Review Article Open Access
Use of Immunostimulants and Nucleotides in Aquaculture: A Review
Einar Ringø1*, Rolf Erik Olsen2, Jose L. Gonzalez Vecino3, Simon Wadsworth3 and Seong Kyu Song41Norwegian College of Fishery Science, Faculty of Biosciences, Fisheries and Economics, University of Tromsø, Tromsø, Norway
2Institute of Marine Research, Matre Research Station, Matredal, Norway
3EWOS Innovation AS, Dirdal, Norway
4School of BioScience, Handong University, Pohang, South Korea
- *Corresponding Author:
- Einar Ringø
Norwegian College of Fishery Science
Faculty of Biosciences, Fisheries and Economics
University of Tromsø, Tromsø, Norway
Tel: +47 907 26125
Fax: +47 776 46114
E-mail: Einar.Ringo@uit.no
Received date: September 09, 2011; Accepted date: October 21, 2011; Published date: October 25, 2011
Citation: Ringø E, Olsen RE, Vecino JLG, Wadsworth S, Song SK (2012) Use of immunostimulants and nucleotides in aquaculture: a review. J Marine Sci Res Development 2:104. doi:10.4172/2155-9910.1000104
Copyright: © 2012 Ringø E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Keywords
Fish; Immunostimulants; Nucleotides
Currently there are numerous gaps in existing knowledge about exogenous nucleotide application to fish including various aspects of digestion, absorption, metabolism, and influences on various physiological responses, especially expression of immunogenes and modulation of immunoglobulin production. Additional information is also needed in regard to age/size-related responses and appropriate doses and timing of administration. Thus further research in these areas should be pursued.
Immunostimulants
On a worldwide scale, fisheries landings remain constant at about 90 million tons of fish whereas commercial aquaculture supplies per se is approximately 60 million tons and is increasing at a rate of about 8% per annum. Use of immunostimulants (natural immune stimulants) is a unique approach for fish culturists to control disease losses in their facilities and numerous polysaccharides from a variety of sources have the ability to stimulate the immune system, and thus behaving as immunostimulants. Immunostimulants have an architecture consisting of repeating units of single molecular forms such as glucose in β-glucans and (deoxy) riboses in DNA/RNA, fatty acid chains in bacterial lipopolysaccharides (LPS) and certain lipoproteins. Such patterns are abundant in microbial communities of prokaryotes, and can be termed pathogen-associated molecular patterns (PAMPs) if they initiate inflammatory responses. It was turned out that commential microbiota also contain those molecular patterns in them and now scientists tend to use a broader term, microbe-associated molecular pattern (MAMP).
Sakai indicated that immunostimulants could be grouped depending on their sources; bacterial, algae-derived, animal-derived, nutritional factors and hormones/cytokines [1]. This sub-grouping changed the concept that immunostimulants instead are divided according to their modes of actions. Previously, an immunostimulant was defined based upon its activity on mononuclear phagocytic system only [2]. However, due to recent discoveries of the pattern recognition receptors (PPRS) of the phagocytic cells, the former definition of immunostimulant needs to be redefined. Different leucocytes possess different PPRs and the immune cells bring about different immune responses depending on the types of ligands PPRs interact with. To define an immunostimulant, all elements of the immune system must be considered, and according to Bricknell and Dalmo [3] the definition could be; “An immunostimulant is a naturally occurring compound that modulates the immune system by increasing the host’s resistance against diseases that in most circumstances are caused by pathogens”. Immunostimulants have been used as feed additives for several years in aquaculture, and yeast β-glucan may be the one with the longest track record. In nature, β-glucans are widespread and have been characterized in microorganisms, algae, fungi and plants [4]. The chemical structure of β-glucan varies with respect to molecular weight and degree of branching. For example, β-glucan from yeast contains a particular carbohydrate consisting of glucose and mannose residues and is a major constituent in the cell membrane.
Nucleotide-supplemented diets are not strictly immunostimulants by definition but provide a dietary supplement that allows improved resistance to a pathogen insult. Although such diets have been reported not to induce measurable immunostimulatory effects [5-7], they nevertheless seems to be able to up-regulate several immune genes in turbot (Scopthalamus maximus L.), which contradicts the earlier claims that these diets are not immunostimulatory [7].
The biological effects of immunostimulants are highly dependent on the receptors on the target cells recognising them as potential highrisk molecules thus triggering various defence pathways. Thus, it is important to increase the knowledge of receptor specificity and the inflammatory processes the different receptors, upon antigen binding, are induced. However, many mammalian receptors reported to bind immunostimulants such as NLR (NOD-like receptors) have yet to be reported in fish. Nevertheless, assuming that fish and mammalian cells share many similar receptors, one may predict the biological outcome of immunostimulants in fish.
In order to evaluate whether immunostimulants may act in synergy with pro– and prebiotics we recommend this option merits further investigations. Probiotics appear to modulate immunity of the host by improving the barrier properties of mucosa and modulating production of cytokines (protein mediators produced by immune cells and contribute to cell growth, differentiation and defence mechanisms of the host)[8]. Viable live probionts are better than the non-viable heat-killed probionts in inducing higher immune responses in rainbow trout (Oncorhynchus mykiss Walbaum), especially enhancing head kidney leucocyte phagocytosis, serum complement activity etc., [9]. In recent years, several in vivo and In vitro studies have investigated the interaction between dietary probiotics and immunocompetence in humans as well as in fish and aquatic animals [8,10-17]. By increasing the host’s adaptive and innate immune mechanisms, lactic acid bacteria (LAB) can protect the host against infection by enteric pathogens and tumour development. Immunological and other mechanisms behind the probiotic action may include;
* Stimulation of antibody secreting cell response [18]
* Enhancement of phagocytosis of pathogens [9,19]
* Modification/enhancement of cytokine production/ natural complement activity [20,21]
* Improvement of the host innate or acquired immune responses, direct effect on other microorganism in the digestive tract, adhesion sites, microbial action or response stemming from microbial products, host products or food components [22]
Consequently, probiotic bacteria may influence both adaptive and innate immune responses. Probiotics may reverse the increased intestinal permeability induced by antigens, but no information is available about long-term effects.
Nutritional factors and the immune response
It is well known that nutrition may affect fish health and immune responses. Nutritional factors are components of the diet essential for normal growth and development of the fish. Many of these undoubtedly have roles in the immune response. Vitamins C, B6, E and A, and the minerals iron and fluoride have been identified as micronutrients that can affect disease resistance. With respect to the strict definition of immunostimulants; vitamins and minerals are not immunostimulants as they enhance the immune system by providing substrates and serving cofactors necessary for the immune system to work properly, but controversy exists [1,23].
Readers with special interest on the effect of nutritional factors on immune response of fish are referred to the comprehensive reviews published [1,24-37]. To our knowledge no information is available about nutritional balance and the immune response and as a natural consequence this topic merits further investigations.
β-1,3/1,6-glucans
Glucans are high molecular-weight substances composed of glucose as building blocks, usually isolated from cell walls of bacteria, mushrooms, algae, cereal grains, yeast and fungi [38]. Glucans in general comprise a great variety of substances common in nature (such as cellulose, glycogen, and starch), most of which do not interact with the immune system. Pharmacologically, they are classified as biological response modifiers (BRM). The common feature of immunomodulatory glucans is a chain of glucose residues linked by β-1.3-linkages, also called beta-glucans. Of the different β-glucans, the products known as β-1.3/1.6-glucans (read as “beta-one-three-one-six-glucans”), derived from baker’s yeast, are suggested to be the most potent immune-system enhancers. β-1.3/1.6-glucans is characterized by side-chains attached to the backbone that radiate outward like branches on a tree.
The primary structure of the β-1,3/1,6-glucan is determinant for its immune-enhancing ability. The frequency and nature of side-chains strongly affect the ability of the glucan to mediate binding to surface receptors on the target cells influencing the effectiveness of the glucan as an immunostimulant.
The effectiveness of MacroGard® in stimulating the immune system combined with improved growth and feed conversion has been proven in some experiments [39,40]. MacroGard® has been suggested to be used in feed for all aquatic organisms and also to enrich live feed such as Artemia. Some studies involving shrimp have also suggested that the product contributes to a better growth in crustaceans [40,41].
MacroGard® is an environmentally sound alternative to feed antibiotics and chemotherapeutics for livestock, pets and cultured aquatic organisms. MacroGard® is based on a well-characterized β-1,3/1,6-glucan that has been in use world-wide for almost 25 years as an immune modulating agent in animal husbandry and aquaculture (see Table 1).
| Immunostimulant | Species | Administration and dose | Length of administ-ration | Mechanism of action/results |
References |
|---|---|---|---|---|---|
| β-1,3 glucans | Carp | i.p; 2-10 mg kg-1 BW | 12 days | ↑ phagocytic activity of kidney leucocytes | [152] |
| Channel catfish |
Injected; 50 and 70 μg fish-1 (100 g BW) suspended in 0.2 ml PBS |
2 weeks | ↑ phagocytic, bacterial activity and serum antibody concentration |
[153] | |
| Marron | 0.08, 0.1, 0.2, 0.4 and 0.8 % supplementations |
12 weeks | ↑ haemocyte count → on physiological parameters |
[154] | |
| β-glucan | Atlantic halibut (larvae) |
Immersion (25 mg l-1) | 5 days | Absorbed laminaran | [155] |
| Carp | i.p (10 mg kg-1) | 15 days | ↑ superoxide dismutase and catalase activities |
[156] | |
| Coho | i.p (5 and 15 mg kg-1) | 36 days | → immune response | [157] | |
| Trout | Oral | 4 weeks | ↑ stress resistance | [158] | |
| Turbot | Oral (2 g kg-1) | 5 weeks | ↑ leukocyte number | [159] | |
| Trout | i.p | 18 days | ↑ resistance to IHNV | [39] | |
| Trout | Oral (0, 0.2 and 0.4 %) | 37 days | ↓ expression of genes involved In acute inflammation reaction |
[160] | |
| Sea bass | Oral (2% wet body weight) | 2 weeks | ↑ complement activation | [161] | |
| Sea bass | Oral (0.1%) | 60 days | ↑ serum complement activity, serum lysozyme, gill and liver HSP | [162] | |
| Sea bass | Oral (250, 500, 1,000 ppm) | 25 days | ↑ respiratory burst activity of head kidney macrophages |
[163] | |
| Snapper | Oral (10g kg-1) | 84 days | ↑ macrophage superoxide Anion production and growth → complement activity |
[164] | |
| Tilapia and Japanese eels |
i.p (10 mg kg-1) | 2 days | ↑ lysozyme activity, phagocytic activities in anterior kidney and peripheral blood phagocytes and classical complement path- way |
[165] | |
| Nile tilapia |
Oral | 6 weeks | ↑ stress resistance | [166] | |
| Red drum | Oral (2% of diet) | 6 weeks | → on stress resistance | [124] | |
| Flounder | Oral (3 g kg-1) | n.i | ↑ respiratory burst activity | [167] | |
| Channel catfish |
Oral (1g/kg) | 4 and 6 weeks |
→ growth performance hematology or immune function Some improvement in stress resistance | [168] | |
| Climbing perch |
Β-glucan suspension (0, 5, 10 15 mg l-1) |
7 days | ↑ lysozyme and bactericidal activities and survival of spawns immersed in 15 mg l-1 when challenged with A. hydrophila | [169] | |
| American White shrimp |
Immersed | 120 hours | ↑ total haemocyte counts and soluble haemocyte protein after 48-120 hours | [170] | |
| Shrimp | Oral (0.2% w/w) | 7 days | ↑ prophenoloxidase and reactive oxygen intern- mediate activity | [171] | |
| White shrimp |
Oral (2000 mg kg-1) | 6 weeks | ↑ total haemocyte counts, phenoloxidase, superoxide anion and superoxide dismu- tase until day 27 | [172] | |
| Carpet shell clam |
i.v (0.05, 0.1, 0.5 and 1.0 mg ml-1) |
Different sampling times |
↑ nitric oxide production Hemolymph treated with β-glucan inhibited growth of Vibrio algynolyticus, Vibriosplendidus and Escherichiacoli | [173] | |
