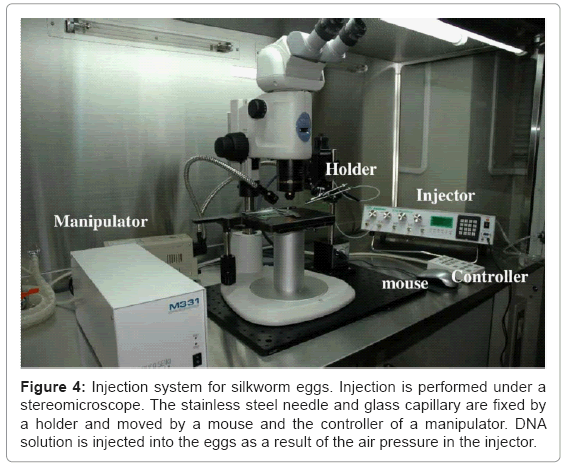
To inject the DNA, a machine first makes a small hole in the egg chorion, inserts the tip of a glass capillary into the egg through the hole, and then forces the DNA into the eggs using air pressure. This injection method enables the precise injection of DNA into silkworm eggs using a thin glass capillary and increases the hatchability of the injected eggs. So far, we have developed three different types of machines [7,8] (Uchino K, personal communication). The most recently developed system is shown in figure 4. In this method, injection of DNA into 400 eggs is generally performed to generate transgenic silkworm lines carrying one target gene, and more than 10 transgenic lines are obtained from the injection, on average (Uchino K, personal communication).
Figure 4: Injection system for silkworm eggs. Injection is performed under a stereomicroscope. The stainless steel needle and glass capillary are fixed by a holder and moved by a mouse and the controller of a manipulator. DNA solution is injected into the eggs as a result of the air pressure in the injector.
Synthesis of silk proteins in the silk gland
The silkworm possesses an ability to synthesize large amounts of silk proteins in its silk gland. The mechanism of silk protein synthesis has been extensively studied at the molecular level [9-11]. Silk consists of two proteins called fibroin and sericin. Fibroin is the main component of silk fibers, while sericin is a kind of glue protein that coats the surfaces of the fibers (Figure 5). Fibroin accounts for about 75% of all silk protein and is produced in the posterior silk gland (PSG). The remaining 25% is sericin, which is synthesized in the middle silk gland (MSG). Fibroin contains three different proteins called fibroin heavy (H) and light (L) chains and fibrohexamarin (FHX), which are produced in molar ratio of 6:6:1, respectively [12]. The character of silk as a fiber is determined by the large fibroin H chain, whose molecular weight is 350-400 kDa. The fibroin L chain and FHX are small proteins with molecular weights of about 25 kDa. The three molecules form a large complex called the fibroin elementary unit, whose molecular weight is 2.3 MDa. Joining of the fibroin H and L chains by an S-S linkage is required for the formation of the complex and the secretion of the fibroin proteins from the silk gland cell to the lumen of the PSG [13]. The fibroin H, L, and FHX genes are single-copy genes and the DNA sequences regulating their PSG-specific expression has been analyzed by in vitro transcription of the cloned gene [14]. The fibroin secreted into the lumen of the PSG moves to the MSG, where it accumulates prior to spinning.
Figure 5: Structure of cocoon silk. The silkworm cocoon is made from a long filament of cocoon silk that consists of fibroin covered with a thin layer of sericin. Since the silk is expelled from the anterior part of a pair of silk glands, two fibroin threads appear in the silk. The silk filament is very long, reaching more than 1500 m in length in the cocoons cultivated by farmers.
Sericin is the name of a group of several different serine-rich proteins synthesized in the MSG. Three sericin genes, sericins 1, 2, and 3, have thus far been identified [15-18]. The three genes are only expressed in the MSG. The localization and timing of the expression of each sericin gene differ within the MSG and during larval development, respectively [19]. The sericin 1 gene is mainly expressed in the posterior part of the MSG at the late stage of the final instar and its expression level is much higher than those of the other two genes. The sericin 2 gene is expressed in the middle part of the MSG throughout the larval stage. The sericin 3 gene is expressed in the anterior part of the MSG at the late stage of the final instar. Moreover, alternative splicing occurs in the sericin 1 and sericin 2 gene transcripts [20,21]. This explains why many different sericin proteins are synthesized from the three sericin genes. The synthesized sericin is secreted into the lumen of the middle part of the silk gland and accumulates as a layer just beneath the MSG cells in the lumen.
