Review Article Open Access
Use of Photodynamic Therapy in Treatment of Bisphosphonate-related Osteonecrosis of the Jaws: Literature Review and Case Series
Petra Rugani*, Astrid Truschnegg, Stephan Acham, Barbara Kirnbauer and Norbert Jakse
Department of Oral Surgery and Radiology, Medical University of Graz, Austria
- *Corresponding Author:
- Petra Rugani
Department of Oral Surgery and Radiology
Medical University of Graz, Austria
Tel: +43 316 385 13792
Fax: +43 316 385 14064
E-mail: petra.rugani@medunigraz.at
Received date: August 01, 2013; Accepted date: August 28, 2013; Published date: August 30, 2013
Citation: Rugani P, Truschnegg A, Acham S, Kirnbauer B, Jakse N (2013) Use of Photodynamic Therapy in Treatment of Bisphosphonate-related Osteonecrosis of the Jaws: Literature Review and Case Series. J Anal Bioanal Tech S1:006. doi: 10.4172/2155-9872.S1-006
Copyright: © 2013 Rugani P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Introduction: Even though surgical intervention is the preferred option in treatment of Bisphosphonate-related Osteonecrosis of the Jaws (BRONJ) the application of low-level-laser therapy (LLLT) has been described either as part of conservative protocols or as adjuvant measure in surgical regimes. So far there are no reports concerning adjuvant photodynamic laser application in this indication in the literature.
Case series: We present the integration of Photodynamic Therapy (PDT) in a stage related treatment concept and report 10/12 cases in which PDT application was beneficial for achieving mucosal healing of BRONJ lesions.
Discussion and conclusion: In treatment of bisphosphonate-related osteonecrosis conservative therapeutic measures like the application of low-level-laser therapy and photodynamic therapy help to manage symptoms or may even promote mucosal healing. They are particularly helpful if surgical procedures are not indicated. Photodynamic therapy additionally provides antimicrobial effects and can therefore be used if complications in postoperative healing occur.
Keywords
Bisphosphonate; Osteonecrosis; Photodynamic therapy
Introduction
Bisphosphonates are important agents for the treatment of skeletalrelated events in cancer therapy, as well as for the treatment of multiple myeloma and osteoporosis. Bisphosphonate-related osteonecrosis of the jaws (BRONJ) is a serious side-effect of bisphosphonate therapy. Its cardinal symptom is exposure of jawbone that does not heal within eight weeks. This condition significantly compromises the patient’s quality of life. It affects mainly patients suffering from solid tumors like breast, prostate, bronchial and renal cell carcinoma or multiple myeloma who receive high-potency compounds in a high dosage intravenously [1], but has also been described in patients with osteoporosis [2].
Conservative regimes aim at enhancing the patient’s quality of life by controlling BRONJ symptoms and preventing progression of disease [3-5]. The main therapeutic measures are systemic medical therapy (antibiotics, anti-inflammatory therapy), local disinfectant rinsing (e.g. 3% hydrogen peroxide, 0.1-0.2% Chlorhexidindigluconate) [5] and low-level laser therapy (LLLT) [6-8].
In recent publications the superiority of surgical regimes for treating this condition was demonstrated [6,9,10]. Surgical treatment consists of the surgical removal of necrotic parts of the jawbone and, in most cases, safe and permanent mucosal coverage of former BRONJ sites. Nevertheless, adjuvant conservative therapeutic measures, such as disinfectant rinsing and medical therapy still help to manage subjective BRONJ symptoms like pain, swelling and purulent drainage, reduce the bacterial colonization prior to surgery and manage postoperative complications like local infections and dehiscences. This is particularly important if surgical therapy cannot be performed due to the patient’s poor general health or patient’s wish.
In 2006, Vescovi et al. [11] introduced the application of LLLT. Several case reports dealing with this issue have been published [7,8,12-14] but controlled trials assessing the effectiveness of LLLT are rare and often not conclusive [9,10,13,15-20] (Table 1).