| Scallop | i.p | 7 days | ↑six enzymes in haemocytes. β-glucan-induced activation was stronger at 15Ë?C than 25Ë?C . | [174] | |
| β-glucan + mannose | Snapper | Oral (0.1-1.0% w/w) | n.i in vitro study |
↑ macrophage activation | [175] |
| β-glucan + LPS | Salmon | 10 μg ml-1 | n.i in vitro study |
↑ lysozyme activity | [176] |
| β-1,3 and β-1,6 glucans |
Sea urchin eggs |
0.01, 0.05, 0.1, 0.25, 0.5 and 1.0 mg ml-1 |
Incubation | ↑ survival of embryos | [177] |
| β-1,3 and β-1,6 inked yeast glucan (M-glucan) |
Salmon | Injected i.p (1 ml of 0.5% (w/v) M-glucan in 0.9% saline) |
22 days | ↑ macrophage and neutrophil numbers, head kidney macrophages and ability to kill A. salmonicida | [178] |
| Salmon | 0, 500 and 1000 mg kg-1 | 70 days | → no detrimental effect ↓lice- infected fish when the fish were fed 14 % soybean meal + 14 % sunflower and yeast extract |
[179] | |
| Trout | Injected i.p 1 ml of 1% M-glucan suspension |
3 weeks | ↑macrophages ability to kill A. salmonicida and serum lysozyme activity | [180] | |
| β-1,3 glucan from Schizophyllum commune |
Black tiger shrimp |
Oral (0 and 2 g kg-1) | 40 days | ↑ survival, haemocyte activity, cell adhesion and superoxide anion production | [181] |
| Glucan (barley extract (Sigma) |
Trout | Injected i.p. 100 μg glucan dissolved in PBS. Immersed the concentration of 100 μg ml-1 glucan for 30 min |
10 days | ↑ phagocytic activity and numbers of circulatory glass-adherent cells | [182] |
| Yeast glucan | Salmon | i.p | 20 days | ↑ resistance to V. anguillarum, V. salmonicida and Y. rückeri | [183] |
| Salmon | i.p | 4 weeks | ↑ complement and lysozyme activity | [184] | |
| Salmon | i.p (0.5 mg/fish) | 43 weeks | ↑ survival against A. salmonicida infection | [185] | |
| Salmon | i.p | 7 weeks | ↑ antibody → resistance to A. salmonicida |
[186] | |
| Salmon | Oral and anal (150 mg kg-1) |
2 days | ↑ acid phosphatase | [187] | |
| Trout | i.p | n.i | ↑ lysozyme activity | [188] | |
| Trout | Diet (0, 0.125 and 0.25 g k-1) |
4 + 4 weeks | 4 weeks ↑ survival and growth 4 – 8 weeks ↑ survival (0.25 g k-1) → feed conversion |
[189] | |
| Indian major carp |
15 + 30 days | ↑ superoxide anion, in vitro phagocytic activity and nitrite production of leucytes (10 days) → heamatocrit |
[190] | ||
| Indian major carp and rohu |
Diet (0 and 5 g kg-1) | 15 + 20 days | ↑ phagocytosis and prolifer- ation of lymphocytes and oxidative radical and nitrate production | [191] | |
| Pacu | Diet (2.5 and 5 %) | 86 days | ↑ feed efficiency → plasma glucose and cortisol |
[192] | |
| Fathead minnows |
Diet (10 g kg-1) | 14 days | ↑ degranulation of primary granules in fish neutrophils | [193] | |
| Shrimp | immersion | 43 days | ↑ growth at 0.5; 1 and 2 mg ml-1, but not at 0.25 mg ml-1 ↑ phenoloxidase activity and resistance to V. vulnificus |
[194] | |
| Black tiger shrimp |
Oral (0.2 %) | 3 days | ↑ phenoloxidase, no. of haemocytes and bacterial killing activity against Vibrio harveyi |
[195] | |
| Pacific white shrimp |
Oral (0, 1 and 2 g kg-1) | 4 weeks | ↑ total haemocyte – and granular haemocyte counts → growth |
[196] | |
| Yeast glucan + Vitamin C |
Trout | Diets containing yeast glucan and vitamin C at 150, 1,000 and 4,000 p.p.m |
4 weeks | ↑ alternative pathway of complement – and macrophage activity and specific Ab response following vaccination with Y. ruckeri |
[197] |
| Saccharomyces cerevisiae | Sea bass | Diet (0, 10, 20, 30 and 40 %) |
12 weeks | ↑ feed conversion (10, 20 and 30 %), N retention (% N intake) → growth and energy retention (% E intake) |
[198] |
| Sea bream | 0 and 10g kg-1 | 6 weeks | ↑ serum peroxidases and complement activity | [199] | |
| Sea bream | In vitro experiment, head kidney leucocytes | 0 – 30 min | ↑ degranulation | [200] | |
| Sea bream | 0, 1, 5 and 10g kg-1 | 6 weeks | ↑ total serum IgM level | [201] | |
| Sea bream | In vitro experiment, blood leucocytes | 30 min | ↑ inhibition of the phagocytic ability | [202] | |
| Sea bream | 0, 11.5 and 23 % (Y2) | 10 weeks | ↑ growth, feed intake, lipid content (Y2) and arginase activity → body composition |
[203] | |
| Gilthead seabream | Lyophilised S. cerevisiae (0, 1, 5 or 10g kg-1) | 4 weeks | ↑ cellular parameters | [204] | |
| Gilthead seabream | S. cerevisiae (0, 10, and 20 %) (BDY protein) instead of fish meal | 12 weeks | → growth, alkaline phospha-tase, blood urea Nitrogen, serum protein, cholesterol, triglyceride, albumin and amylase ↓ plasma glucose (10 % BDY) |
[205] | |
| Nile tilapia | Diet (0 and 0.1 %) | 9 weeks | ↑growth and feed efficiency | [206] | |
| Nile tilapia | S. cerevisiae(0, 25, 50, 75 and 100 %) (BDY protein) instead of soy bean meal (SBM) protein |
6 weeks + 5 days | → growth and feed utilization when 50 % BDY protein instead of SBM protein | [207] | |
| Nile tilapia | S. cerevisiae(0, 25, 50, 75 and 100 %) (BDY protein) instead of soy bean meal (SBM) protein |
122 days | → growth and feed utilization when 25 % BDY protein instead of SBM protein | [208] | |
| Hybrid striped bass | Diet (0, 1, 2 and 4 %) | 8 weeks | ↑growth and feed efficiency | [209] | |
| Hybrid striped bass | Diet (0, 1 and 2 %) | 7 weeks (trial 1) and 4 weeks (trial 2) | → growth (trial 1) Trial 2 ↑ resistance against Streptococcus iniae and extra-cellular superoxide anion → growth and feed efficiency |
[210] | |
| Hybrid striped bass | Diet (0, 1 and 2 %) | 16 and 21 weeks | ↑growth, feed efficiency, serum peroxidase and extracellular superoxide anion of head kidney macrophages (16 weeks) → resistance against mycobacterial infection |
[211] | |
| Pacu | S. cerevisiae (0, 30, 35, 50, 70 and 100 %) (BDY protein) instead of fish meal | 54 days | ↑growth and feed utilization (until 50 % replacement). Protein retention was higher in fish fed 35 and 50 5 replace- ment → protein digestibility |
[212] | |
| Galilee tilapia | 0 and 10g kg-1 | 6 weeks | ↑growth, feed utilization and resistance against waterborne Cu toxicity |
[213] | |
| Beluga | Diet (0, 1 and 2 %) | 6 weeks | 2 % inclusion ↑final weight, weight gain, SGR and FCR ↑ autochthonous LAB levels →survival rate, haematological and serum biochemical parameters resist |
[97] |
Salmon – Atlantic salmon; Trout – rainbow trout; Coho – Coho salmon (Oncorhynchus kisutch), Turbot – Scophthalmus maximus; Sea bass - Dicentrachus labrax, Snapper
– pink snapper (Pagrus auratus); Flounder – Japanese flounder (Paralychthis olivaceus), Nile tilapia - Oreochromis niloticus; Red drum - Sciaenops ocellatus; Channel
catfish - Ictalurus punctatus
ROS – reactive oxygen species; IHNV – infectious hematopoietic necrosis virus
Table 1: Glucans as immunostimulants in fish, shrimp and scallop.
When included in feed or administered on mucosal surfaces, MacroGard® modulates immune responses and affects biological functions in a favourable way. The products are extracted and purified from food-grade baker’s yeast by patented processes. The use of MacroGard® may be regarded as a natural input that compensates for a possible sub-optimal function of the immune system of farm animals, companion animals and aquatic species in intensive culture. The product has also been suggested to reduce the use of antibiotics in animal husbandry and consequently the concern that developing antibiotics resistance that may undermine the health security effective antibiotics represents. In aquaculture, glucans have been successfully been used to enhance the resistance of fish and crustaceans against bacterial and viral infections and (Table 1) present an overview of papers using glucans as immunostimulants in aquaculture.
In Norway, β-glucan, as prebiotics and nucleotides, has been used in the pancreas disease (PD) diets; to fed fish suffering from PD (Gonzalez Vecino, personal communication). In this case, the β-glucans are a very small part of the diet composition, and the benefits of the diets come mainly from a very specific formulation concentrated to recover pancreas and reduce inflammation in muscle and heart.
Baker’s yeast, Saccharomyces cerevisiae, is the 2nd major by-product from brewing industry and contains various immunostimulating compounds such as β-glucans (the cell walls are constructed almost entirely by β-1,3-D-glucan, β-1,6-D-glucan, mannoproteins and chitin bound together by covalent linkages), nucleic acids and oligosaccharides [42] and it has the capacity to enhance growth and increase both humoral (myeloperoxidase and antibody titer) and cellular (phagocytosis, respiratory burst and cytotoxicity) immune responses, and to increase or confer resistance against pathogenic bacteria in various fish species (Table 1).
Bioactive alginate (high-M alginate) and Ergosan
The adaptive immune system is poorly developed in early developmental stages of fish [43,44], which is why immunostimulants and probiotics have been used in an attempt to increase survival of larvae against microbial pathogens [45-47]. In this respect, alginate has been proposed as a potential candidate. Alginate is a polysaccharide composed of β-1,4-D-mannuronic acid (M) and C5-epimer α-Lglucuronic acid (G) [48]. The monomers are usually arranged in M-blocks, G-blocks and alternating MG-blocks [49]. Alginate also binds various cations found in the seawater such as Mg2+, Sr2+, Ba2+, and Na+. Commercial alginates are extracted from three species of brown algae; Laminaria hyperborean, Ascophyllum nodosum and Macrocystis pyrifera, in which alginate comprises up to 40% of the dry weight [50]. Furthermore, bacterial alginates, not commercial products, have also been isolated from Azotobacter vinelandii and several Pseudomonas species [51].
Commercially available alginates today have M-content ranging between 30 and 70%. Alginates with even higher M-content, typically higher than 80%, have also been shown to be potent stimulators of immune cells such as human monocytes [52]. High-M alginate has also been used as an immunostimulant for enhancement of innate immune resistance in fish larvae and fry [53-56].
Ergosan is an algal based product and contains 1 % alginic acid extracted from Laminaria digitata. The first study on Ergosan in aquaculture, to the author’s knowledge, was reported by Miles and coauthors on striped snakehead (Channa striata) [57]. In a study with rainbow trout, Peddie et al. reported that a single injection of 1 mg of Ergosan significantly augmented the proportion of neutrophils in the peritoneal wall, increased the degree of phagocytosis, respiratory burst activity and expression of interleukin-1β (IL-1β), interleukin-8 (IL-8) and one of the two known isoforms of tumour necrosis factor-alpha (TNF-α) in peritoneal leucocytes one day post-injection [58]. However, humoral immune parameters were less responsive to intraperitoneal alginate administration with complement stimulation only evident in the 1 mg-treated group at 2 days post-injection. (Table 2) presents an overview of reports using alginates and Ergosan in fish studies.
| Immunostimulant | Fish | Administration and dose | Length of administ-ration | Mechanism of action/results |
References |
|---|---|---|---|---|---|
| High-M alginate | Atlantic halibut |
Immersion | n.i | ↑ survival | [214] |
| Atlantic halibut |
Bioencapsulated in Artemia | Different periods during initial feeding | ↑ growth | [215] | |
| Atlantic cod and spotted wolffish |
Diet | 59 days 55 days |
↑ growth Uptake of 125I-labelled molecule in the stomach and intestine |
[56] | |
| Turbot | Enriched in Artemia | 1 week | ↑ survival | [53] | |
| Alginate | Atlantic salmon |
Macrophage culture |
n.i | ↑ phagocytic activity and respiratory burst |
Rokstad et al. (unpublished data) cited in Vadstein [54] |
| Atlantic salmon | Diet | Six months | ↑ lysozyme activity → survival, colour, taste consistency and grading |
[216] | |
| Turbot | Diet | 13 days | ↑ protein synthesis and protein turnover → survival and larval size |
[217] | |
| Ergosan | Striped snakehead | i.p | 14 days | ↑ inhibition index of macrophages and serum on in vitro growth of Aphanomyces invidans | [57] |
| Rainbow trout | i.p | 1 day | ↑ neutrophils and respiratory burst | [58] | |
| Sea bass | Diet (0.5 %) | 60 days | ↑ serum complement activity, serum lysozyme, gill and liver HSP |
[162] | |
| AquaVac Ergosan | Rainbow trout | Diet (0.5 %) | Three cycles (95 days in total) | ↑ growth (95 days) and the growth related gene expression of IGFI, TRαmRNA and TRβ, and gene expression of IL-1β, IL8 and TLR3 in spleen ↓ cortisol, HSP70 gene expression in liver, MYO gene expression |
[218] |
Symbols represent an increase (↑) in the specified response; no change (→); decrease (↓).
n.i – no information given
Table 2: Bioactive alginate (high-M alginate) and Ergosan as immunostimulants in fish.
Plant extracts
Some immunostimulants cannot be used because of various disadvantages, such as high cost and limited effectiveness upon parenteral administration. Numerous plants have on the other hand long been used in traditional medicine for the treatment and control of several diseases [59]. As herbs have little side effects and are easily degradable and abundantly available in farm areas, numerous investigations have investigated the effect of plant products on innate and adaptive immune response and to prevent and control fish and shellfish diseases [60].