Systems for producing recombinant proteins in the silk gland
The system for producing recombinant proteins in the transgenic silkworm uses the silk synthesis system in the silk gland and the silk genes that are strongly expressed in the silk gland. Until now, production systems using the fibroin L and H chain and FHX genes have been used for production in the PSG. In the MSG, two different systems using the sericin 1 gene have been developed. Each has advantages and disadvantages depending on the aim of producing the protein. Careful selection of the production system is required for proper use of transgenic silkworms.
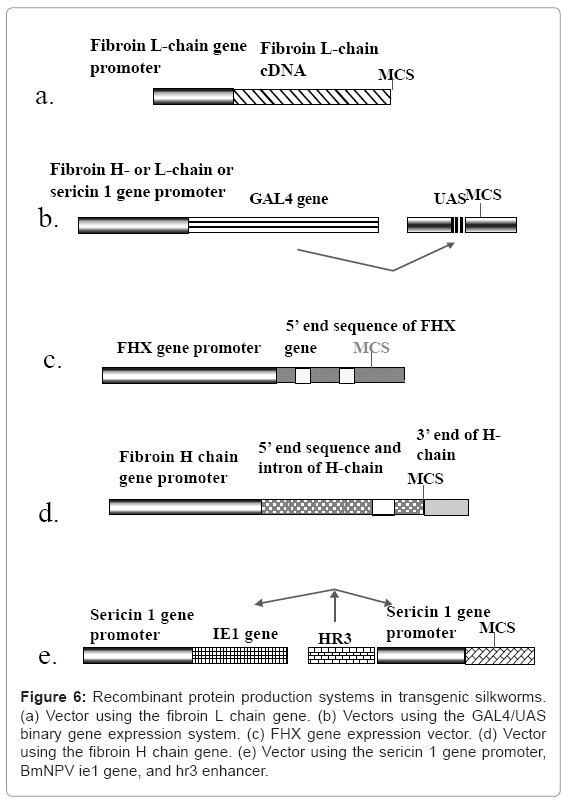
Recombinant protein production system using the fibroin L chain gene: The first expression system for the production of recombinant proteins uses the fibroin L chain gene (Figure 6a). The system has been applied to the production of human collagen and enhanced green fluorescent protein (EGFP) [22,23]. The expression vector contains the promoter and full cDNA sequence of the fibroin L chain gene. The fusion protein comprising the target protein and the fibroin L-chain is produced in the PSG, secreted into the lumen, accumulates in the lumen of the MSG, and is finally expelled in the silk filament at the spinning stage. The fibroin L gene promoter of the fusion gene works well without the help of other factors, although the expression level of the transgene is less than 1/10 of that of the endogenous fibroin L chain gene. Since the formation of a disulfide bond between the product of the transgene and the fibroin H-chain is required for the secretion of the product from PSG cells into the lumen, the system can only produce fusion proteins containing the fibroin L chain. When a C-propeptidedeleted human type III procollagen gene fused to an EGFP-coding sequence is used, the transgenic silkworm produces mini-collagen in PSG cells and secretes it into the cocoon [22]. However, the amounts of recombinant proteins produced from the fibroin L chain gene vector are much smaller compared to those produced from the endogenous gene, even when an EGFP-fibroin L chain fusion protein is used.
Figure 6: Recombinant protein production systems in transgenic silkworms. (a) Vector using the fibroin L chain gene. (b) Vectors using the GAL4/UAS binary gene expression system. (c) FHX gene expression vector. (d) Vector using the fibroin H chain gene. (e) Vector using the sericin 1 gene promoter, BmNPV ie1 gene, and hr3 enhancer.
The fusion protein produced in the silkworm cannot compete with the endogenous normal fibroin L chain in the process of S-S linking with the fibroin H chain since the normal L chain has much stronger affinity for the H chain. In an attempt to increase the yield, a fibroin secretion-deficient silkworm mutant, Nd-s, was used [23]. The Nd-s mutant has a deletion in the fibroin L chain gene and produces an abnormal L chain that does not form an S-S linkage with the fibroin H chain. The mutant produces a cocoon consisting only of sericin because the mutant fibroin L chain does not form an S-S linkage with the H chain and the fibroin is not secreted into the lumen of the PSG [13]. Therefore, only recombinant proteins fused to the normal L chain can form the S-S linkage with the H chain in the mutant. The use of this mutant increased production of the fusion protein to about 10 mg per larva [23].
The system using the fibroin L chain gene is useful for the production of modified silk. A transgenic silkworm carrying a fusion protein consisting of human fibroblast growth factor and the fibroin L chain spun silk with the biological activity of the growth factor, indicating that the silk produced may be useful as a new biomaterial for tissue engineering [24]. An experiment using fusion proteins consisting of the fibroin L chain and a peptide containing a partial collagen or fibronectin sequence also showed that the transgenic silkworm produced silk containing the fusion protein and that the film made from the silk had higher cell adhesion activity compared to unmodified silk. Notably, the silk produced in the transgenic Nd-s mutant silkworm contains a larger amount of the fusion protein with much higher cell adhesion activity than that of the recombinant silk produced in the normal silkworm with the transgene [25]. Thus, the system using the fibroin L chain gene can be adapted to produce silk as a biomaterial. For production of soluble proteins, using another system may be better because purification of the target protein in the fibroin may be laborious and expensive.