| Authors | Indication | LLLT + settings | Treatment | Outcome |
|---|---|---|---|---|
| Da Guarda et al. [12] | 1 pat. PC | GaAlAs diode; 860 nm; 70 mW; SS 4 mm2. Fl: 4.2 J/cm2; 5x – 10d | STL EP:MH | 1 case healed |
| Vescovi et al. [10] | BM: Stage 1 (18), 2(48) and 3(3) MM: Stage 1(12), 2(44) and 3(3) | Nd:YAG; 1.25 W; 15 Hz; fd320 μm; dist: 2 mm; 5x 1min, 1/w, 2 mo | MT vs. MTL EP:MH | 18.2% vs. 21.4 % |
| STL + LLLT as described above or Er:YAG – Laser supported ST | ST vs. STL vs. Er:YAG-ST; EP: MH | 53.8% vs. 70.6% vs. 90.3%; | ||
| Vescovi et al. [9] | 128 pat.; MM(52), BM(53), OP(23); Stage 1 (17), 2 (92), 3 (19); 101 treated sites | Nd:YAG 1064 nm; 1.25 W; 15 Hz; DC: 0.15%; fd320 μm; dist: 2 mm; 5x1 min (PD 268.81 W/cm2, Fl:14.37 J/cm2) | MT vs. MTL; EP: CI | 25% vs. 66% |
| ST vs.STL; EP: CI | 53%vs 89% | |||
| Vescovi et al. [15] | 151 pat.; 139 treated sites; MM (56); BM (65); OP (30) | Nd:YAG 1064 nm, 1.25 W; 15 Hz; fd: 320 μm; 1/w, 2 mo | MT vs. MTL EP: MH | 7.85% (5/28) vs. 28.125% (9/32) |
| Nd:YAG laser; 1064 nm; 1.25 W; 15 Hz; fd: 320 μm; 1/w, 2 mo; | ST vs. STL EP: MH | 64.7% (11/17) vs. 72.72% (24/33) sign. | ||
| Martins et al. [13] | 22 pat.; BC (13); PC (4); LC (1); MM (2) | InGaAlP diode laser; 660 nm; 40 mW, SS 0.042 cm2, Fl: 6 J/cm2; 6 s; PD: 0.24 J/point; 1/d. | MT (3) vs. ST (5) vs. PRP + LLLT+ suturing (14) | 1/3 (33.3%) vs. 3/5 (60%) vs. 12/14 (85.7%) |
| Luomanen et al. [8] | 1 pat., MM | Nd: YAG 1,064 nm; 1.25 W; 15 Hz; fd: 320 μm; dist: 1-2 mm; 1 min; 9x in 6 mo; 5x/session, PD:1.555 W/cm2; Fl/min 167.94 J/cm2 | LLLT | MH(9 w) |
| Atalay [16] | 20 pat.; BC (11); PC (1); MM(7); NEUR (1); stage 1 + 2, MT ineffective | Nd:YAG; 1 min, dist: 4 cm; R24 950 μm fiber handpiece; 0.25 W, 10 Hz; 2.5 J. Fl: 6.25 J/cm2. | ST vs. Er:YAG -STL | 4/10 vs. 7/10, signif. |
| Romeo et al. [7] | 12 pat.; MM (4); BM (7); OP (1); 7 pat with BRONJ | Double diode, 650 nm + 904-910 nm; SS=8mm; Fl: 0.053J/cm2; 5x15min, 1/3d, 2w; dist: 1mm | LLLT EP: CI | Sign. pain reduction 6/7 |
| Manfredi et al. [19] | 25 pat.; OP; Stage 1 (4), 2 (19), 3 (2) | Nd:YAG; 1064 nm, dist: 2 mm; 5×1 min; PD 268.57 W/cm2; 1.25 W; 15 Hz; fd: 320 nm; 1x/w, 2 mo; | MT vs. MTL EP: MH (6mo) | 3/5 (40%) vs. MTL: 4/7 (57%) |
| Pat. not responsive to 3 AB cycles | Er:YAG laser (2940 nm) ST, LLLT as described above; 1/w, 1 mo; | ST vs. STL EP: MH (6 mo) | 4/4 vs. 6/6 | |
| Rugani et al. [14] | 5 Pat.; BRONJ stage 2; 2 Pat. dehiscences BC + MM, | ErCrYSGG-ST; PDT: diode laser; 75 mW; 680 nm, 90s, dist: 2 mm; PS: methylene blue | MH after 12 mo | 2/2 MH |
| Vescovi et al. [17] | 91 pat.; 55 BRONJ sites MM (27), BM (18), OP(10), Stage 1(8), 2(41), 3(6) | Nd:YAG; 1,064 nm; 1.25 W; 15 Hz; fd 320 μm; dist 2 mm; 1 min; 5x; PD: 268.57 W/cm2; Fl: 2.0175 J/cm2 | MT vs. MTL 3 Mo EP: MH | 0/13 vs. 7/17 (41%) (P= 0.033) |
| Er:YAG laser supported surgery Nd:YAG laser-irridation as above | ST+ vs. Er:YAG – STL;EP: MH | 6/13 (46%) vs. 10/12 (83.3%) | ||
| Scoletta et al. [6] | 20 pat.; BC (6), MM (6), OP (5), PC (3); Stage 1 (2), 2 (16), 3 (2/20) | Pulsed diode(GaAs); 904 nm; 50 kHz, Fl: 28.4 J/cm2; dc40%; SS 0.8 cm; 5 min: 59 J - 60 kHz + 10 min 120 J - 40 kHz 10x in 20 d | LLLT; BRONJ symptoms EP: CI | sign: pain, clinical size, edema, pus, fistulas |
| Vescovi et al. [18] | 19 pat.; 20 sites; MM (9), BM (8), OP (2); 5 treated sites MT or MTL | Nd:YAG; 1064 nm; 1.25 W, 15 Hz, fd 320 μm; dist 1-2 mm; 1 min; PD 1555: W/cm2, Fl/min 167.94 J/cm2); 5x/session; twice; | Mucosal healing Follow-up at least 4 (to 9 months) | Medical 1/3 vs. (33.3%) vs. Medical + LLLT 2/2 (100%) |
| 15 sites ST after ineffective MT | Nd:YAG as above; twice; (2 pat. some mo later) | ST vs. STL EP: MH (4 mo) | 4/8 (50%) vs. 6/7 (85.7%) | |