Dügenci and co-authors investigated the effects of mistletoe, nettle and ginger on dietary intake of rainbow trout [61]. The diets contained lyophilized extracts of these plants at two inclusions levels, 0.1 and 1%, in a 3 week experiment. At the end of the experimental period, various parameters of innate defence mechanisms, including extracellular and intracellular respiratory burst activities, phagocytosis in blood leukocytes, total plasma protein level, specific growth rates and condition factors were examined. Inclusion of the plant materials increased the extracellular respiratory burst activity (P<0.001) compared to control. Furthermore, fish fed the diet containing 1% ginger roots exhibited a significant innate immune response. Phagocytosis and extracellular burst activities of blood leukocytes were significantly higher in this group compared to the control group. All plant extracts increased plasma protein level except for the 0.1% ginger supplemented diet. The highest level of plasma proteins was observed in the group fed with 1% ginger extract.
Oral administration of the medical plant, Eclipta alba, on the non-specific immune responses and disease resistance of tilapia (Oreochromis mossambicus) has been investigated [62]. The results indicated that E. alba administrated in the diet significantly enhanced the non-specific immune parameters tested. Furthermore, when tilapia was challenged with Aeromonas hydrophila mortality was significantly reduced in E. alba treated fish.
Lectins are proteins or glycoprotein substances, usually of plant origin, and they are sugar-binding proteins which are highly specific for their sugar moieties. Lectins are also known to play important roles in the immune system by recognizing carbohydrates that are found exclusively on pathogenic bacteria, or that are inaccessible to host cells [63]. Examples are the lectin complement activation pathway, and mannose binding lectin (MBL), also named mannose- or mannanbinding protein (MBP). MBL recognizes carbohydrate patterns, found on the surface of a large number of pathogenic micro-organisms, including bacteria, viruses, protozoa and fungi. Readers with special interest in lectins and immune response are referred to the recent comprehensive reviews [64-65].
In a recent paper, Galina and co-authors reviewed studies currently being carried out on herbs and herbal extracts that have been shown to modulate the immune system in fish and special attention was given to; Astragalus, Ganoderma, Lonicera and Chinese and Indian herbs [66]. Based on the results available per se Galina and co-authors suggested that herb extracts might have a potential application as an immunostimulant is fish, due to that they can easily be obtained, are as not expensive and act against a broad spectrum of pathogenic bacteria, and could be alternatives to vaccines, antibiotics or S. In a more recent study, Soosean and co-authors reported that mangosteen (Garcinia mangostana) extracts as feed additive to African catfish (Clarias gariepinus) had no significant effect on growth parameters, feed conversion ratio and haemoglobin content. However, significantly higher red blood cells and white blood cell counts were recorded in fish fed the shoot extract [67]. In their study on kelp grouper (Epinephelus bruneus), Harikrishnan and co-authors evaluated the efficacy of dietary doses of Lactuca indica extracts on immunological parameters and disease resistance against Streptococcus iniae infection [68]. Based on their results, the authors suggested that supplementation of L. indica enhanced the immune system and the disease resistance against pathogenic infection. Enhanced immune response of tiger shrimp (Penaeus monodon) by the medical herb black nightshade (Solanum nigrum) and disease resistance against Vibrio harveyi were investigated by Harikrishnan and co-authors [69]. In this study the authors displayed improved immune response including respiratory bursts, PO-, SOD – and GPx activities and disease resistance by feeding tiger shrimp black nightshade at 0.1 and 1 % doses. This herb has also been shown to have inhibitory In vitro growth effect on A. hydrophila and improved survival and increased haematological parameters of spotted snakehead (Channa punctatus) infected with A. hydrophila [70].
Readers with special interest in the topic plant products on innate and adaptive immune response of fish and shellfish are referred to the recent review of Harikrishnan et al. [60].
Mechanisms of Action
Phagocytic activity of kidney leucocytes
Phagocytic activity is the principle function of kidney leucocytes of teleosts. Monocytes, macrophages, dendritic cells, and neutrophils belong to the phagocytic leucocytes called phagocytes. In host defense, phagocytes respond to microbes in a sequential manner: active recruitment of the cells to the sites of infection through inflammatory responses, ingestion of microbes by the process of phagocytosis, and destruction of the ingested microbes. Phagocytosis is an active, energyrequiring engulfment process of large particles (>0.5 μm in diameter). The first step in phagocytosis is the recognition of microbe by the specific receptors expressed on the phagocyte such as TLRs (toll-like receptors). The bound microbes are ingested into vesicles forming phagosomes inside of the cells and the phagosomes are then fused with lysosomes that contain many different proteases. The internalized microbes will be killed there through proteolytic processes [71].
Macrophage activation
Macrophage activation is initiated upon binding of the microbe to the cell. The recognition of microbes by phagocytes such as macrophages, neutrophils, and dendritic cells is selective and mediated by receptors on phagocytic cells. Upon microbe binding, the receptors cooperatively activate the cells through a receptor-mediated signal transduction to kill ingested microbes. The receptors responsible for microbe recognition include TLR (toll-like receptors), G protein-coupled receptors, antibody Fc and complement C3 receptors, and receptors for cytokines, mainly IFN-γ. Activated macrophages kill phagocytosed microbes by the action of microbicidal molecules in phagolysosomes of phagocytes. In addition, activated macrophages secrete cytokines such as TNF, IL-1, and IL-12, which cause inflammation and bridge to activate the adaptive immune system.
Respiratory burst activity
Respiratory burst (sometimes called oxidative burst) is the rapid release process of reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical and hydrogen peroxide from activated macrophages and neutrophils. The respiratory burst, releasing important bactericidal ROS molecules, plays a crucial role to remove microbes from the body. The respiratory burst is mediated by the enzyme phagocyte oxidase, which converts molecular oxygen to ROS and is usually triggered by bacterial products or by inflammatory mediators. The respiratory burst activity is an indicator of the status of macrophage and neutrophil activation [71].
Acid phosphatase
Acid phosphatase is a ubiquitous enzyme that removes phosphate groups from other molecules. The acid phosphatase is one of the lysosomal hydrolases which exist in phagolysosomes of macrophages (lysosomal acid phosphatases). When the macrophage is activated, the inside of phagosome becomes acidic. In an acidic environment, lysosomal hydrolases including acid phosphatase are activated to kill microbes. Acid phosphatase activity is an indicator of the microbicidal activity of macrophages and a high concentration of acid phosphatase in the blood is a sign of a chronic infection [71,72]. Recently, acid phosphatase activity tests are used for the diagnosis of prostate cancer and breast cancer in human.
Serum complement activity
The complement system is one of the major effector mechanisms of the humoral immunity as well as the adaptive immunity, which involves the clearing of pathogens from an organism. The complement system consists of serum and cell surface proteins that are activated by microbes (the alternative pathway) and antibodies (the classical pathway). Activation of the complement system involves the sequential proteolysis of proteins to generate new enzyme complexes with proteolytic activity, which is a characteristic of a proteolytic enzyme cascade. The products of the complement activation become covalently attached to microbes, antibodies bound to microbes, and to other antigens. The binding of complement products to the target molecules cause lysis of the microbe, facilitation of opsonization (the process of attaching IgG or complement fragments to microbial surfaces for phagocytosis) and phagocytosis, and inflammation [71].
Phenoloxidase activity
Phenoloxidases are known to be involved in the innate immune response of invertebrates such as shrimps and crabs. Phenoloxidases are composed of tyrosinases, catecholases and laccases which are the terminal components of the prophenoloxidase (proPO) system, the modified complement system of several phyla of invertebrates [73]. The proPO system is present in the blood of a wide range of marine invertebrates, especially crustaceans. Phenoloxidases play a role in bactericidal activity and the enhancement of phagocytosis in crustaceans [74-76].
Serum antibody concentration
B lymphocytes recognize antigens such as microbes using their receptors (membrane-bound antibody molecules). The engagement of antigen receptors and other signals trigger the lymphocytes to expand their numbers and to differentiate into antibody-secreting mast cells, which secrete different classes of antibodies with distinct functions. The functionally different antibodies have the same antigen specificity, through which the antibodies recognize the same microbes despite their different classes. Antibodies bind to microbes and prevent them from infecting cells (neutralization). The binding of antibodies to microbes also promotes opsonization and phagocytosis of microbes to kill and mediate antibody-dependent cellular cytotoxicity (ADCC). In addition, antibodies trigger activation of the complement system (the classical pathway), which in turn causes to increase phagocytosis of microbes opsonized with complement fragments and inflammation. Serum antibody concentration is an indicator of B lymphocyte activation against specific microbes [71].
Leukocyte number
Leukocytes (white blood cells) are immune cells involved in defending the body against both infectious microbes and foreign materials. Leucocytes consist of neutrophil, eosinophil, basophil, monocyte, and lymphocyte. They are all derived from a hematopoietic stem cell in the bone marrow. Neutrophils and monocytes are involved in the innate immune response as the first line of the defense system. Effector cells of the innate immune system circulate in the blood and migrate into tissues and kill microbes through phagocytosis. The innate immune cells also play a role in the inflammatory response by releasing cytokines. Lymphocytes including T cells and B cells are the only cells in the body capable of specifically recognizing and distinguishing different antigenic determinants (epitopes). When these adaptive immune effector cells recognize the microbes, the number of lymphocytes is significantly increased as a part of their effector functions. Although the number of the innate immune cells are also expanded upon activation, the level of increase is much lower than that of the adaptive immune cells. The number of leukocytes in the blood is often an indicator of the activation of the immune system or of leukemia [71].
Lysozyme activity
Lysozyme is a cationic enzyme that attacks the β-1,4 glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine in the peptidoglycan of bacterial cell walls. This activity causes the lysozyme to lyse certain gram-positive bacteria and even some gram-negative bacteria. Lysozyme activity has been detected in mucus, serum, organs and eggs of several fish species [77]. Lysozyme is produced mainly by macrophages and its production is induced in response to microbial components such as microbial lipopolysaccharide (LPS) [78] and many other immunostimulants.
Do immunostimulants raise the level of protection against disease?
A large number of reviews have been published during the last 20 years concerning the advantages of immunostimulants in fish and the immune system [1,3,23,28,30,32,79-90]. In this section an attempt has been made to compile the recent developments in the field, mainly over the last five years, besides the earlier work done in the field of disease resistance in fish with exposure to immunostimulants.
Several types of β-glucan have been successfully used to enhance resistance of fish and crustacean against bacterial and viral infections. An overview is presented in (Table 3). Most pathogens used in these challenge experiments are bacteria reported in commercial aquaculture and include; A. hydrophila, Aeromonas salmonicida, Aphanomyces invadans, Edwardsiella ictaluri, Edwardsiella tarda, Photobacterium damselae ssp. piscicida, Vibrio (Listonella) anguillarum, Vibrio harvei, Yersinia ruckeri, Lactococcus garvieae, S.iniae and Streptococcus sp. as well as infectious hematopoietic necrosis virus (IHNV), white spot syndrome virus (WSSV) and parasites. Readers with special interest in the topic immune response and aquatic viruses are referred to the recent reviews [91,92].