The GAL4/UAS binary transgene expression system: A GAL4/UAS binary gene expression system has been established in the silkworm [26]. In this system (Figure 6b), the activator line carries the GAL4 gene under the control of a native gene promoter and expresses the activator in specific tissues and at a particular time. The effecter line harbors the target gene under the control of an upstream activation sequence (UAS). Target gene expression is stimulated by the presence of GAL4. Generally, the silkworm does not possess GAL4. Therefore, the effecter gene is expressed only when GAL4 is present. The system was designed to construct tools for the analysis of gene function because the use of separate activator and effecter lines makes the control of target gene expression easier.
Expression of the same transgene in different tissues and developmental stages can be achieved by crossing the effecter line with the different GAL4 lines and evaluating the gene’s function without creating several different target genes constructs. In addition, the system can be used to generate transgenic lines that carry lethal or toxic genes because the transgene is not expressed in the effecter line. Furthermore, the binary gene expression system can be used to increase transgene expression. The system has been used to analyze the function of the gene that controls insect hormone titers, identify mutant genes, and investigate insect behavior [27-30]. Additionally, many activator lines that drive expression in different tissues and different stages have been constructed via enhancer traps [31] and are available for the study of gene function [32].
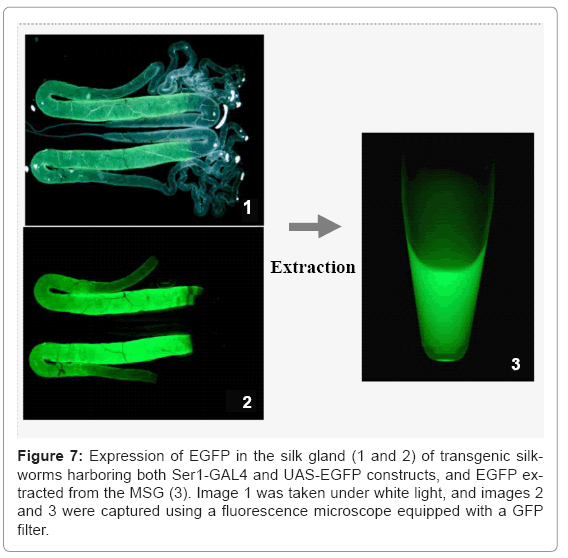
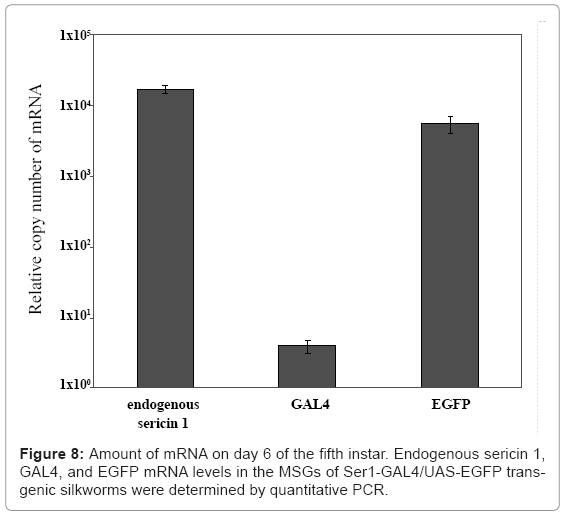
Our group has also used the binary system to produce useful proteins. To produce recombinant proteins, we first constructed an activator line harboring GAL4 under the control of the fibroin L chain promoter [26]. The line constructed specifically drives expression of the effecter gene in the PSG; no expression was observed in other tissues. In the transgenic line, the target gene is under the control of the UAS and is therefore not expressed in the transgenic silkworm until it is crossed with the GAL4 activator line. To construct a system for producing recombinant proteins in the MSG, the promoter activity of the sequences upstream of the sericin 1, 2, and 3 genes was studied. The sericin 1 gene promoter was found to have stronger activity than the other two gene promoters [33]. The sericin 2 gene promoter had no activity and the sericin 3 gene promoter drove only weak expression in the anterior part of the MSG in larvae at the late stage of the fifth instar. The activity of an activator line with the GAL4 gene under the control of the sericin 1 promoter (Ser1-GAL4) is shown in figure 7. An EGFP gene under the control of the UAS was used to construct the effecter line and to detect the transactivational activity of Ser1-GAL4 (Figure 7). Generally, transgenes directly controlled by the region upstream of the sericin 1 gene are not strongly expressed. Only small amounts of recombination proteins can be produced from these transgenes. For example, the expression of GAL4 mRNA in silkworms with the GAL4 gene under the direct control of the sericin1 gene promoter was less than 1/1000 that of the endogenous sericin 1 gene, whereas the amount of EGFP mRNA in silkworms with both the GAL4 and effecter genes was similar to that of endogenous sericin 1 mRNA (Figure 8). Use of the GAL4/UAS binary gene expression system increased target gene expression >1000-fold. Furthermore, the product in Ser1-GAL4/UASEGFP silkworms is secreted into the lumen of the MSG and transferred to the cocoon [33].When the same EGFP construct is expressed in the PSG using the GAL4 activator line with the fibroin L chain promoter, the product remains in PSG cells; the secretion and transfer of the product into the lumen does not occur [26]. Recombinant proteins produced in the MSG accumulate in the lumen of the MSG and are expelled with the cocoon silk.