| Miglorati et al. [58] | 1 pat. PC, stage 0 | diode laser 815 nm; 1.0 W; fd 400 μm; pockets 5 x + fistulas 3x 15s, after 4w CO2-Laser | EP: CI | Progress Stage 1 |
| Merigo et al. [20] | BM (14), MM (12), OP (3) | Nd:YAG, settings not specified | MT vs. ST vs. LLLT | Partial success, not specified |
| Vescovi et al. [11] | 26 pat., MM (9), BM (14), OP (3) | Nd:YAG; 125 W, 15Hz, 60 s, 5 x; fd 320 μm | 6 MT + 6 ST vs. 6 MTL + 6 STL EP: MH | 4/12 (33.3%) vs. 9/14 (64.3%) |
pat: Patient; BM: Bone metastasis; PC: Prostate cancer; BC: Breast cancer; LC: Lung cancer; MM: Multiple Myeloma; NEUR: Neuroendocrine tumor; AB: Antibiotics; fd: fibre diameter; dist: distance; PD: Power Density; Fl: Fluence; DC: duty cycle; SS: Spot size; PS: Photo sensitizer; iv. Intravenous; BP: Bisphosphonate; vs.: versus; MT: Medical therapy; MTL: Medical therapy and LLLT; LPM: Long pulse mode; ST: Surgical therapy; STL: Surgical therapy and LLLT; d: day(s); w week(s); mo: month(s); EP: Endpoint; MH: Mucosal healing; CI: Clinical improvement
Table 1: Published reports on use of low-level-laser therapy (LLLT), preferred endpoint: Mucosal healing.
Basically, the effect of lasers in the mW range is classified in two categories: Biostimulation (corresponding with LLLT) and photodynamic therapy (PDT). In biostimulation (LLLT) the laser acts directly on the tissue and aims to support tissue healing. In PDT the laser acts on a chemical medium (photosensitizer), which subsequently induces cell (e.g. bacteria) and tissue damage as a chemical effect.
From a clinical point of view LLLT has in general become a wellaccepted adjuvant medical tool for enhancing wound-healing processes, although the exact biochemical mechanisms are not quite clear so far. Numerous clinical studies report about laser-induced stimulation especially of soft tissue healing, in particular to promote healing of ulcers and postoperative wound dehiscences [21,22]. A meta-analysis of the literature revealed a highly significant positive effect on wound healing in general and a significant shortening of healing time [23].
More recently the potency of LLLT on hard tissue regeneration has become a focus of scientific interest. Numerous clinical and experimental studies indicate enhancement of bone regeneration by laser irradiation [24-32]. Dörtbutak et al. [27] demonstrated significantly stimulated in vitro bone matrix formation in osteoblast cultures by low energy laser irradiation. In vitro laser irradiation of osteoblast cultures evidently enhances both cellular proliferation and cellular differentiation, which results in an increase of the number of more differentiated osteoblastic cells and finally an increase in bone matrix formation [33]. An in vitro study of Stein et al. [32] confirms evidence that low energy laser irradiation promotes proliferation and maturation of human osteoblasts. Biochemically the stimulating effect of LLLT is explained by an increase of ATP synthesis in irradiated cells [34].
Consisting of the additional application of photosensitizers, photodynamic therapy (PDT) was introduced in particular to treat cancer and several studies have shown its antimicrobial properties [35-38]. A study of the effects of PDT on osteoblast growth showed that whether PDT results in biostimulation of osteoblastic cell cultures or a cytotoxic effect may depend only on the applied dose [39]. PDT is so far clinically established in the adjuvant local treatment of infected wounds and ulcers [40-42]. Furthermore periodontitis and peri-implantitis could be potential indications for the application of PDT [36,43-45]. BRONJ could be another possible indication.