| Resistance to pathogen |
Agent | Species | Route of exposure |
Length of administration | Mechanism of action/results |
References |
|---|---|---|---|---|---|---|
| Aeromonas hydrophila |
Yeast glucan | Tilapia and grass carp | i.p | 14 days | ↑ protection in both species and number of NTB-positive staining cells | [219] |
| Glucan | Rohu | Oral | 7 days | ↑ phagocytotic activity, bactericidal activity, antibody titre and agglutinin level |
[220] | |
| Glucan | Rohu | i.p | 28 days | ↑ phagocytotic activity, lysozyme activity, bactericidal activity, complement activity and resistance |
[221] | |
| Garlic | Rohi | i.p | 10 days | ↑superoxide production, lysozyme activity and resistance | [222] | |
| Glucan | Catla | i.p | 30 days | ↑ antibody titre and macrophage activating factor | [223] | |
| Glucan from S. cerevisiae |
Carp (25-30 g) |
i.p (0, 100, 500 and 1000 µg fish-1) |
14 days | ↑ total leukocyte count and neutrophil and monocytes, increase survival in a challenge experiment with Aeromonas hydrophila | [224] | |
| Glucan from S. cerevisiae |
Carp (25-30 g) |
i.p (0, 100, 500 and 1000 µg fish-1) |
14 days | ↑ total leukocyte count and neutrophil and monocytes, superoxide anion production by kidney macrophages and resistance (500 µg) against A. hydrophila | [225] | |
| β-glucan in combination with LPS |
Carp (25-30 g) |
i.p, bathing and oral ad-ministration | 14 | ↑ total blood leukocyte count and neutrophil and monocytes and resistance against A. hydrophila when i.p and oral administration were used | [226] | |
| Levamisole | carp | Diet (0, 125, 250 and 500 mg kg-1) |
70 days | ↑ resistance and erythrocyte count, haemoglobin content, haematocrit, total serum protein, albumin and globulin of fish fed 250 mg kg-1 |
[227] | |
| β-glucan and S. uvarum |
Carp | i.p | 15 days | ↑ resistance and serum lysozyme activity | [228] | |
| Glucan | Asian catfish |
Oral | 30 days | ↑ antibody titre and protection | [229] | |
| Glucan | Blue gourami | i.p (5, 10, 20 and 40 mg kg-1) | 22 days | ↑ chemiluminescent response of head-kidney phagocytic cell isolated from fish i.p with a dose of 20 mg kg-1 and resistance | [230] | |
| Glucan | Indian major carp | i.p | 1 week | ↑superoxide production and phagocytic activity resistance unclear | [231] | |
| S. cerevisiae | zebrafish | i.p (0.5, 2 and 5 mg ml-1) |
6 + 4 days | ↑ resistance (5 mg), myelomonocytic cells in kidney and enhanced the ability of kidney cells to kill the pathogen | [232] | |
| S. cerevisiae | Nile tilapia | Oral (0, 0.25, 0.5, 1, 2 and 5 g kg-1) and i.p |
12 weeks + 10 days |
12 weeks ↑ growth, feed utiliza-tion and protein turn-over (1-5 g kg-1) and resistance (5 g kg-1) |
[233] | |
| S. cerevisiae | Nile tilapia | Oral (2 % autolyzed yeast; 0.3 % cell wall) | 70 days | ↑thrombocytes and nonspecific acute inflammatory response ↓neutrophils, macro-phages an lymphocytes |
[234] | |
| Green tea (Camellia sinensis) |
Nile tilapia | Oral (0, 0.125, 0.25, 0.5, 1 and 2 g kg-1) | 12 weeks + 10 days (i.p challenge) |
↑ growth and feed utilization (highest at 0.5 G kg-1), haematological and biochemical parameters (0.25-2 g kg-1) and resistance with increasing inclusion levels |
[235] | |
| Debaryomyces hansenii |
Leopard grouper |
Oral (106 yeast g-1) |
5 weeks | ↑ resistance and growth → immunological para-meters after 4 weeks |
[236] | |
| Macrogard | Tench | Oral (0, 0.5, 1 and 2 g kg-1) |
1 month | ↑ resistance and phagocytic activity (1 and 2 g kg-1) | [237] | |
| Schizophyllan | Two orna- mental fish species |
i.p (108 cells ml-1) | 24 days | ↑ resistance against both pathogens | [238] | |
| A.hydrophila and Pseudomonas fluorescens | Glucan | Brook trout | i.p and 30 min immersion | 4 weeks | ↑ resistance (up to 7 days). By day 14 no resistance was reduced | [239] |
| Aeromonas salmonicida |
Macrogard | Trout | Oral | 1 week | ↑ MPO activity, phagocytic activity, superoxide production and Ig level | [240] |
| Aeromonas salmonicida and Vibrio salmonicida |
Laminaran | Atlantic salmon | i.p (23.6 mg kg-1) | 35 days | ↑resistance against V. salmonicida → resistance against A. salmonicida |
[241] |
| Aphanomyces invadans |
Ergosan | Striped snakehead | Injected intramuscularly | 2 weeks | ↑ inhibitory effect of Serum and macrophage activating factor | [57] |
| Edwardsiella ictaluri | Yeast glucan | Channel catfish |
i.p | 2 weeks | ↑ resistance and phagocytic activity | [153] |
| Glucan | Channel catfish |
Oral | 2 weeks | ↑ macrophage and neutrophil migration → resistance |
[242] | |
| Edwardsiella tarda |
Glucan | Carp | i.p | 12 days | ↑ resistance | [152] |
| Glucan | Rohu | i.p | 10 days | ↑ bactericidal activity ↑resistance |
[243] | |
| Glucan | Rohu | i.p | 28 days | ↑ phagocytotic activity, lysozyme activity, bactericidal activity, complement activity and resistance |
[221] | |
| Glucan - injections |
Rohu | i.p | 28 days | ↑ phagocytotic activity, lysozyme activity, bactericidal activity, complement activity and resistance | [222] | |
| Yeast glucan | Tilapia and grass carp | i.p | 14 days | ↑ protection in both species and number of NTB-positive staining cells | [219] | |
| Pasteurella piscicida | Glucan | Yellowtail | i.p | 10 days | → resistance | [244] |
| Photobacterium damselae ssp. piscicida |
Glucan | Gilthead sea bream | Immersion (107 cells l-1) |
10 days | ↑ resistance (10 g kg-1) → resistance (5 g kg-1) and phagocytic activity |
[245] |
| Vibrio alginolyticus | Glucan | White shrimp | Oral | 120 hours | ↑ resistance, phenol- oxidase and respiratory burst | [246] |
| Glucan | Fresh-water prawn | Bath exposure with glucan. | 4 hours + 1 week immersion with the pathogen | ↑ haemocyte lysate supernatant, lysozyme and phosphatase active-ties and increased resistance → total protein content | [247] | |
| Vibrio anguillarum | Yeast glucan | Salmon | i.p | 20 days | ↑ resistance | [183] |
| High-M alginate | Turbot | Immersion (105 cells ml-1) |
1 week | ↑ resistance | [53] | |
| High-M alginate | Atlantic halibut | Immersion (5x105 or 1.4x107 cells ml-1) |
Different periods during initial feeding | ↑ resistance at the highest dose → resistance at lowest dose |
[215] | |
| Cationic cod milt protein | Atlantic cod (5 g) | Bath challenge (5x106 cells ml-1) |
4 weeks | ↑ resistance | [248] | |
| Vibrio anguill-arum and Vibrio campbellii |
Glucan from S. cerevisiae |
Gnoto-biotic Artemia | Immersion (5x106 cells ml-1) |
6 days | ↑ resistance against both pathogens | [249] |
| Vibrio campbellii | Glucan from S. cerevisiae |
Artemia | Immersion (5x106cells ml-1) |
5 days | Protection against Vibrio campbellii when supplied in advance of the challenge | [250] |
| Glucan from S. cerevisiae |
Artemia | Immersion (5x106 cells ml-1) |
6 days | ↑ survival and resistance against V. campbellii | [251] | |
| Vibrio damsela | Yeast glucan | Turbot | Yeast glucan was used as adjuvant |
42 days | ↑ activities of the immune parameters when glucan was injected after the bacterin | [252] |
| Vibrio harvei | Glucan | Croaker | i.p | 8 weeks | ↑ lysozyme activity, phagocytic activity and resistance (0.09%) → resistance (0.18%) |
[253] |
| Vibrio vulnificus | Glucan | Tiger shrimp | Immerse | 43 days | ↑ resistance in shrimp treated with 0.5 and 1 mg/ ml glucan but no in groups treated with 0.25 and 2 mg/ ml glucan | [194] |
| Vibrio sp. 90-69B3 |
Yeast Culture | Pacific white shrimp | Oral | i.p after 4 weeks |
↑ resistance | [254] |
| Yersinia ruckeri | Yeast glucan | Salmon | i.p | 20 days | ↑ resistance | [183] |
| S. cerevisiae | Rainbow trout |
i.p | 14 days | ↑ resistance, growth, lysozyme and comple-ment activities | [255] | |
| Lactococcus garviae | Alginate micro- particles |
Trout | i.p | 3 weeks | → resistance | [256] |
| Streptococcus sp. | Glucan | Yellowtail | i.p | 10 days | ↑ resistance and serum complement ↑ lysozyme activity |
[244] |
| Sodium alginate | Grouper | i.p | 6 days | ↑ lysozyme activity, respiratory bursts, phagocytic activity and resistance |
[257] | |
| Streptococcus iniae |
Glucan | Hybrid striped bass | Oral | 3 weeks | → resistance | [258] |
| Glucan | Nile tilapia | Oral | 14 weeks | ↑ resistance when fish were fed 100 and 200 mg glucan | [259] | |
| Glucan | Shark | Oral | 2 weeks | ↑ resistance | [260] | |
| Virus | ||||||
| IHNV | Glucan | Rainbow trout | i.p | 18 days | ↑ resistance → respiratory bursts and TNF-α-expression |
[39] |
| WSSV | β-1,3-glucan | Penaeus monodon | Oral (0 and 2g kg-1) |
15 days | ↑ resistance | [261] |
| β-1,3-glucan | Penaeus monodon | i.p | 20 days | ↑ resistance and the Immune system | [262] | |
| Marine yeast (Candida sake) |
White prawn | Oral (10 %) | 40 + 10 days | ↑ immunity index and resistance | [263] | |
| Marine yeast glucan |
Penaeus monodon | Oral | 21 days | ↑ resistance, but higher molecular weight and lower degree of branching acts better | [264] | |
| Parasite | ||||||
| Loma morhua | Glucan | Atlantic cod | Oral | n.i | ↑ lymphocyte density | [265] |
| Loma salmonae | β-1,3/1,6 glucan |
Rainbow trout | i.p (0, 50, 100, 500 and 1000 µg) | 10 weeks | During week 8-9 post exposure a significant reduction in no. of xenoma-positive fish was noticed. | [266] |
i.p – intraperitoneal injection
n.i – no information given
IHNV – infectious hematopoietic necrosis virus
IHNV – infectious hematopoietic necrosis virus
WSSV - White spot syndrome virus
Table 3: Immunostimulants and disease susceptibility in fish and shrimps.
Recognition of invasive pathogens is an essential step for the activation of the immune system. This is accomplished by binding of PRRs to PAMPs. The PRRs and PAMPs activate appropriate immune responses [93]. β-glucan binding proteins (β-GBP) are known as one of the most important PRRs in invertebrates [94] and fish [95]. The presence of these proteins in invertebrates and fish may indicate that the defence systems against β-glucan containing microorganisms are crucial for these organisms.
Effect of immunostimulants on gut microbiota
Immunostimulants seem to be valuable for the control of fish diseases. However, knowledge regarding the ability of immunostimulants to modulate the gut microbiota in a healthier way and decrease the infection pressure by improving the gut function is scarce. To our knowledge only two studies, Gildberg and Mikkelsen [46] and Skjermo et al. [96] have been done on the topic; immunostimulants and modulation of the gut microbiota. In their study on Atlantic cod (Gadus morhua) fry, Gildberg and Mikkelsen evaluated the effect of supplementation of LAB either alone or in combination with immunostimulating peptides [46]. After three weeks of feeding, the fish were challenged by bath exposure to V. anguillarum (107 ml- 1; 1hour). Twelve days after infection significantly (p<0.05) reduced cumulative mortality was recorded in fish fed diet supplemented with LAB, originally isolated from Atlantic salmon (Salmo salar) and with immunostimulating peptides. No synergistic or cumulative effects were achieved by combining LAB and immunostimulating peptides. Four weeks after infection similar cumulative mortality (80–84%) was observed in all groups. LAB seems to colonize the internal mucus layer of the cod fry pyloric caeca, and a significant number of the bacteria survived the passage of the whole gastrointestinal tract.
In a study with Atlantic cod larvae, Skjermo and co-authors [96] evaluated the effect of β-(1→3, 1→6)-glucans (chrysolaminaran) from the marine diatom Chaetoceros mülleri, a commercial yeast-glucan product and high-M alginate (high content of mannuronic acid isolated from Durvillaea antarctica), on the microbial community in larval gut and water. Total colony forming units (CFU) were evaluated on marine agar, and Vibrio - and Pseudomonas - like species on selective agars (TCBS and marine Pseudomonas agar with CFC - supplement). The larvae were rapidly colonised after hatching, but no or weak effects of the stimulants were observed on the bacterial colonisation rates or the bacterial community. The total CFU varied from 101 to 102 CFU per μg larva after initiation of the first feeding. Bacteria belonging to Pseudomonas appeared to increase throughout the period, whereas the level of Vibrio-like bacteria was low and stable. However, in this investigation the authors only focused on characterisation of Pseudomonas- and Vibrio-like bacteria and did not use molecular methods to evaluate the entire bacterial community.
Hoseinifar and co-authors evaluated the effect of brewer’s yeast (S.cerevisiae var. ellipsoideus) on gut microbiota of juvenile beluga (Huso huso) and displayed that juveniles fed 2 % S. cerevisiae var. ellipsoideus increased the levels of autochthonous LAB [97]. The authors suggested that this interesting finding might occur as a result of the provision of enzymes, RNA and free nucleotides, B-complex vitamins and/ or amino acids by the dietary yeast. Whether supplementation of S. cerevisiae var. ellipsoideus improved disease resistance against pathogens was not investigated and merits further evaluation.
Nucleotides
Dietary nucleotides have attracted attention as key ingredients missing from nutritional formulae for many years. They are the building blocks of tissue RNA and DNA and of ATP, and their presence in breast milk has stimulated research in babies which has indicated that supplementation of infant formula milk leads to improved growth and reduced susceptibility to infection [108-110]. Nucleotide fortification of breast milk substitutes has been recommended to the U.S. Food and Drug Administration for approval [111]. There is increasing evidence that nucleotides administered intravenously or in the diet are capable of modifying immune responsiveness and recovery of organs that have undergone a metabolic or inflammatory insult.
It is generally accepted that nucleotides have essential physiological and biochemical functions including encoding and deciphering genetic information, mediating energy metabolism and cell signalling as well as serving as components of coenzymes, allosteric effectors and cellular agonists [112,113]. However, controversy has existed for many years over the roles of nucleotides administered exogenously. As neither overriding biochemical malfunctions nor classical signs of deficiency are developed in endothermic animal models, nucleotides have traditionally been considered to be non-essential nutrients. However, this opinion has been challenged by several research publications during the last decade which suggest that dietary nucleotide deficiency may impair liver, heart, intestine and immune functions [114]. The modulatory effects of dietary nucleotides on lymphocyte maturation, activation and proliferation, macrophage phagocytosis, immunoglobulin responses, gut microbiota as well as genetic expression of certain cytokines have been reported in endothermic animals [115,116]. Nucleotide supplementation has been one important aspect of research on clinical nutrition and functional food development for humans [109,110].