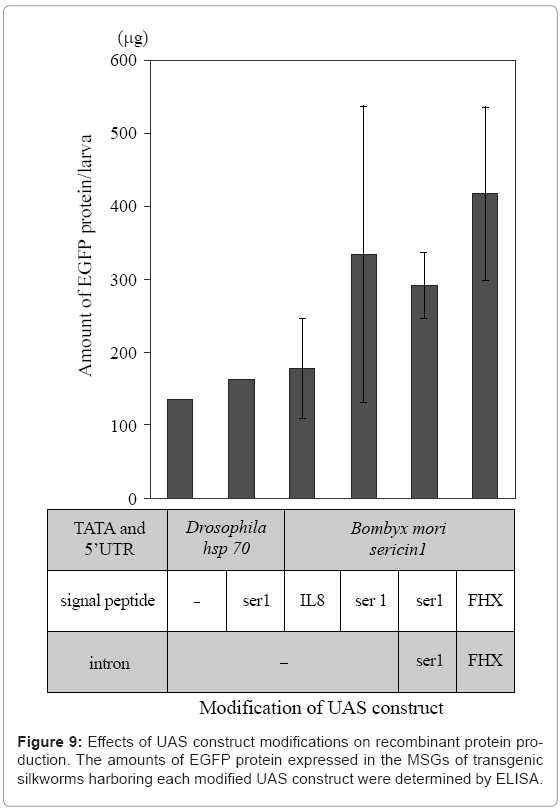
Recombinant proteins produced in the MSG can easily be extracted from the MSG or cocoon without using protein denaturants (Figure 7). The MSG production systems have been adapted for the production of recombinant proteins that function normally. In addition, mammalian signal peptide sequences are recognized in the MSG, and proteins are secreted into the lumen after signal peptide cleavage [33]. Modification of the signal peptide sequence and optimization of the UAS and intron sequences are useful for increasing target protein yield (Figure 9).
To increase the expression of target genes under the control of the UAS, the transactivational activity of the modified GAL4 variant was tested in cultured cells and transgenic silkworms [34]. The application of the GAL4 variants GAL4Δ, GAL4VP16, and GAL4NFκB markedly increased the expression of target genes in cultured cells and transgenic silkworms. The use of these variants in the production of recombinant proteins in the MSG may increase yields. A new vector carrying the silkworm kynurenine 3-monooxygenase gene as a marker for the detection of transgenic silkworms is also available to facilitate binary gene expression [35]. Moreover, a GAL4/UAS binary gene expression system using the B. mori fibroin H chain and Antheraea yamamai fibroin gene promoter is available for the analysis of gene function in the PSG [36].
The recombinant protein production system has been shown to be useful in many fields (Tamura T, personal communication). However, publications are few. Tateno et al. [37] reported production of the human mu-opioid receptor in different organs using the GAL4/UAS gene expression system and showed that the receptor produced has a similar ligand affinity to an authentic sample.
The FHX gene expression system: The FHX gene is also used for the production of recombinant proteins [38]. A fusion construct containing the second exon of the FHX gene and the coding sequence for the red fluorescent protein DsRed was inserted into the silkworm (Figure 6c). The transgenic silkworms generated harbored the fusion gene under the control of the FHX gene promoter. The expression of DsRed was studied. A cysteine codon in the FHX signal peptide sequence of the construct was replaced with isoleucine to increase secretion of the product. The transgenic silkworms produced DsRed in the lumen of the PSG and exported it to the cocoon. The protein was spread over the whole silk fiber, showing the presence of globular protein at the surface of the thread. This suggests that this construct system can be adapted for the production of globular proteins for pharmaceutical use.