The following section describes a potential role of photodynamic therapy in a stage-related treatment concept (Table 2) and demonstrates its beneficial application in a case series of 12 patients.
| Stage | Related Treatment |
|---|---|
| Stage 0 | Antiseptic mouth rinses; medical therapy (antibiotic, antimycotic, antiphlogistic, analgetic); LLLT (altered neurosensory function); PDT (fistulas; HelboMinilaser 2075 F dent [HELBO Photodynamic Systems, Austria], 75 mW, 680 nm; photosensitizer: methylene-blue;) |
| Stage 1 | Intital CoTr for max. 8 weeks; LRST; impaired healing: Disinfectant rinsing, PDT |
| Stage 2 | Preoperatively: CoTr, PDT; LRST; impaired healing: Disinfectant rinsing, PDT |
| Stage 3 | Radical surgery; impaired healing: Disinfectant rinsing, PDT |
Staging: AAOMS classification [46]
LRST: limited-resective surgical therapy; CoTr: conservative treatment;
Table 2: BRONJ Treatment concept [14].
Treatment Concept
In case of evident BRONJ the choice of treatment options relies on staging according to AAOMS staging system (Table 2) [46]. Photodynamic therapy is additionally applied for symptomatic treatment in stage 0, preoperatively to reduce the bacterial burden and, in cases of healing deficiencies, after surgical intervention in stages 1, 2 and 3.
Furthermore PDT is the adjuvant conservative treatment of choice for palliative therapy of compromised patients or if patients refuse surgery to control symptoms and avoid progression of disease.
Presentation of Cases
We describe the benefit of photodynamic therapy in five cases of BRONJ stage 0 and seven cases of BRONJ stage 2.
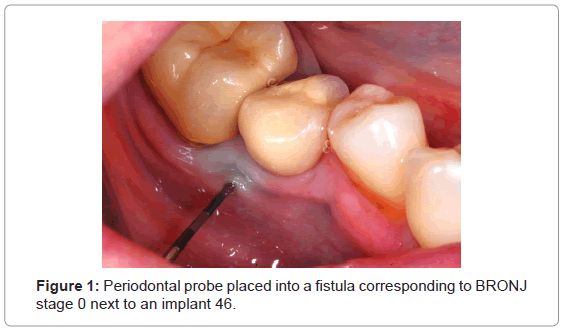
Five women with breast cancer who participated in a study to assess BRONJ after adjuvant administration of zoledronate in a lowdose protocol (7 infusions over three years, totaling 28 mg) displayed fistulas and further clinical and radiological symptoms corresponding to stage 0 (Figure 1 and Table 1) [47]. According to our standardized protocol fistulas were rinsed with antiseptic fluids and photodynamic therapy was applied twice a week for the first two weeks. We used a diode laser emitting 680 nm visible red light at 2 mm distance for 90 seconds (Helbo Mini laser 2075 F dent [HELBO Photodynamic Systems, Austria], 75 mW) and methylene blue (methylthioninium chloride 50 mg/5 ml, Monico, Italy) as photosensitizer (Power density: 1.25 W/cm2; fluence: 112.5 J/cm2; total energy: 6.75 J). The methylene blue was applied topically on the surface of all affected tissue and no incubation time was awaited. After two weeks all five fistulas healed and correlating clinical symptoms vanished.
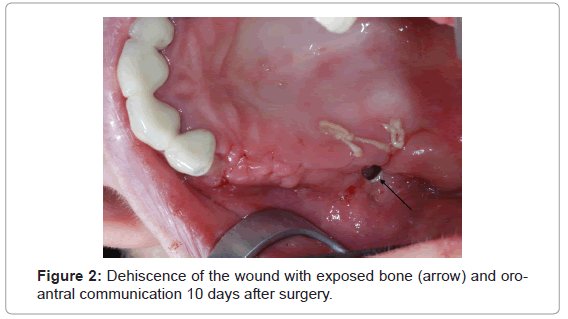
In seven of 27 surgically treated patients with BRONJ stage 2, mucosal closure did not remain stable postoperatively. They developed dehiscences to various extents (Figure 2). As a consequence photodynamic therapy, as described above was started immediately. Five of seven lesions are healed by secondary granulation (75%). In two cases the lesion did not heal. One patient was scheduled for another, more invasive surgical intervention indicated by the appearance of a further sequester. In the second patient, suffering from breast cancer, general health worsened significantly and therefore conservative maintenance therapy was applied (Table 3).