Although initial efforts in evaluation of dietary supplementation of nucleotides for fishes could be traced to the early 1970s, research at that time mainly focused on the possible chemo-attractive effects of these compounds [117-119]. However, the pioneer investigations by Burrels and co-authors resulted in increased attention on nucleotide supplementation for fishes as their studies indicated that dietary supplementation of nucleotides enhanced resistance of salmonids to viral, bacterial and parasitic infections as well as improved efficacy of vaccination and osmoregulation capacity [5,6]. To date, research related to nucleotide nutrition in fishes has to some extent showed consistent and encouraging beneficial results in fish health management (Table 4), although most of the suggested explanations put forward by the authors remain hypothetical. Systematic research on fishes is therefore needed.
| Nucleotide form | Dose and/or feeding regime | Length of administration | Species | Initial size | Effecta | Authors |
|---|---|---|---|---|---|---|
| Ascogen S | 2 and 5 g kg−1 diet | 16 weeks | Hybrid tilapia | 21 days old | ↑growth and survival | [267] |
| Ascogen P | 5 g kg−1 diet, fixed ration approaching satiation daily | 7 weeks | Hybrid striped bass | 7.1; 9.1 g | ↑ neutrophil oxidative radical production and survival after challenge with S. iniae |
[268-269] |
| Ascogen | 5 g kg−1 diet | 120 days | Hybrid tilapia | 30 days old | ↑ antibody titer after vaccination and mitogenic response of lymphocyte | [270] |
| 0.62, 2.5 and 5 g kg−1 diet at 1% bw day−1 | 37 days | Rainbow trout | 163.4–169.7 g fish−1 | ↑ growth | [134] | |
| Nucleic acid | 5.8 and 11.5 % | 10 weeks | Sea bream | 12.7 g | ↑ growth, ornithine carbamyltransferase activity → body composition, hepatic glutamate dehydrogenase and uricase activities |
[203] |
| Optimûn | 2 g kg−1 diet, containing 0.03% NT, 2% bw day−1 | 3 weeks | Rainbow trout | 217 ± 62 g | ↑ survival after challenge with V. anguillarum |
[5] |
| 2 g kg−1 diet, containing 0.03% NT, 1% bw day−1 | 2 weeks | Rainbow trout | 53–55 g | ↑ survival after challenge with infectious salmon anaemia virus | [5] | |
| 2 g kg−1 diet, containing 0.03% NT, 2% bw day−1 | 3 weeks | Coho salmon | 100 g | ↑ survival after challenge with Piscirickettsia salmonis | [5] | |
| 2 g kg−1 diet, containing 0.03% NT, 2% bw day−1 | 3 weeks | Atlantic salmon | 60 g | ↓ sea lice infection | [5] | |
| 2 g kg−1 diet, containing 0.03% NT at 1.5% bw day−1 | 3 weeks before vaccination and 5 weeks post-vaccination | Atlantic salmon | 34.7 ± 9.6 g | ↑ antibody titer ↓ mortality |
[6] | |
| 2 g kg−1 diet, containing 0.03% NT at 1.5% bw day−1 | 8 weeks | Atlantic salmon | 43 ± 3.0 g | ↑ growth ↓ plasma chloride |
[6] | |
| 2 g kg−1 diet, containing 0.03% NT | 10 weeks | Atlantic salmon | 205 g | ↑ intestinal fold | [6] | |
| NA | 120 days | “all-female” rainbow trout | 80–100 g | ↑ B lymphocytes and resistance to IPN virus ↓ plasma cortisol |
[271] | |
| 2 g kg−1 diet, containing 0.03% NT to hand satiation daily | 15 weeks | Turbot | 120.9 ± 5.1 g | Altered immunogene expression in various tissues | [7] | |
| 2 g kg−1 diet | 6 weeks | Red drum | 1 g | → Growth, whole body composition and in situ challenge with Amyloodinium ocellatum | [126] | |
| Ribonuclease-digested yeast RNA | 15 mg fish−1, by intubation | 3 days | Common carp | 100 g | ↑ phagocytosis, respiratory burst, complement and lysozyme ↓ A. hydrophila infection |
[273] |
| Aquagen | Oral | 2 weeks | Shark | 1.4 ± 0.2 g | ↑ resistance after challenge with S. iniae |
[260] |
| Nucleotide mixture | 4 g kg−1 diet | 5 weeks | Pacific white shrimp | ca. 0.83 g | ↑ growth | [274] |
| 4 g kg−1 diet | 4 weeks | Red drum | 10.2 ± 0.2 g | ↑ growth, neutrophil oxidative radical production and survival after challenge with V. harveyi |
[275] |
Table 4: Research on dietary supplementation of nucleotides on fishes.
Because increasing concerns of antibiotic use have resulted in a ban on subtherapeutic antibiotic usage in Europe and the potential for a ban in the US and other countries [120], research on immunonutrition for aquatic animals is becoming increasingly important [121]. Research on nucleotide nutrition in fish and shrimp is needed to provide insights concerning interactions between nutrition and physiological responses as well as provide practical solutions to reduce basic risks from infectious diseases for the aquaculture industry. Devresse hypothesized that nucleotides are a key nutrient for the shrimp immune system and supplementation of nucleotides or other nucleic acid-rich ingredients such as yeast or yeast extract may enhance disease resistance and growth of shrimp [122]. Although yeast products have been used in shrimp diet formulations, the role of yeast nucleotides remains largely unanswered. In their comprehensive review, Li and Gatlin summarized and evaluated knowledge of nucleotide nutrition in fishes as compared with that of terrestrial animals [123].
The roles of nucleotides and metabolites in fish diets have been sparingly studied for nearly 20 years. Beside possible involvement in diet palatability, fish feeding behaviour and biosynthesis of nonessential amino acids, exogenous nucleotides have shown promise most recently as dietary supplements to enhance immunity and disease resistance of fish produced in aquaculture. Research on dietary nucleotides in fishes has shown they may improve growth in early stages of development, enhance larval quality via broodstock fortification, alter intestinal structure, increase stress tolerance as well as modulate innate and adaptive immune responses. Fishes fed nucleotidesupplemented diets generally have shown enhanced resistance to viral, bacterial and parasitic infection. Despite occasional inconsistency in physiological responses, dietary supplementation of nucleotides has shown rather consistent beneficial influences on various fish species. Although nucleotide nutrition research in fishes is in its infancy and many fundamental questions remain unanswered, observations thus far support the contention that nucleotides are conditionally or semi-essential nutrients for fishes, and further exploration of dietary supplementation of nucleotides for application in fish culture is warranted. Hypothesized reasons associated with these beneficial effects include dietary provision of physiologically required levels of nucleotides due to limited synthetic capacity of certain tissues (e.g. lymphoid), inadequate energetic expenditure for de novo synthesis, immunoendocrine interactions and modulation of gene expression patterns.
The numbers of scientific publications dealing with dietary nucleotides in fish has been relatively limited since the comprehensive review of Li and Gatlin [123] was published. The section below summarises the dietary effects of nucleotides on a number of fish species such as red drum (Sciaenops ocellatus), red-tail black shark (Epalzeorhynchos bicolor), Asian carp (Catla catla), barramundi (Lates calcarifer), cobia (Rachycentron canadum), grouper (Epinephelus malabaricus) and salmonids such as rainbow trout and Atlantic salmon published post 2006.
The use of dietary nucleotides in red drum Sciaenops ocellatus has also been evaluated on growth and feed utilisation [124]. Juvenile fish (ca. 122g) were fed purified a commercial nucleotide product 0.2% (Optimûn, Chemoforma, August, Switzerland) or nucleotides mixtures, which contained equal amounts of CMP (cytidine monophosphate), UMP (uridine monophosphate), AMP (adenosine monophosphate), IMP (inosine monophosphate) and GMP (guanosine monophosphate), at 0.03%, 0.1% and 0.3% of the diet for a period of four weeks [125]. Fish fed dietary nucleotides had showed a clear trend towards increased growth, measured as SGR at the end of the trial. Growth improvements ranged from 5-6% up to 8% higher than the control diet for the fish fed the nucleotide mixtures and commercial product respectively. A similar trend towards improved feed efficiencies was observed in all the groups fed diets containing added nucleotides. No diet effect on body composition was observed in contrast to previous observations. A clear trend of diet effect on neutrophil oxidative radical production was also observed that could suggest that excessive dose could inhibit immune responses. Fish were challenged against V.harveyi at the end of the four-week feeding period but no differences were observed in terms of survival when compared to the control diet. The authors reported on unexpected high mortalities within the 3 days postchallenge in contrast to previous experiments so it was concluded that further studies are needed in order to evaluate potential effect of dietary nucleotides on red drum resistance against V. harveyi [126].
Red drum was also the fish species selected by Cheng and co-authors to assess the effects of dietary nucleotides on intestinal morphology and immune responses [127]. The authors used around 7g fish to compare a commercial nucleotide product, Ascogen P® (Canadian Byosystems Inc., Calgary, Alberta, Canada), at 0.5% and 1% inclusion levels against a control diet not enriched with nucleotides. Evaluation of immune response after six weeks of feeding showed increased extracellular superoxide anion production at the highest dose. In general intestinal morphology, measured as fold height, enterocytes height and microvillus height, in the pyloric caeca, proximal, mid- and distal intestine, was positively influenced by dietary nucleotides. Thus, fold height in the proximal intestine was increased by nucleotides as previously reported for Atlantic salmon [5,6]. Enterocyte height was increased in the pyloric caeca, proximal and distal intestine, not in the mid intestine, by nucleotides. The microvillus height was also increased in the pyloric caeca, proximal, mid and distal intestine.
Russo et al. examined the effects of commercial beta-glucans (Macroguard®, Biotec Pharmacon ASA, Tromsø, Norway) and nucleotide product (AquagenTM, NOVARTIS-Aqua Health Ltd., Charlottetown, Canada) on disease resistance of red-tail black shark (Epalzeorhynchos bicolor) against S.iniae infection [128]. Two trials were conducted using 1.4g fish feeding control, beta-glucan (0.1% inclusion) or nucleotide diet (0.2% product inclusion) for 24 days. In the first trial both vaccinated and non-vaccinated fish were used while in the second experiment nucleotide- or beta-glucan-enriched diets were fed only to vaccinated fish and results compared against a nonvaccinated fish fed control diet. Results of the first trial showed that both commercial products were able to reduce mortalities compared to the fish fed control diet in both vaccinated and non-vaccinated fish. In the second experiment vaccinated fish fed beta-glucans and nucleotide suffered lower mortalities than the vaccinated fish fed control diet and despite slightly lower mortality in the beta-glucan group compared to nucleotide group no statistical difference could be claimed. Growth performance was influenced significantly by dietary treatment in any of the trials conducted.
Jha et al. tested yeast nucleotides, in the form of RNA (Sisco Research Laboratories, India), at 0.4% and 0.8% inclusion levels on Catla catla fingerlings (ca. 8g start weight) to assess potential changes in haemato-immunological responses followed by a challenge trial against A. hydrophila after 60 days feeding [129]. Feeding dietary nucleotides increased leucocyte counts, total protein, globulins, and albumin: globulin ratio, lysozyme activity and respiratory burst activity compared to controls. When comparing differences between nucleotide doses only the respiratory burst activity was increased by augmenting the dietary nucleotides from 0.4% to 0.8% dose. Conversely, the highest survival was observed in the fish fed the highest dose of nucleotides. Fish fed 0.4% nucleotides also showed significantly higher survival than control fish.
At 30°C barramundi fed diets supplemented with nucleotides (Optimûn, Chemoforma, August, Switzerland) grew significantly better and had lower FCR than those fed the reference diet with no additional nucleotides added [130]. In the same study it is noted that the supplementation of dietary nucleotide at 30°C significantly improved the retention efficiencies of protein, energy, lysine and histidine compared to the reference diet. However at 37°C , under heat-stress conditions, despite dietary nucleotides reduced FCR an improvement in growth was not observed.
Salze et al. supplemented cobia diets with a nucleotide-rich yeast extract (Nupro, Alltech, Nicholasville, KY, USA) part of a zero-fishmeal feed [131]. The 104g fish fed the zero-fish meal diet, which was based on soy protein concentrate, worm meal and mannan-oligosaccharides, had similar growth as a control diet with 25.3% fish meal. However the experimental design tested was insufficient to separate the effects of the different raw materials and to evaluate whether cobia benefited from nucleotide supplementation.
Lin et al. reported the benefits of supplementing dietary nucleotides on growth and immune responses of grouper (Epinephelus malabaricus) juveniles [132]. The first experiment assessed different inclusion levels of a nucleotide mixture containing equal amounts of IMP, AMP, GMP, UMP and CMP. Weight gain and feed efficiency were the highest in the group of grouper fed 0.15% of the nucleotide mixture; with head kidney leucocyte superoxide anion production ratio and plasma total immunoglobulin concentration also being higher than the fish fed the control diet. In the second experiment a diet containing 0.15% nucleotide mixture was compared against a control diet and also diets containing 0.15% of IMP, AMP, GMP, UMP or CMP. From the second experiment it was concluded that juvenile grouper diets containing 0.15% AMP seemed to have better effects than other diets supplemented with different single-nucleotides. Overall conclusion showed that both growth and immune responses of juvenile grouper were enhanced in grouper diet with 0.15% nucleotide mixture.