Production system using the fibroin H chain gene: The fibroin H chain gene is also useful for producing recombinant proteins since ordinal recombinant proteins are not efficiently produced in the PSG because formation of the disulfide bond between the cysteine residues of the fibroin L chain and the C-terminal region of the H chain is required. A vector carrying a fusion protein containing the N- and C-terminal ends of the fibroin H chain and the upstream region of the gene as the promoter was constructed [39-41] (Figure 6d). The fibroin H chain gene consists of exon 1, a 970-bp intron, and exon 2, which contains a large core sequence that encodes the repetitious amino acid sequence [42,43]. Since the fibroin H chain gene promoter has good activity in the transgenic silkworm, the constructed system can be used to produce large amounts of the transgene product.
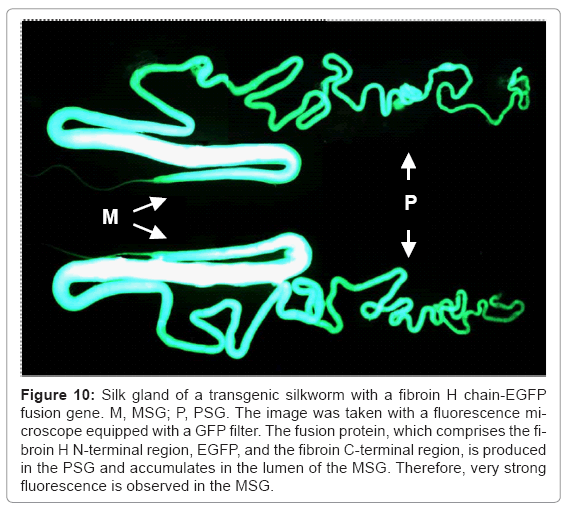
When EGFP is used as the target protein, secretion of the product into the lumen of the silk gland of the transgenic silkworm is very clearly observed (Figure 10). The product of the target gene is secreted into the lumen of the PSG, accumulates in the lumen of the MSG, and is expelled with cocoon silk when the expression system is applied to the production of recombinant proteins. The system has been adapted for the production of modified silk, especially in making textiles and biomaterials for medical purposes. Genetically modified fluorescent silk was produced using this system [41,44], and this system will be used to produce fluorescent fabrics.
Figure 10: Silk gland of a transgenic silkworm with a fibroin H chain-EGFP fusion gene. M, MSG; P, PSG. The image was taken with a fluorescence microscope equipped with a GFP filter. The fusion protein, which comprises the fibroin H N-terminal region, EGFP, and the fibroin C-terminal region, is produced in the PSG and accumulates in the lumen of the MSG. Therefore, very strong fluorescence is observed in the MSG.
This system has also been applied to silk containing spider silk protein and may improve the mechanical properties of the silk [44,45]. The possibility of improving the characteristics of silk for use as a biomaterial for medical purposes was also tested [46,47]. Genetically modified silk containing polyglutamic acid was shown to have high Ca-binding activity and to accelerate the mineralization of implanted bone [47]. The system was also shown to be useful for producing active recombinant proteins [40]. A silkworm carrying a transgene encoding feline interferon fused with the N- and C-terminal sequences of the fibroin H chain was generated. The transgenic silkworm produced normal-sized cocoons containing large amounts of the fusion protein. Interferon separated from the fibroin H chain derived from N- and C- terminal sequences through treatment with PreScission enzyme showed very high antiviral activity.
Expression system involving the sericin 1 gene promoter, a BmNPV ie1 gene, and a hr3 enhancer sequence: A recombinant protein production system involving the sericin 1 gene promoter and baculovirus enhancing factors has also been developed for the production of recombinant proteins in the MSG [48,49] and can be used to produce many different proteins. The system uses a BmNPVderived enhancer sequence, hr3, and the transactivator IE1 to enhance sericin 1 gene promoter activity (Figure 6e). The system was applied to the production of EGFP and human serum albumin. Both proteins were produced in the MSG and expelled in the cocoon, from which they were very easily purified. The system can be used to produce nonfusion proteins that are secreted after cleavage of the signal peptide. Recombinant human serum albumin was very easily purified from the cocoons and possessed the same characteristics as native human serum albumin.