| Patients with early BRONJ and bone exposure via fistulas | ||||||||
| D.M. | f | 60 | 27.05.10 | BC | Zol | Stage 0 region 36 | Desinfectant rinsing, LLLT | Mucosal healing |
| S.R. | f | 57 | 23.02.10 | BC | Zo | Stage 0 region 44-45 | Desinfectant rinsing, LLLT | Mucosal healing |
| Sc.E. | f | 48 | 25.03.10 | BC | Zo | Stage 0 (pus) region 37, 27 | Desinfectant rinsing, LLLT | Mucosal healing |
| St.E. | f | 51 | 10.05.10 | BC | Zo | Stage 0 region 46 | Desinfectant rinsing, LLLT | Mucosal healing |
| vH.W. | f | 54 | 23.02.10 | BC | Zo | Stage 0 (pus) region 47 | Desinfectant rinsing, LLLT | Mucosal healing |
| Patients with impaired healing after BRONJ surgery | ||||||||
| B.T. | f | 69 | 17.08.10 | OP | Al | Stage 2, 35-38, | Er:YAG Surgery 18.10.10; Dehiscence 10 days after surgery → LLLT | Mucosal healing |
| C.E. | f | 65 | 31.05.11 | BC | Zo | Stage 2, 35-38 lingually | Er:YAG Surgery 18.07.11 Dehiscence 38 PDprogredient | No success |
| D.A. | f | 86 | 26.06.07 | MM | Zo | Stage 2, region 44-45 | ErCrYSGG Surgery 13.01.09; Postoperative dehiscence → LLLT | Mucosal healing |
| H.W. | m | 69 | 22.11.10 | PC | Zo | Stage 3, region 45 | Er:YAG surgery 07.03.11 Fistula | No success |
| K.M. | f | 74 | 05.10.10 | BC | Zo | Stage (0) 2, 46-47 | Er:YAG Surgery 23.05.11; Dehiscence 10 days after surgery → LLLT | Mucosal healing |
| S.E. | f | 68 | 30.07.10 | BC | Zo | Stage 2, region 16-17 | Er:YAG Surgery 17.1.11; Dehiscence 10 days after surgery → LLLT | Mucosal healing - 1 month |
| S.W. | f | 66 | 03.08.10 | BC | Zo→Ib | Stage (0) 2, region 13 | Er:YAG Surgery 28.02.11; Dehiscence 10 days after surgery → LLLT | Mucosal healing |
Pat: Patient; S: Sex; m: male; f: female; PD: Primary disease; BC: Breast cancer; BrC: Bronchial Carcinoma; MM: Multiple Myeloma; PC: Prostate cancer; OP: Osteoporosis; BP: Bisphosphonate; Zo: Zoledronate; Ib: Ibandronate; Al: Alendronate; CMT: conservative maintenance therapy; Tooth numbering: World Dental Federation notation
Table 3: Patients-Use of Photodynamic Therapy in BRONJ treatment.
Discussion
The illustrated cases demonstrate that photodynamic therapy can be an effective adjuvant tool for treating BRONJ in the very early stages or for managing healing impairment in surgically treated BRONJ sites.
Application of LLLT in cases of BRONJ has been described in numerous studies, but to our knowledge the issue of photodynamic therapy is not reported in the literature so far (Table 1). LLLT was introduced by Vescovi et al. [11] in 2006 who used Nd:YAG laser biostimulation in addition to medical or surgical therapy and were, albeit not conclusively able to demonstrate a better healing tendency. Shortly thereafter the group confirmed this statement with the publication of a controlled study of 20 BRONJ sites in 19 patients. These were treated with medical therapy alone, medical therapy and LLLT, surgery or surgery and LLLT. Patients received laser stimulation only twice and healing improved from 33.3% to 100% and from 50% to 85.7% respectively. In a further report in 2010 that compared 13 medically treated BRONJ sites to 17 sites where LLLT was additionally applied improvement was significant [17]. In this study Vescovi et al. also took the next step of using Er:YAG radiation for minimal invasive bony ablation, which is also known to have bactericidal [48] and detoxification effects [49] and to promote better healing [50], and which may also bebio-stimulant [51]. This observation has also been reported by other authors [52]. Atalay et al. [16] used this regime in patients with malignant diseases and Manfredi et al. [19] in patients with osteoporosis and BRONJ. And recently Vescovi et al. [9,10] published two further reports dealing with this issue. All of them reported better healing if medical or surgical therapy was supplemented with LLLT. Romeo et al. [7] used LLLT to treat patients experiencing pain in necrotic areas and reached significant pain reduction in 6 of 7 patients. Luomanen and Alaluusua [8] treated one multiple myeloma patient with Nd:YAG LLLT only and accomplished mucosal closure. Notably this was not done until a bony sequester protruded by itself. Surgical removal may have been beneficial to achieve this result earlier. Martins et al. [13] combined the use of LLLT with platelet-rich plasma and primary wound closure and reached better results than with medical or surgical therapy, although group sizes were not comparable. Recently da Guarda et al. [12] reported a case of successful BRONJ treatment with the GaAIAs diode laser in combination with bone curettage.