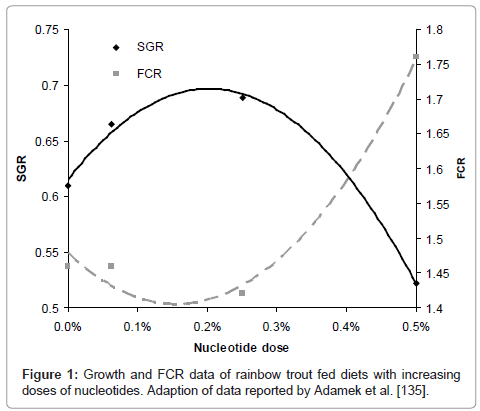
Due to its economical impact as a high-value species, research on salmonids species and nucleotides has received lots of attention in the last years. Tahmasebi-Kohyani and co-authors conducted a dose response trial on rainbow trout fingerlings (ca. 23g start weight) in order to evaluate their effects on growth, humoral immune responses and resistance against S.iniae [133]. A basal diet was supplemented with Optimûn (Chemoforma, August, Switzerland) at 0.05%, 0.1%, 0.15% and 0.2% inclusion levels and fish were fed for eight weeks. Results showed that 0.05% dose was too low and thereafter increased growth, FCR, alternative complement activity, lysozyme activity and IgM as nucleotide dose was augmented, reaching highest values for each of them at the highest nucleotide dose. Similar results were observed at the end of the challenge trial and increased survival was observed with increasing dietary level of nucleotides from 0.05% to 0.2%. This is in agreement with previous work on rainbow trout by Adamek and co-authors that used a wider range of concentrations of the same commercial product, from 0% up to 0.5% and reported approximately 0.2% as the optimal dose (Figure 1)[134].
The efficacy of dietary nucleotides (Optimûn, Chemoforma, August, Switzerland) increasing survival (+39%) against Piscirickettsia salmonis was first reported by Burrells and co-authors on 100g coho salmon (Oncorhynchus kisutch) after feeding for three weeks before challenge [5]. The efficacy of dietary nucleotides against P. salmonis has also been confirmed in other salmonid species such as Atlantic salmon. González Vecino et al. reported increased protection against P. salmonis when Atlantic salmon were fed diets containing a combination of dietary nucleotides with a bacterial cell-wall extract (Sanictum®, Chemoforma, Augst, Switzerland) [135]. Thus, a synergistic effect of the combination of nucleotides and Sanictum® was observed since survival was significantly better than feeding nucleotides or bacterial cell-wall extract separately.
Dietary nucleotides in broodstock diets
Fish reproduction, and egg and larval quality are affected by broodstock nutrition. Nucleotides, the building blocks of nucleic acids, are now considered as semiessential nutrients during periods of food deficiency, stress, rapid growth and immunological stress. González Vecino reported that since oogenesis is a process of intensive cell division with high nucleic acid formation and a concomitant high requirement for nucleotides, supplementation of broodstock diets with nucleotides was beneficial for fish [136]. The results showed that Atlantic halibut (Hippoglossus hippoglossus L.) and haddock (Melanogrammus aeglefinus L.), two cold water species with different life histories, had improved fecundity, egg quality, larval quality and survival when broodstock diets were supplemented with nucleotides. In addition it was observed that haddock larvae from broodfish fed nucleotides had a significantly better developed gut and first feeding success that those from broodstock fed diets not supplemented with nucleotides. Similar beneficial effects have been observed in salmon broodstock diets supplemented with nucleotides (González Vecino, unpublished data).
Dietary nucleotides against sea lice
Immune modulation of salmon can significantly affect the infection intensity of sea lice both Caligus spp. and Lepeophtheirus salmonis. Salmon with reduced or compromised immunity have higher infection with sea lice [137-139]. In addition it is known that during the attachment period sea lice release immunosupressive compounds, including prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) (cytokines) creating a local immunosuppression at the infestation site allowing the lice to attach unhindered [139-144]. Salmon with enhanced immunity have lower infection with sea lice, thus a range of products such as alginates, ß-glucans and nucleotides have shown to reduce sea lice infections. Burrells et al. [5] reported a 38% reduction in sea lice (L. salmonis) infestation on 60g Atlantic salmon fed diets containing nucleotides for three weeks. This was in agreement with reports of 38% reduction on attached stages of L. salmonis on 700g Atlantic salmon after feeding nucleotides for 3 weeks [145]. In the same study Burrells et al. demonstrated that the combination of dietary nucleotides with anti-sea lice bath treatments increased the efficacy of the treatment by 53% [145]. Later work on Caligus rogercresseyi also reported increased efficacy of deltamethrin (bath treatment) and emamectin benzoate (oral treatment) when applied in combination with diets containing nucleotides Optimûn and bacterial cell-wall extract Sanictum® (Chemoforma, August, Switzerland) [146].
Sea lice are well adapted to develop resistance against medicines. Even within a fully sensitive population a number of sea lice will survive treatment. Wadsworth et al. reported that the use of pyrethroids at the recommended dose showed a survival of 20% of attached stages in a naïve population [147]. Although these lice survived the sub-lethal toxicity of the compound and were able to complete their life cycle, the pyrethroid still had a significant, negative effect upon the rate of development, dramatically increasing the time required to reach pre adult I stage. Increasing the time the lice takes to reach pre adults will expose them to the host immunity during this extended period. In their weakened state, it is unlikely the lice are deploying the full range of immune suppressive compounds as effectively as untreated lice. Therefore, it is recommended that immune modulating compounds such as dietary nucleotides are deployed against lice during treatments with anti-sea lice medicines. It is essential that attached lice surviving treatments are targeted to prevent the development of resistant populations.
Furthermore a sea lice infestation represents a substantial stress to the infected animal. An anti-sea lice bath treatment is in its nature a severe multi stress event including starvation, handling, crowding, hypoxia and exposure to a toxic pharmaceutical all within a short period of time. Even a presumably soft action such as feeding diets containing emamectin benzoate has been shown to trigger stress. Thus, Olsvik and co-authors reported that dietary treatment with Slice® (Schering-Plough Animal Health, Boxmeer, The Netherlands) in salmon triggers the expression of stress coding genes in the fish due to sheer metabolisation and degrading of emamectin benzoate in the liver [148]. Since dietary nucleotides have been shown to reduce levels of cortisol its use could also be suggested with emamectin benzoate treatment.
The use of dietary nucleotides as an immune modulating tool against sea lice should be utilised in an integrated pest management programme with rotation of compounds, resistance monitoring, coordinated treatments, cleaner fish as well as effective monitoring and control of other diseases.
Conclusions and Recommendations
Immunostimulants
It is generally accepted that immunostimulants used in fish experiments induce beneficial effects such as disease protection due to increased cellular and humoral responses. However, precautions have to be taken regarding issues such as tolerance, non-wanted side effects such as immunosuppression using too high doses of immunostimulants or non-desirable effects caused by a prolonged use of such compounds. In future, it is hoped that following the development of genomic and proteomic tools for several fish species, many issues with special attention to immune response polarisation after receptor binding of immunostimulants will be unveiled.
Possible effect of immunostimulants on potential probiotics ability to adhere to intestinal mucus should be given high priority in future studies. New and vital information on this topic is needed as the gastrointestinal tract is a potential port of entry for pathogenic bacteria.
Both prebiotics Bio-MOS® and β-1.3 glucan are two commercially available products, derived from the cell wall of S.cerevisiae, in which the use of Bio-MOS® in aquaculture has increased during the last years [149]. However, as less information per se in available on the effect of Bio-MOS® and β-1.3 glucan in aquatic animals [150], this topic should be given high priority in future studies.
Nucleotides
Based on available knowledge and experience within nucleotides, demonstrating that nucleotide supplementation improves the composition of the gut microbiota in formula-fed infants should encourage bacteriologists working with fish to investigate interaction between nucleotides and gut microbiota. Furthermore, we recommend that the following topics should be given high priority in future; (1) in broodstock during oogenesis to improve fecundity, egg and larval quality, (2) during sea water transfer: feeding pre- and post- seawater transfer provides fish with a better osmoregulation and adaptation to marine environment, (3) combination with vaccines: feeding nucleotides during (pre- and post-) the vaccination period modulates the immune response and reduces the negative side effects caused by vaccines (e.g. growth suppression), (4) improved immune response and protection against pathogens such as; V. anguillarum, A. salmonicida, IPN, ISA, rickettsia-like bacteria, Flavobacterium, Moritella viscosus and (5) protection against sea lice and improved (higher) efficacy of pyrethroids when used combined with nucleotide feed.
Acknowledgements
This review is an extended revision of the section immunostimulants and nucleotides in the Ad-hoc report “A risk-assessment on the use of plant ingredients in diets for carnivorous fish” financed by the Norwegian Scientific Committee of Food Safety. The report is available at: www.vkm.no
References
- Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172: 63-92.
- Seljelid R (1990) Immunomodulators – medicine for the 90-ies? In: Wadström T, et al. (Eds.), Workshop on Pathogenesis of Wound and Biomaterial-Associated Infections. Aug. 30–Sep. 01, 1989, Springer Verlag, New York, pp. 107–113.
- Bricknell I, Dalmo RA (2005) The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol 19: 457-472.
- Volman JJ, Ramakers JD, Plat J (2008) Dietary modulation of immune function by β-glucans. Physiol Behavior 94: 276-284.
- Burrells C, Williams PD, Forno PF (2001) Dietary nucleotides: a novel supplement in fish feeds. 1. Effects on resistance to disease in salmonids. Aquaculture 199: 159–169.
- Burrells C, Williams PD, Southgate PJ, Wadsworth SL (2001) Dietary nucleotides: a novel supplement in fish feeds. 2. Effects on vaccination, salt water transfer, growth rates and physiology of Atlantic salmon (Salmo salar L.). Aquaculture 199: 171–184.
- Low C, Wadsworth S, Burrells C, Secombes CJ (2003) Expression of immune genes in turbot (Scophthalmus maximus) fed a nucleotide-supplemented diet. Aquaculture 221: 23-40.
- Nayak SK (2010) Probiotics and immunity: A fish perspective. Fish Shellfish Immunol 29: 2-14.
- Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, et al. (2005) The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 243: 241-254.
- Gomez GD, Balcazar JL (2007) A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol 52: 145- 154.
- Franca FM, de Carla Dias D, Teixeira PC, Marcantonio AS, De Stefani MV, et al. (2008) Effect of the probiotic Bacillus subtilis on growing, survival and fisiology in the bullfrog (Rana catesbeiana). Boletim Do Instituto de Pesca 34: 403-412.
- Shida K, Nanno M (2008) Probiotics and immunology. Separating the wheat from the chaff. Trends Immunol 29: 565-573.
- Borchers, AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Borchers, AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44: 26-46 J Gastroenterol 44: 26-46.
- Panigrahi A, Azad IS, Das BK, Dandpat J, Das G, et al. (2009) Probiotic induced immunomodulation. Investigation into the cellular and molecular mechanism involved. Res J Biotechnol 4: 7-13.
- Zanello G, Meurens F, Mustapha B, Salmon H (2009) Saccharomyces boulardii effects on gastrointestinal diseases. Curr Iss Mol Biol 11: 47-58.
- Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, et al. (2010) Microbial manipulations to improve fish health and production – a Mediterranean perspective. Fish Shellfish Immunol 30: 1-16.
- Ganguly S, Paul I, Mukhopadhayay SK (2010) Application and effectiveness of immunostimulants, probiotics, and prebiotics in aquaculture: A review. The Israeli J Aquacult – Bamidgeh 62: 130-138.
- Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, et al. (1992) Enhanchement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Ped Res 32: 141-144.
- Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, et al. (2004) Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunopathol 102: 379–388.
- Panigrahi A, Kiron V, Satoh S, Hirono I, Kobayashi T, et al. (2007) Immune modulation and expression of cytokine genes in rainbow trout Oncorhynchus mykiss upon probiotic feeding. Dev Comp Immunol 31: 372-382.
- Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, et al. (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 25: 114-123.
- Oelschlaeger TA (2010) Mechanisms of probiotic actions – A review. Int J Med Microbiol 300: 57-62.
- Suchner U, Kuhn KS, Fürst P (2000) The scientific basis of immunonutrition. Proc Nutr Soc 59: 553-563.
- Landolt ML (1989) The relationship between diet and the immune responses of fish. Aquaculture 79: 193-206.
- Anderson DP (1992) Immunostimulants, adjuvants, and vaccine carriers in fish: application to aquaculture. Ann Rev Fish Dis 281-307.
- Blazer VS (1992) Nutrition and disease resistance in fish. Ann Rev Fish Dis 2: 309-323.
- Waagbø R (1994) The impact of nutritional factors on the immune system in Atlantic salmon Salmo salar L.: A review. Aquacult Fish Manag 25: 175-197.
- Raa J (1996) The use of immunostimulatory substances in fish and shellfish farming. Rev Fish Sci 4: 229-288.
- Galeotti M (1998) Some aspects of the application of immunostimulants and critical review of methods for their evaluation. J Appl Ichthyol 14: 189-199.
- Gannam AL, Schrock RM (1999) Immunostimulants in fish diets. J Appl Aquacult 9: 53-89.
- Sohn KS, Kim MK, Kim JD, Han IK (2000) The role of immunostimulants in monogastric animal and fish – review. Asian-Australasian J Anim Sci 13: 1178- 1187.