Mouse IgG monoclonal antibodies can also be produced in the silkworm [50]. IgG antibodies consist of two light chains and two heavy chains; the light chain binds to the heavy chains and the two heavy chains are connected to each other by disulfide bonds. Protein complexes linked by S-S bonds can be produced in the MSG and secreted into the cocoon, showing the broad applicability of the transgenic silkworm to the production of recombinant proteins [50,51]. The antibody produced in the silkworm was easily purified from the cocoons and possessed an antigen-binding affinity that was almost identical to that of the native antibody. A large amount of non-triple helical collagen α1 chain was also produced using the system [52]. The cocoons of transgenic silkworms are 8% collagen and the protein is easily recovered. The secondary structure of the purified collagen is the same as that of denatured collagen (gelatin). The product could be used to coat tissue and cell culture dishes.
Conclusions and Future Prospects
Transgenic silkworms can be used as bioreactors to produce recombinant proteins. They can be easily generated using the DNA transposon piggyBac as a vector by injecting helper and vector plasmid DNA into eggs just after oviposition. The recombinant protein production systems were constructed using silk genes expressed in the silk gland. The expression system in the PSG is suitable for the production of genetically modified silk, and the silk made by transgenic silkworms can be used to produce fabrics and biomaterials for medical purposes. The system in the MSG is suitable for the production of recombinant proteins that can be used for pharmaceutical purposes. The latter system can yield up to 4 mg of recombinant protein per silkworm. This yield is quite high compared to other systems and producing the transgenic silkworms is much cheaper and faster than producing transgenic livestock.
Posttranslational modification and folding of the proteins is more similar to that in mammals compared to that in microorganisms or plants. The protein produced in the MSG forms the S-S linkage between the recombinant proteins and is secreted into the cocoon. The N-linked glycans of proteins produced in cultured insect cells and baculoviruses are generally of the paucimanose type, with the α-1,3 and α-1,6 fucose sugars being attached to the core N-acetylglucosamine. In contrast, the protein produced in the MSG does not have the α-1,3 fucose sugars and the end of the sugar chain contains N-acetylglucosamine, indicating that glycosylation in the MSG is more similar to that in mammalian cells [50,51].
This is an important reason for producing recombinant proteins in the silkworm, although improvements to the system are still required. To increase the protein yields, analysis of the factors controlling protein expression and translational efficiency is necessary, as are studies to modify glycosylation. The development of a method for targeting and replacing endogenous silk genes is also important for the production of recombinant silks and increasing protein yields. In fact, targeted gene disruption can be achieved by injecting zinc finger nuclease mRNA [53]. Moreover, the development of a system for rearing transgenic silkworms in the field is important for commercialization of the products. Efforts to rear transgenic silkworms on farms are ongoing.
References
- Dyck MK, Lacroix D, Pothier F, Sirard MA (2003) Making recombinant proteins in animals--different systems, different applications. Trends Biotechnol 21: 394-399.
- Liénard D, Sourrouille C, Gomord V, Faye L (2007) Pharming and transgenic plants. Biotechnol Annu Rev 13: 115-147.
- Tamura T, Thibert C, Royer C, Kanda T, Abraham E, et al. (2000) Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 18: 81-84.
- Uchino K, Imamura M, Shimizu K, Kanda T, Tamura T (2007) Germ line transformation of the silkworm, Bombyx mori, using the transposable element Minos. Mol Genet Genomics 277: 213-220.
- Fraser MJ (2000) The TTAA-specific family of transposable elements: identification, functional characterization, and utility for transformation of insects. In Handler AM and James JJ eds., Insect Transgenesis: Methods and Applications 356-361.
- Horn C, Jaunich B,Wimmer EA (2000) Highly sensitive, fluorescent transformation marker for Drosophila transgenesis. Dev Genes Evol 210: 623-629.
- Kanda T, Tamura T (1991) Microinjection method for DNA in early embryos of the silkworm, Bombyx mori, using air-pressure. Bulletin of the National Institute of Sericultural and Entomological Science 2: 31-46.
- Tamura T, Kuwabara N, Uchino K, Kobayashi I, Kanda T (2007) An Improved DNA Injection Method for Silkworm Eggs Drastically Increases the Efficiency of Producing Transgenic Silkworms. J Insect Biotechnol Sericology 76: 155-159.
- Mizuno S (1987) Regulation of fibroin gene expression and secretion of fibroin in the silk gland. Seikagaku 59: 1308-1320.
- Hui CC, Suzuki Y (1995) Regulation of the silk protein genes and the homeobox genes in silk gland development. In Goldsmith MR and Wilkins AS eds., Molecular model systems in the Lepidoptera, Cambridge University Press 249-271.
- Julian E, Coulon-Bublex A, Garel A, Royer C, Chavancy G, et al. (2005) Silk Gland Development and Regulation of Silk Protein Genes. In Gilbert LI, Iatrou K, Gill SS eds., Comprehensive Molecular Insect Science 2: 369-384.
- Inoue S, Tanaka K, Arisaka F, Kimura S, Ohtomo K, et al. (2000) Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J Biol Chem 275: 40517-40528.