Summarizing the literature we conclude that the use of LLLT seems to be beneficial for treatment of BRONJ, although to date there are no larger-scale studies that demonstrate significant improvement. We combined the low-level laser beam with the use of a photosensitizer as defined by photodynamic therapy.
PDT is primarily used in the treatment of cancer and due to its antimicrobial properties it is also indicated in bacterial and fungal infections. Further indications may be psoriasis, actinic keratosis, rheumatoid arthritis and age-related macular degeneration [53].
It has been hypothesized that the presence of microflora is a triggering or promoting factor of BRONJ. Biopsies and cultures sampled from BRONJ lesions noted the presence of species such as Fusobacterium, Eikenella, Bacillus, Actinomyces, Staphylococcus and Streptococcus [3,54-56]. The majority of microbes in affected patients seem to be facultative anaerobes. These organisms are predisposed to survive in oxygen-depleted areas of necrotic bone that lack adequate blood supply, as typical in BRONJ [56].
The antimicrobial activity of photosensitizers is mediated by singlet oxygen. The high chemical reactivity of molecular oxygen results in a direct effect on extracellular molecules. Thus, the polysaccharides present in the extracellular matrix of a bacterial biofilm are also sensitive to photodamage. This is a significant advantage. Breaking down biofilms may inhibit plasmid exchange involved in the transfer of antibiotic resistance and interrupt colonization [53].
Even though surgery is our first treatment option to deal with BRONJ we are applying photodynamic therapy for several purposes. Prior to surgery we treat the symptoms that come along with infection of the BRONJ site. Pain, swelling and purulent discharge can be well managed and the bio-stimulative effect of the laser beam is appropriate if neurosensory dysfunction is already evident. This is particularly helpful in patients with advanced primary disease or suffering from other severe sicknesses resulting in a general poor health condition where a surgical treatment is not indicated.
At very early BRONJ stages photodynamic therapy might be even sufficient to promote secondary granulation and the formation of mucosal coverage and consequently surgery is not even necessary. This was also demonstrated with the application of LLLT as reported by Scoletta et al. [57] who showed that the presence of fistulas significantly decreased after four weeks. Controlled studies opposing LLLT and PDT using exactly the same laser parameters are needed to evaluate if the additional use of a photosensitizer is beneficial.
Applying photodynamic therapy immediately after surgical intervention may increase success rates as complications linked to impaired healing of the wound can be effectively handled. Nevertheless, we are aware of a possible bias that may arise from the fact that those patients are recalled much more often to get irradiated, which may also affect possible relapse factors such as poor oral hygiene or pressure marks. A controlled study evaluating the effects of PDT after BRONJ surgery should therefore contain the same recall interval for both groups and leave patients and doctors blindfold.
Conclusion
Photodynamic therapy can be a supportive tool in BRONJ treatment. It may be used as an adjuvant before or after BRONJ surgery or as the primary treatment option in cases of very early BRONJ or if surgery is not indicated.
References
- Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, et al. (2008) Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23: 826-836.
- Rizzoli R, Burlet N, Cahall D, Delmas PD, Eriksen EF, et al. (2008) Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone 42: 841-847.
- Marx RE, Sawatari Y, Fortin M, Broumand V (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63: 1567-1575.
- Suleman YF, Meer S, Lurie R (2007) Bisphosphonate-induced osteonecrosis of the jaws: review, clinical implications and case report. Head Neck Pathol 1: 156-164.
- Nicolatou-Galitis O, Papadopoulou E, Sarri T, Boziari P, Karayianni A, et al. (2011) Osteonecrosis of the jaw in oncology patients treated with bisphosphonates: prospective experience of a dental oncology referral center. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112: 195-202.
- Scoletta M, Arduino PG, Dalmasso P, Broccoletti R, Mozzati M (2010) Treatment outcomes in patients with bisphosphonate-related osteonecrosis of the jaws: a prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110: 46-53.
- Romeo U, Galanakis A, Marias C, Vecchio AD, Tenore G, et al. (2011) Observation of pain control in patients with bisphosphonate-induced osteonecrosis using low level laser therapy: preliminary results. Photomed Laser Surg 29: 447-452.
- Luomanen M, Alaluusua S (2012) Treatment of bisphosphonate-induced osteonecrosis of the jaws with Nd:YAG laser biostimulation. Lasers Med Sci 27: 251-255.
- Vescovi P, Manfredi M, Merigo E, Guidotti R, Meleti M, et al. (2012) Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): a retrospective analysis of 101 treated sites with long-term follow-up. Photomed Laser Surg 30: 5-13.