- Galindo-Villegas J, Hosokawa H (2004) Immunostimulants: Towards temporary prevention of diseases in marine fish. In: Cruz Suarez LE, Ricque MD, Nieto Lopez MG, Villareal D, Scholz Y, Gonzalez M (Eds.), Advances en Nutricion. Acuicola VII Memorias del VII Simposium Internationale de Nutricion Acuícola, 16-19 279-319.
- Trushenski JT, Kasper CS, Kohler CC (2006) Challenges and opportunities in finfish nutrition. North Am J Aquacult 68: 122-140.
- Panigrahi A, Azad IS (2007) Microbial intervention for better fish health in aquaculture: the Indian scenario. Fish Physiol Biochem 33: 429-440.
- Sahoo PK (2007) Role of immunostimulants in disease resistance of fish. CAB Reviews: Perspectives in Agriculture. Vet SciNutrNat Res 2: no. 045.
- Galli C, Calder PC (2009) Effects of fat and fatty acid intake on inflammatory and immune responses. A critical review. Ann Nutr Metab 55: 123-139.
- Verlhac TV (2010) Nutrition and immunity. Aquaculture Research 41: 356-372.
- Zekovic DB, Kwiatowski S (2005) Natural and modified (1->3)-beta-D-glucans in health promotion and disease alleviation. Crit Rev Biotechnol 25: 205-230.
- Sealey WM, Barrows FT, Hang A, Johansen KA, Overturf K, et al. (2008) Evaluation of the ability of barley genotypes containing different amounts of β-glucan to alter growth and disease resistance of rainbow trout Oncorhynchus mykiss. Anim Feed Sci Technol 141: 115-128.
- Soltanian S, Stuyven E, Cox E, Sorgeloos P, Bossier P (2009) Beta-glucans as immunostimulants in vertebrates and invertebrates. Crit Rev Microbiol 35: 109-138.
- Supamattaya K, Pongmaneerat J, Klowklieng T (2000) The effect of β-glucan (MacroGard®) on growth performance, immune response and disease resistance in black tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J Sci Technol 22: 677-688.
- Ferreira IMPLVO, Pinho O, Vieira E, Tavarela JG (2010) Brewer`s Saccharomyces yeast biomass: characteristics and potential applications. Trend Food Sci Technol 21: 77-84.
- Schrøder MB, Villena AJ, Jørgensen TØ (1998) Ontogeny of lymphoid organs and immunoglobulin producing cells in Atlantic cod (Gadus morhua L.). Dev Comp Immunol 22: 507–517.
- Grøntvedt RN, Espelid S (2003) Immunoglobulin producing cells in the spotted wolffish (Anarhichas minor Olafsen): localization in adults and during juvenile development. Dev Comp Immunol 27: 569–578.
- Gildberg A, Johansen A, Bøgwald J (1995) Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida. Aquaculture 138: 23–34.
- Gildberg A, Mikkelsen H (1998) Effects of supplementing the feed to Atlantic cod (Gadus morhua) fry with lactic acid bacteria and immuno-stimulating peptides during a challenge trial with Vibrio anguillarum. Aquaculture 167: 103- 113.
- Nikoskelainen S, Ouwehand A, Salminen S, Bylund G (2001) Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture 198: 229–236.
- Remminghorst U, Rehm B (2006) Bacterial alginates: from biosynthesis to applications. Biotechnol Lett 28: 1701-1712.
- Haug A, Myklestad S, Larsen B, Smidsrød O (1967) Correlation between chemical structure and physical properties of alginates. Acta Chemica Scand 21: 768–778.
- Smidsrød O, Skjåk-Bræk G (1990) Alginate as immobilization material for cells. Trend Biotechnol 8: 71-78.
- Remminghorst U, Rehm B (2006) Remminghorst U, Rehm B (2006) Bacterial alginates: from biosynthesis to applications. Biotechnol Lett 28: 1701-1712 Biotechnol Lett 28: 1701-1712.
- Skjåk-Bræk G, Flo T, Halaas Ø, Espevik T (2000) Immune stimulating properties of de-equatorially β(1–4) linked poly-uronides. Bioactive Carbohydrate Polymers. Kluwer Academic Publishers, 85–93.
- Skjermo J, Defoort T, Dehasque M, Espevik T, Olsen Y, et al. (1995) Immunostimulation of juvenile turbot (Scophthalmus maximus L.) using an alginate with high mannuronic acid content administrated via the live food organism Artemia. Fish Shellfish Immunol 5: 531-534.
- Vadstein O (1997) The use of immunostimulation of marine larviculture: possibilities and challenges. Aquaculture 155: 401-417.
- Skjermo J, Vadstein, O (1999) Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture 177: 333–343.
- Vollstad D, Bøgwald J, Gaserød O, Dalmo RA (2006) Influence of high-M alginate on the growth and survival of Atlantic cod (Gadus morhua L.) and spotted wolffish (Anarhichas minor Olafsen) fry. Fish Shellfish Immunol 20: 548–561.
- Miles DJC, Polchana J, Lilley JH, Kanchanakhan S, Thompson KD, et al. (2001) Immunostimulation of striped snakehead Channa striata against epizootic ulcerative syndrome. Aquaculture 195: 1-15.
- Peddie S, Zou J, Secombes CJ (2002) Immunostimulation in the rainbow trout (Oncorhynchus mykiss) following intraperitoneal administration of Ergosan. Vet Immunol Immunopathol 86: 101–113.
- Duke JA (1987) CRC Handbook of Medicinal Herbs (5th ed.). CRC Press, Boca Raton, FL.
- Harikrishnan R, Balasundaram C, Heo MS (2011) Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317: 1-15.
- Dügenci SK, Arda N, Candan A (2003) Some medicinal plants as immunostimulants for fish. J Ethnopharmacol 88: 99-106.
- Christybapita D, Divyagnaneswari M, Michael RD (2007) Oral administration of Eclipta alba leaf aqueous extract enhances the non-specific immune responses and disease resistance of Oreochromis mossambicus. Fish Shellfish Immunol 23: 840-852.
- Dam TK, Brewer CF (2010) Lectins as pattern recognition molecules: The effects epitope density in innate immunity. Glycobiol 20: 270-279.
- Boshra H, Li J, Sunyer JO (2006) Recent advances on the complement system of teleost fish. Fish Shellfish Immunol 20: 239-262.
- van Kooyk Y, Rabinovich GA (2008) Protein-glycan interactions in the control of innate and adaptive immune responses. Nature Immunol 9: 593-601.
- Galina J, Yin G, Ardo, Jenny Z (2009) The use of immunostimulating herbs in fish. An overview of research. Fish Physiol Biochem 35: 669-676.
- Soosean C, Marimuthu K, Sudhakaran S, Xavier R (2010) Effect of mangosteen (Garcinia mangostana L.) extracts as a feed additive on growth and haematological parameters of African catfish (Clarias gariepinus). Eur Rev Med Pharmacol Sci 14: 605-611.
- Harikrishnan R, Kim JS, Kim MC, Balasundaram C, Heo MS (2011) Lactuca indica extract as feed additive enhances immunological parameters and disease resistance in Epinephelus bruneus to Streptococcus iniae. Aquaculture 318: 43-47.
- Harikrishnan R, Balasundaram C, Jawahar S, Heo MS (2011) Solanum nigrum enhancement of the immune response and disease resistance of tiger shrimp, Penaeus monodon against Vibrio harveyi. Aquaculture 318: 67-73.
- Rajendiran A, Natarajan E, Subramanian P (2008) Control of Aeromonas hydrophila infection in spotted snakehead, Channa punctatus, by Solanum nigrum L., a medical plant. J World Aquacult Soc 39: 375-383.
- Abbas AK, Lichtman AH, Pillai S (2010) Cellular and molecular immunology (updated 6/E). Saunders Elsevier Philadelphia, PA, USA
- Møller M, Rath MF, Klein DC (2006) The perivascular phagocyte of the mouse pineal gland. Chronobiol Int 23: 393-401.
- Beck G, Habicht GS (1996) Immunity and the invertebrates. Sci Am November 60~66.
- Smith, VJ, Soderhall K (1991) A comparison of phenoloxidase activity in the blood of marine invertebrates. Dev Comp Immunol 15: 251-261.
- Hellio C, Bado-Nilles A, Gagnaire B, Renault T, Thomas-Guyon H (2007) Demonstration of a trae phenoloxidase activity and activation of a ProPO cascade in pacific oyster, Crassostrea gigas (Thunberg) In vitro. Fish Shellfish Immunol 22: 433-440.
- Liu H, Jiravanichpaisal P, Cerenius L, Lee BL, Söderhöll I, et al. (2007) Phenoloxidase is an important component of the defense against aeromonas hydrophila infection in a crustacean, Pacifastacus leniusculus. J Biol Chem 282: 33593-33598.
- Alexander JB, Ingram GA (1992) Noncellular nonspecific defence mechanisms of fish. Ann Rev Fish Dis 2: 249–280.
- Goethe R, Phi-van L (1998) Posttranscriptional lipopolysaccharide regulation of the lysozyme gene at processing of the primary transcript in myelomonocytic HD11 cells. J Immunol 160: 4970–4978.
- Robertsen B, Engstad R, Jørgensen JB (1994) β-glucans as immunostimulators in fish. In: Stolen, JS, Fletcher, TC (Eds.), Modulators of Fish Immune Responses. SOS Publication, Fair Haven, NJ, USA, Vol. 1, pp. 83-99.
- Robertsen B (1999) Modulation of the non-specific defence of fish by structurally conserved microbial polymers. Fish Shellfish Immunol 9: 269-290.
- Sahoo PK, Mukherjee SC (1999) Immunostimulants in aquaculture. In: Mohapatra, BC, Ingole, PG, Bharad, GM (Eds.), Aquaculture with Special Reference to Vidarbha. Maharashtra State, Akola, India, pp. 282-293.
- Philip R, Sahoo PK, Sreekumar PK, Anas A, Mukherjee SC, et al. (2003) Immunostimulants – source, diversity and mode of application. In: Aquaculture Medicine. Bright Singh IS, Somnath Pai, S, Philip, R, Mohandas, A (ds.) p 135- 147 Centre for Fish Disease Diagnosis and Management, Cochin University of Science and Technology (CUSAT), Cochin, India.
- Peddie S, Secombes CJ (2005) An overview of fish immunostimulant research. Fish Vet J 8: 1-31.
- Tassakka ACMAR, Sakai M (2005) Current research on the immunostimulatory effects of CpG oligodeoxynucleotides in fish. Aquaculture 246: 25-36.
- Magnadóttir B (2010) Immunological control of fish diseases. Mar Biotechnol 12: 361-379.
- Smith VJ, Brown JH, Hauton C (2003) Immunostimulation in crustaceans: does it really protect against infections? Fish Shellfish Immunol 15: 71-90.
- Magnadóttir B, Gudmundsdottir BK, Lange S, Steinarsson A, Oddgeirsson M, et al. (2006) Immunostimulation of larvae and juveniles of cod, Gadus morhua L. J Fish Dis 29: 147-155.
- Whyte SK (2007) The innate immune response of fish – A review of current knowledge. Fish Shellfish Immunol 23: 1127-1151.
- Dalmo RA, Bøgwald J (2008) β-glucans as conductors of immune symphonies. Fish Shellfish Immunol 25: 384-396.
- Rombout J, Abelli L, Picchietti S, Scapigliati G, Kiron V (2010) Teleost intestinal immunology. Fish Shellfish Immunol early view. Doi:10.1016/j.fsi.2010.09.001.
- Walker PJ, Mohan CV (2009) Viral disease emergence in shrimp aquaculture: origins, impact and the effectiveness of health management strategies. Rev Aquacult 1: 125-154.
- Workenhe ST, Rise ML, Kibenge MJT, Kibenge FSB (2010) The fight between the teleost fish immune response and aquatic viruses. Mol Immunol 47: 2525- 2536.
- Palm NW, Medzhitov R (2009) Pattern recognition receptors and control of adaptive immunity. Immunol Reviews 227: 221-233.
- Johansson MW, Keysera P, Sritunyalucksanaa K, Söderhäll K (2000) Crustacean haemocytes and haematopoiesis. Aquaculture 191: 45-52
- Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20: 137-151
- Skjermo J, Størseth TR, Hansen K, Handå A, Øie G (2006) Evaluation of β-(1→3, 1→6)-glucans and high-M alginate used as immunostimulatory dietary supplement during first feeding and weaning of Atlantic cod (Gadus morhua L.). Aquaculture 261: 1088-1101.
- Hoseinifar SH, Mirvaghefi AM, Merrifield DL (2011) The effect of dietary inactive brewer`s yeast Saccharomyces cerevisiae var. ellipsoideus on the growth, physiological responses and gut microbiota of juvenile beluga (Huso huso). Aquaculture 318: 90-94.
- Ringø E, Jutfelt F, Kanapathippillai P, Bakken Y, Sundell K, et al. (2004) Damaging effect of the fish pathogen Aeromonas salmonicida ssp. salmonicida on intestinal enterocytes of Atlantic salmon (Salmo salar L.). Cell Tiss Res 318: 305-311.
- Birkbeck TH, Ringø E (2005) Pathogenesis and the gastrointestinal tract of growing fish. In: Holzapfel W, Naughton P (Eds.), Microbial Ecology in Growing Animals. Elsevier, Edinburgh, UK, pp 208-234.