- Mori K, Tanaka K, Kikuchi Y, Waga M, Waga S, et al. (1995) Production of a chimeric fibroin light-chain polypeptide in a fibroin secretion-deficient naked pupa mutant of the silkworm Bombyx mori. J Mol Biol 251: 217-228.
- Suzuki Y, Tsuda M, Takiya S, Hirose S, Suzuki E, et al. (1986) Tissue-specific transcription enhancement of the fibroin gene characterized by cell-free systems. Proc Natl Acad Sci U S A 83: 9522-9526.
- Okamoto H, Ishikawa E, Suzuki Y (1982) Structural analysis of sericin genes. Homologies with fibroin gene in the 5' flanking nucleotide sequences. J Biol Chem 257: 15192-15199.
- Garel A, Deleage G, Prudhomme JC (1997) Structure and organization of the Bombyx mori sericin 1 gene and of the sericins 1 deduced from the sequence of the Ser 1B cDNA. Insect Biochem Mol Biol 27: 469-477.
- Takasu Y, Yamada H, Tamura T, Sezutsu H, Mita K, et al. (2007) Identification and characterization of a novel sericin gene expressed in the anterior middle silk gland of the silkworm Bombyx mori. Insect Biochem Mol Biol 37: 1234-1240.
- Kludkiewicz B, Takasu Y, Fedic R, Tamura T, Sehnal F, et al. (2009) Structure and expression of the silk adhesive protein Ser2 in Bombyx mori. Insect Biochem Mol Biol 39: 938-946.
- Michaille JJ, Garel A, Prudhomme JC (1989) The expression of five middle silk gland specific genes is territorially regulated during the larval development of Bombyx mori. Insect Biochem 19: 19-27.
- Michaille JJ, Couble P, Prudhomme JC, Garel A (1986) A single gene produces multiple sericin messenger RNAs in the silk gland of Bombyx mori. Biochimie 68: 1165-1173.
- Couble P, Michaille JJ, Garel A, Couble ML, Prudhomme JC (1987) Developmental switches of sericin mRNA splicing in individual cells of Bombyx mori silkgland. Dev Biol 124: 431-440.
- Tomita M, Munetsuna H, Sato T, Adachi T, Hino R, et al. (2003) Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat Biotechnol 21: 52-56.
- Inoue S, Kanda T, Imamura M, Quan GX, Kojima K, et al. (2005) A fibroin secretion-deficient silkworm mutant, Nd-sD, provides an efficient system for producing recombinant proteins. Insect Biochem Mol Biol 35: 51-59.
- Hino R, Tomita M, Yoshizato KL (2006) The generation of germline transgenic silkworms for the production of biologically active recombinant fusion proteins of fibroin and human basic fibroblast growth factor. Biomaterials 27: 5715-5724.
- Yanagisawa S, Zhu Z, Kobayashi I, Uchino K, Tamada Y, et al. (2007) Improving cell-adhesive properties of recombinant Bombyx mori silk by incorporation of collagen or fibronectin derived peptides produced by transgenic silkworms. Biomacromolecules 8: 3487-3492.
- Imamura M, Nakai J, Inoue S, Quan GX, Kanda T, et al. (2003) Targeted gene expression using the GAL4/UAS system in the silkworm Bombyx mori. Genetics 165: 1329-1340.
- Tan A, Tanaka H, Tamura T, Shiotsuki T (2005) Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc Natl Acad Sci U S A 102: 11751-11756.
- Sakudoh T, Sezutsu H, Nakashima T, Kobayashi I, Fujimoto H, et al. (2007) Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the Yellow blood gene. Proc Natl Acad Sci U S A 104: 8941-8946.
- Fujii T, Daimon T, Uchino K, Banno Y, Katsuma S, et al. (2010) Transgenic analysis of the BmBLOS2 gene that governs the translucency of the larval integument of the silkworm, Bombyx mori. Insect Mol Biol 19: 659-667.
- Sakurai T, Mitsuno H, Haupt SS, Uchino K, Yokohari F, et al. (2011) A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genet 7: e1002115.
- Uchino K, Sezutsu H, Imamura M, Kobayashi I, Tatematsu K, et al. (2008) Construction of a piggyBac-based enhancer trap system for the analysis of gene function in silkworm Bombyx mori. Insect Biochem Mol Biol 38: 1165-1173.
- Shimomura M, Minami H, Suetsugu Y, Ohyanagi H, Satoh C, et al. (2009) KAIKObase: an integrated silkworm genome database and data mining tool. BMC Genomics 10: 486.