- Vescovi P, Merigo E, Meleti M, Manfredi M, Fornaini C, et al. (2012) Surgical Approach and Laser Applications in BRONJ Osteoporotic and Cancer Patients. J Osteoporos 2012: 585434.
- Vescovi P, Merigo E, Meleti M, Manfredi M (2006) Bisphosphonate-associated osteonecrosis (BON) of the jaws: a possible treatment? J Oral Maxillofac Surg 64: 1460-1462.
- da Guarda MG, Paraguassú GM, Cerqueira NS, Cury PR, Farias JG, et al. (2012) Laser GaAlAs (λ860 nm) photobiomodulation for the treatment of bisphosphonate-induced osteonecrosis of the jaw. Photomed Laser Surg 30: 293-297.
- Martins MA, Martins MD, Lascala CA, Curi MM, Migliorati CA, et al. (2012) Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: a preliminary study. Oral Oncol 48: 79-84.
- Rugani P, Acham S, Truschnegg A, Obermayer-Pietsch B, Jakse N (2010) Bisphosphonate-associated osteonecrosis of the jaws: surgical treatment with ErCrYSGG-laser. Case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110: e1-6.
- Vescovi P, Merigo E, Meleti M, Manfredi M, Guidotti R, et al. (2012) Bisphosphonates-related osteonecrosis of the jaws: a concise review of the literature and a report of a single-centre experience with 151 patients. J Oral Pathol Med 41: 214-221.
- Atalay B, Yalcin S, Emes Y, Aktas I, Aybar B, et al. (2011) Bisphosphonate-related osteonecrosis: laser-assisted surgical treatment or conventional surgery? Lasers Med Sci 26: 815-823.
- Vescovi P, Manfredi M, Merigo E, Meleti M, Fornaini C, et al. (2010) Surgical approach with Er:YAG laser on osteonecrosis of the jaws (ONJ) in patients under bisphosphonate therapy (BPT). Lasers Med Sci 25: 101-113.
- Vescovi P, Merigo E, Meleti M, Fornaini C, Nammour S, et al. (2007) Nd:YAG laser biostimulation of bisphosphonate-associated necrosis of the jawbone with and without surgical treatment. Br J Oral Maxillofac Surg 45: 628-632.
- Manfredi M, Merigo E, Guidotti R, Meleti M, Vescovi P (2011) Bisphosphonate-related osteonecrosis of the jaws: a case series of 25 patients affected by osteoporosis. Int J Oral Maxillofac Surg 40: 277-284.
- Merigo E, Manfredi M, Meleti M, Guidotti R, Ripasarti A, et al. (2006) Bone necrosis of the jaws associated with bisphosphonate treatment: a report of twenty-nine cases. Acta Biomed 77: 109-117.
- Mester E, Mester AF, Mester A (1985) The biomedical effects of laser application. Lasers Surg Med 5: 31-39.
- Liao HF, Chen QJ, Yi JL, Feng Z, Zhang XR, et al. (2004) [Semiconductor low level laser irradiation for exposure of hydroxyapatite orbital implants]. Zhonghua Zheng Xing Wai Ke Za Zhi 20: 177-179.
- Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, et al. (2004) The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg 22: 241-247.
- Trelles MA, Mayayo E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7: 36-45.
- Barushka O, Yaakobi T, Oron U (1995) Effect of low-energy laser (He-Ne) irradiation on the process of bone repair in the rat tibia. Bone 16: 47-55.
- Yaakobi T, Maltz L, Oron U (1996) Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He-Ne) irradiation. Calcif Tissue Int 59: 297-300.
- Dörtbudak O, Haas R, Mallath-Pokorny G (2000) Biostimulation of bone marrow cells with a diode soft laser. Clin Oral Implants Res 11: 540-545.
- Ueda Y, Shimizu N (2001) Pulse irradiation of low-power laser stimulates bone nodule formation. J Oral Sci 43: 55-60.
- Dörtbudak O, Haas R, Mailath-Pokorny G (2002) Effect of low-power laser irradiation on bony implant sites. Clin Oral Implants Res 13: 288-292.
- Khadra M, Kasem N, Haanaes HR, Ellingsen JE, Lyngstadaas SP (2004) Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97: 693-700.
- Rochkind S, Kogan G, Luger EG, Salame K, Karp E, et al. (2004) Molecular structure of the bony tissue after experimental trauma to the mandibular region followed by laser therapy. Photomed Laser Surg 22: 249-253.
- Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23: 161-166.
- Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22: 347-354.
- Karu T, Pyatibrat L, Kalendo G (1995) Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 27: 219-223.
- Hamblin MR, Hasan T (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3: 436-450.