- Tatematsu K, Kobayashi I, Uchino K, Sezutsu H, Iizuka T, et al. (2010) Construction of a binary transgenic gene expression system for recombinant protein production in the middle silk gland of the silkworm Bombyx mori. Transgenic Res 19: 473-487.
- Kobayashi I, Kojima K, Uchino K, Sezutsu H, Iizuka T, et al. (2011) An efficient binary system for gene expression in the silkworm, Bombyx mori, using GAL4 variants. Arch Insect Biochem Physiol 76: 195-210.
- Kobayashi I, Uchino K, Sezutsu H, Iizuka M, Tamura T (2007) Development of New piggyBac Vector for Generating Transgenic Silkworms using the Kynurenine 3-mono Oxygenase Gene. J Insect Biotechnol Sericology 76: 145-148.
- Sezutsu H, Uchino K, Kobayashi I, Tatematsu K, Iizuka T, et al. (2009) Conservation of fibroin gene promoter function between the domesticated silkworm Bombyx mori and the wild silkmoth Antheraea yamamai. J Insect Biotechnol Sericology 78: 1-10.
- Tateno M, Toyooka M, Shikano Y, Takeda S, Kuwabara N, et al. (2009) Production and characterization of the recombinant human mu-opioid receptor from transgenic silkworms. J Biochem 145: 37-42.
- Royer C, Jalabert A, Da Rocha M, Grenier AM, Mauchamp B, et al. (2005) Biosynthesis and cocoon-export of a recombinant globular protein in transgenic silkworms. Transgenic Res 14: 463-472.
- Kojima K, Kuwana Y, Sezutsu H, Kobayashi I, Uchino K, et al. (2007) A new method for the modification of fibroin heavy chain protein in the transgenic silkworm. Biosci Biotechnol Biochem 71: 2943-2951.
- Kurihara H, Sezutsu H, Tamura T, Yamada K (2007) Production of an active feline interferon in the cocoon of transgenic silkworms using the fibroin H-chain expression system. Biochem Biophys Res Commun 355: 976-980.
- Zhao A, Zhao T, Zhang Y, Xia Q, Lu C, et al. (2010) New and highly efficient expression systems for expressing selectively foreign protein in the silk glands of transgenic silkworm. Transgenic Res 19: 29-44.
- Tsujimoto Y, Suzuki Y (1979) The DNA sequence of Bombyx mori fibroin gene including the 5' flanking, mRNA coding, entire intervening and fibroin protein coding regions. Cell 18: 591-600.
- Zhou CZ, Confalonieri F, Medina N, Zivanovic Y, Esnault C, et al. (2000) Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res 28: 2413-2419.
- Kojima K, Kuwana Y, Sezutsu H (2007) NMR analysis of silk produced by transgenic silkworm which expresses spider fiber protein in silk. Kobunshi Ronbunshu 64: 817-819.
- Teulé F, Miao YG, Sohn BH, Kim YS, Hull JJ, et al. (2012) Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc Natl Acad Sci U S A 109: 923-928.
- Zhu Z, Kikuchi Y, Kojima K, Tamura T, Kuwabara N, et al. (2010) Mechanical properties of regenerated Bombyx mori silk fibers and recombinant silk fibers produced by transgenic silkworms. J Biomater Sci Polym Ed 21: 395-411.
- Nagano A, Tanioka Y, Sakurai N, Sezutsu H, Kuboyama N, et al. (2011) Regeneration of the femoral epicondyle on calcium-binding silk scaffolds developed using transgenic silk fibroin produced by transgenic silkworm. Acta Biomater 7: 1192-1201.
- Ogawa S, Tomita M, Shimizu K, Yoshizato K (2007) Generation of a transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon: production of recombinant human serum albumin. J Biotechnol 128: 531-544.
- Tomita M, Hino R, Ogawa S, Iizuka M, Adachi T, et al. (2007) A germline transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon. Transgenic Res 16: 449-465.
- Iizuka M, Ogawa S, Takeuchi A, Nakakita S, Kubo Y, et al. (2009) Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS J 276: 5806-5820.
- Tomita M (2011) Transgenic silkworms that weave recombinant proteins into silk cocoons. Biotechnol Lett 33: 645-654.
- Adachi T,Wang X, Murata T, Obara M, Akutsu H, et al. (2010) Production of a non-triple helical collagen alpha chain in transgenic silkworms and its evaluation as a gelatin substitute for cell culture. Biotechnol Bioeng 106: 860-870.
- Takasu Y, Kobayashi I, Beumer K, Uchino K, Sezutsu H, et al. (2010) Targeted mutagenesis in the silkworm Bombyx mori using zinc finger nuclease mRNA injection. Insect Biochem Mol Biol 40:759-765.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 28515
- [From(publication date):
specialissue-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 23147
- PDF downloads : 5368