- Meisel P, Kocher T (2005) Photodynamic therapy for periodontal diseases: state of the art. J Photochem Photobiol B 79: 159-170.
- Komerik N, MacRobert AJ (2006) Photodynamic therapy as an alternative antimicrobial modality for oral infections. J Environ Pathol Toxicol Oncol 25: 487-504.
- Donnelly RF, McCarron PA, Tunney MM, David Woolfson A (2007) Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J Photochem Photobiol B 86: 59-69.
- Zancanela DC, Primo FL, Rosa AL, Ciancaglini P, Tedesco AC (2011) The effect of photosensitizer drugs and light stimulation on osteoblast growth. Photomed Laser Surg 29: 699-705.
- Morley S, Griffiths J, Philips G, Moseley H, O'Grady C, et al. (2013) Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br J Dermatol 168: 617-624.
- Peplow PV, Chung TY, Baxter GD (2012) Photodynamic modulation of wound healing: a review of human and animal studies. Photomed Laser Surg 30: 118-148.
- Simonetti O, Cirioni O, Orlando F, Alongi C, Lucarini G, et al. (2011) Effectiveness of antimicrobial photodynamic therapy with a single treatment of RLP068/Cl in an experimental model of Staphylococcus aureus wound infection. Br J Dermatol 164: 987-995.
- Haas R, Dörtbudak O, Mensdorff-Pouilly N, Mailath G (1997) Elimination of bacteria on different implant surfaces through photosensitization and soft laser. An in vitro study. Clin Oral Implants Res 8: 249-254.
- Takasaki AA, Aoki A, Mizutani K, Schwarz F, Sculean A, et al. (2009) Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000 51: 109-140.
- Raghavendra M, Koregol A, Bhola S (2009) Photodynamic therapy: a targeted therapy in periodontics. Aust Dent J 54 Suppl 1: S102-109.
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, et al. (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg 67: 2-12.
- Rugani P, Luschin G, Jakse N, Kirnbauer B, Lang U, et al. (2013) Prevalence of bisphosphonate-associated osteonecrosis of the jaw after intravenous zoledronate infusions in patients with early breast cancer. Clin Oral Investig.
- Sasaki KM, Aoki A, Ichinose S, Ishikawa I (2002) Ultrastructural analysis of bone tissue irradiated by Er:YAG Laser. Lasers Surg Med 31: 322-332.
- Pourzarandian A, Watanabe H, Aoki A, Ichinose S, Sasaki KM, et al. (2004) Histological and TEM examination of early stages of bone healing after Er:YAG laser irradiation. Photomed Laser Surg 22: 342-350.
- de Mello ED, Pagnoncelli RM, Munin E, Filho MS, de Mello GP, et al. (2008) Comparative histological analysis of bone healing of standardized bone defects performed with the Er:YAG laser and steel burs. Lasers Med Sci 23: 253-260.
- Takasaki AA, Aoki A, Mizutani K, Kikuchi S, Oda S, et al. (2007) Er:YAG laser therapy for peri-implant infection: a histological study. Lasers Med Sci 22: 143-157.
- Angiero F, Sannino C, Borloni R, Crippa R, Benedicenti S, et al. (2009) Osteonecrosis of the jaws caused by bisphosphonates: evaluation of a new therapeutic approach using the Er:YAG laser. Lasers Med Sci 24: 849-856.
- Konopka K, Goslinski T (2007) Photodynamic therapy in dentistry. J Dent Res 86: 694-707.
- Hansen T, Kunkel M, Weber A, James Kirkpatrick C (2006) Osteonecrosis of the jaws in patients treated with bisphosphonates - histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med 35: 155-160.
- Hansen T, Kunkel M, Springer E, Walter C, Weber A, et al. (2007) Actinomycosis of the jaws--histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch 451: 1009-1017.
- Sedghizadeh PP, Yooseph S, Fadrosh DW, Zeigler-Allen L, Thiagarajan M, et al. (2012) Metagenomic investigation of microbes and viruses in patients with jaw osteonecrosis associated with bisphosphonate therapy. Oral Surg Oral Med Oral Pathol Oral Radiol 114: 764-770.
- Scoletta M, Arduino PG, Reggio L, Dalmasso P, Mozzati M (2010) Effect of low-level laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: preliminary results of a prospective study. Photomed Laser Surg 28: 179-184.
- Migliorati CA, Hupp WS, Migliorati EK (2007) Treatment of bisphosphonates-associated osteonecrosis. Clin Cases Miner Bone Metab 4: 62-68.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15493
- [From(publication date):
specialissue-2013 - Apr 20, 2025] - Breakdown by view type
- HTML page views : 10752
- PDF downloads : 4741