- Ringø E, Myklebust R, Mayhew TM, Olsen RE (2007). Bacterial translocation and pathogenesis in the digestive tract of larvae and fry. Aquaculture 268: 251-264.
- Ringø E, Salinas I, Olsen RE, Nyhaug A, Myklebust R, et al. (2007) Histological changes in Atlantic salmon (Salmo salar L.) intestine following In vitro exposure to pathogenic and probiotic bacterial strains. Cell Tiss Res 328: 109-116.
- Salinas I, Myklebust R, Esteban MA, Olsen RE, Meseguer J, Ringø E (2008) In vitro studies of Lactobacillus delbrueckii ssp. lactis in Atlantic salmon (Salmo salar L.) foregut: tissue responses and evidence of protection against Aeromonas salmonicida ssp. salmonicida epithelial damage. Vet Microbiol 128: 167-177.
- Sugita H, Mizuki H, Itoi S (2008) Prevalence of a fish pathogen, Listonella anguillarum in the intestinal tract of fish collected off the coast of Japan. Aquacult Res 39: 103-105.
- Nayak SK (2010) Role of gastrointestinal microbiota in fish. Aquacult Res 41: 1553-1573.
- Ringø E, Løvmo L, Kristiansen M, Bakken M, Salinas I, et al. (2010) Lactic acid bacteria vs. pathogens in the gastrointestinal tract of fish: a review. Aquacult Res 41: 451-467.
- Harikrishan R, Balasundaram C (2005) Modern trends in Aeromonas hydrophila disease management with fish. Rev Fish Sci 13: 281-320.
- Liu Y, Zhou Z, Yao B, Shi P, He S, et al. (2008) The effect of intraperitoneal injection of immunostimulatory substances on allochthonous gut microbiota of Atlantic salmon (Salmo salar L.) determined using Denaturing Gradient Gel Electrophoresis (DGGE). Aquacult Res 39: 635-646.
- Grimble GK (1996) Why are dietary nucleotides essential nutrients? Br J Nutr 76: 475–478.
- Grimble GK, Westwood OMR (2001) Nucleotides as immunomodulators in clinical nutrition. Curr Op Clin Nutr Metabolic Care 4: 57-64.
- Gutierrez-Castellon P, Mora-Magana I, Diaz-Garcia L, Jiménez-Gutierrez C, Ramirez-Mayanas J, et al. (2007) Immune response to nucleotidesupplemented infant formulae: systematic review and meta-analysis. Br J Nutr 98: S64-S67.
- Aggett P, Leach JL, Rueda R, MacLean WC (2003) Innovation in infant formula development: a reassessment of ribonucleotides in 2002. Nutrition 19: 375–384.
- Carver JD, Walker WA (1995) The role of nucleotides in human nutrition. J Nutr Biochem 6: 58–72.
- Cosgrove M (1998) Nucleotides. Nutrition 14: 748–751.
- Grimble GK, Westwood OMR (2000) Nucleotides In: German JB, Keen CL (Eds.), Nutrition and Immunology: Principles and Practice. Humana Press Inc., Totowa, NJ, USA, pp. 135–144.
- Gil A (2002) Modulation of the immune response mediated by dietary nucleotides. Eur J Clin Nutr 56: S1–S4.
- Singhal A, Macfarlane G, Macfarlane S, Lanigan J, Kennedy K, et al. (2008) Dietary nucleotides and fecal microbiota in formula-fed infants: a randomized controlled trial. Am J Clin Nutr 87: 1785-1792.
- Mackie AM (1973) The chemical basis of food detection in the lobster Homarus gammarus. Mar Biol 21: 103–108.
- Kiyohara S, Hidaka I, Tamura T (1975) Gustatory response in the puffer-II. Single fiber analysis. Bull Jap Soc Sci Fish 41: 383–391.
- Mackie AM, Adron JW (1978) Identification of inosine and inosine 5′-monophosphate as the gustatory feeding stimulants for the turbot, Scophthalmus maximus. Comp Biochem Physiol - A 60: 79–83.
- Patterson JA, Burkholder KM (2003) Application of prebiotics and probiotics in poultry production. Poult Sci 82: 627–631.
- Gatlin DM (2002) Nutrition and fish health. In: Halver JE, Hardy RW (Eds.), Fish Nutrition, Academic Press. San Diego, CA, USA, pp. 671–702.
- Devresse B (2000) Nucleotides–a key nutrient for shrimp immune system. Feed Mix 8: 20–22.
- Li P, Gatlin DM (2006) Nucleotide nutrition in fish: Current knowledge and future applications. Aquaculture 251: 141-152.
- Li P, Gatlin DM, Neill WH (2007) Dietary supplementation of a purified nucleotide mixture transiently enhanced growth and feed utilization of juvenile red drum, Sciaenops ocellatus. J World Aquacult Soc 38: 281-286.
- Li P, Burr GS, Goff J, Whiteman KW, Davis KB, et al. (2005) A preliminary study on the effects of dietary supplementation of brewers yeast and nucleotides, singularly or in combination, on juvenile red drum (Sciaenops ocellatus). Aquacult Res 36: 1120-1127.
- Liu P, Chuang CWH, Lee KK (2003) Infectious gastroenteritis caused by Vibrio harveyi (V. carchariae) in cultured red drum, Sciaenops ocellatus. J Appl Ichthyol 19: 59–61.
- Cheng, Z, Buentello A, Gatlin DM (2011) Dietary nucleotides influence immune responses and intestinal morphology of red drum Sciaenops ocellatus. Fish Shellfish Immunol 30: 143-147.
- Russo R, Yanong RPE, Mitchell H (2007) Dietary beta-glucans and nucleotides enhance resistance of red-tail black shark (Epalzeorhynchos bicolor, fam. Cyprinidae) to Streptococcus iniae infection. J World Aquacult Soc 37: 298- 306
- Jha AK, Pal AK, Sahu NP, Kumar S, Mukherjee SC (2007) Haematoimmunological responses to dietary yeast RNA, ω-3 fatty acid and β-carotene in Catla catla juveniles. Fish Shellfish Immunol 23: 917-927.
- Glencross B, Rutherford N (2010) Dietary strategies to improve the growth and feed utilization of barramundi, Lates calcarifer under high water temperature conditions. Aquacult Nutr 16:4 343-350.
- Salze G, McLean E, Battle PR, Schwarz MH, Craig SR (2010) Use of soy protein concentrate and novel ingredients in the total elimination of fish meal and fish oil in diets for juvenile cobia, Rachycentron canadum. Aquaculture 298: 294-299.
- Lin YH, Wang H, Shiau SY (2009) Dietary nucleotide supplementation enhances growth and immune responses of grouper, Epinephelus malabaricus. Aquacult Nutr 15: 117-122.
- Tahmasebi-Kohyani A, Keyvanshokooh S, Nematollahi A, Mahmoudi N, Pasha-Zanoosi H (2011) Dietary administration of nucleotides to enhance growth, humoral immune responses, and disease resistance of the rainbow trout (Oncorhynchus mykiss) fingerlings. Fish Shellfish Immunol 30: 189-193.
- Adamek Z, Hamackova J, Kouril J, Vachta R, Stibranyiova I (1996) Effect of Ascogen probiotics supplementation on farming success in rainbow trout (Oncorhynchus mykiss) and wels (Silurus glais) under conditions of intensive culture. Krmiva (Zagreb) 38: 11–20.
- González VJL, Wadsworth S, González J, Ruohonen K (2010) The combined used of nucleotides and immune-stimulant (Sanictum®) in feed against salmon rickettsia syndrome. Poster presentation at the 14th International Symposium on Fish Nutrition and Feeding, Qingdao, China Book of abstracts page 356.
- González Vecino JL (2005) Nucleotide enhancement of diets, fish reproduction and egg quality. PhD Thesis. Open University, UHI Millennium Institute, Scottish Association for Marine Science, UK.
- MacKinnon BM (1998) Host factors important in sea lice infections. ICES J Mar Sci 55. 188–192.
- Johnson S, Margolis L (1993) Efficacy of invermectin for the control of the salmon louse Lepeophtheirus salmonis on Atlantic salmon. Dis Aquatic Org 17: 101-105.
- Fast MD, Johnson S, Eddy T, Pinto D, Ross N (2008) Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long term stress. Fish Shellfish Immunol 24: 194–204.
- Fast MD, Burka JF, Johnson SC, Ross NW (2003) Enzymes released from Lepeophtheirus salmonis in response to mucus from different salmonids. J Parasitol 89: 7-13.
- Fast MD, Ross NW, Craft CA, Locke SJ, MacKinnon SL, et al. (2004) Lepeophtheirus salmonis: characterization of prostaglandin E-2 in secretory products of the salmon louse by Rp-Hplc and mass spectrometry. Exp Parasitol 107: 5-13.
- Fast MD, Ross NW, Muise DM, Johnson SC (2006) Differential gene expression in Atlantic salmon, Salmo salar, infected with Lepeophtheirus salmonis (Copepoda: Caligidae). J Aquatic Health 18: 116–127.
- Fast MD, Johnson S, Eddy T, Pinto D, Ross N (2007) Lepeophtheirus salmonis secretory / excretory products and their effects on Atlantic salmon immune gene regulation. Parasite Immunol 29: 179–189.
- Wagner G, Fast M, Johnson SC (2008) Physiology and immunology of Lepeophtheirus salmonis infections of salmonids. Trend Parasitol 24: 176- 183.
- Burrells C, Williams PD, Southgate PJ, Wadsworth SL (2001) The effects of a nucleotide-enriched diet on experimental infestation with sea lice (Lepeophtheirus salmonis) and on re-infestation rates following anti-louse bath treatment with cyperthin. SCI Conference, Aberdeen.
- González J, Troncoso J (2009) Reducing Caligus rogercresseyi infestation in Salmo salar fed a diet supplemented with microbial based nucleotides and immunostimulants. Oral presentation at World Aquaculture 2009, Veracruz, Mexico. Book of abstracts p25.
- Wadsworth S, Lygren B (2009) Nukleotider reduserer påslag av lus og forhindrer resistens. Norsk Fiskeoppdrett, Special sea lice Issue June 09, 50- 53.
- Olsvik PA, Lie KK, Mykkeltvedt E, Samuelsen OB, Petersen K, et al. (2008) Pharmakinetics and transcripcional effects of the anti-salmon lice drug emamectin benzoate in Atlantic salmon (Salmo salar L). BMC Pharmacol 8:16.
- Ringø E, Olsen RE, Gifstad TØ, Dalmo RA, Amlund H, et al. (2010) Prebiotics in aquaculture: a review. Aquacult Nutr 16: 117-136.
- Hai NV, Fotedar R (2009) Comparison of the effect of the prebiotics (Bio- MOS® and β-1,3-D-glucan) and the customised probiotics (Pseudomonas synxantha and P. aeruginosa) on the culture of juvenile western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture 289: 310-316.
- George M, Abraham TE (2006) Pylyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan – a review. J Cont Rel 114: 1-14.
- Yano T, Mangindaan REP, Matsuyama H (1989) Enhancement of the resistance of carp Cyprinus carpio to experimental Edwardsiella tarda infection, by some β-1,3 glucans. Nipp Sui Gakk 55: 1815-1819.
- Chen D, Ainsworth, AJ (1992) Glucan administration potentiates immune defence mechanisms of channel catfish, Ictalurus punctatus Rafinesque. J Fish Dis 15: 295-304.
- Sang HM, Fotedar R (2010) Effects of dietary β – 1.3 – glucan on the growth, survival, physiological and immune response of marron, Cherax tenuimanus (smith, 1912). Fish Shellfish Immunol 28: 957-960.
- Strand HK, Dalmo RA (1997) Absorption of immunomodulating β(1,3)-glucan in yolk sac larvae of Atlantic halibut, Hippoglossus hippoglossus (L). J Fish Dis 20: 41-49.
- Kim Y-s, Ke F, Zhang Q-Y (2009) Effect of β-glucan on activity of antioxidant enzymes and Mx gene expression in virus infected grass carp. Fish Shellfish Immunol 27: 336-340.
- Nikl L, Albright LJ, Evelyn TPT (1991) Influence of seven immunostimulants on the immune response of coho salmon to Aeromonas salmonicida. Dis Aqua Org 12: 7-12.
- Jeney G, Galeotti M, Volpatti D, Jeney Z, Anderson DP (1997) Prevention of stress in rainbow trout (Oncorhynchus mykiss) fed diets containing different doses of glucan. Aquaculture 154: 1-15.
- Ogier de Baulny M, Quentel C, Fournier V, Lamour F, Le Gouvello R (1996) Effect of long-term oral administration of β-glucan as an immunostimulant or an adjuvant on non-specific parameters of the immune response of turbot Scophthalmus maximus. Dis Aqua Org 26: 139-147
- Djorddjevic B, Skugor S, Jørgensen SM, Øverland M, Mydland LT, et al. (2009) Modulation of splenic immune responses to bacterial lipopolysaccharide in rainbow trout (Oncorhynchus mykiss) fed lentinan, a beta-glucan from mushroom Lentinula edodes. Fish Shellfish Immunol 26: 201-209.
- Bagni M, Archetti L, Amadori M, Marino G (2000) Effects of long-term oral administration diet on innate immunity in sea bass (Dicentrarchus labrax). J Vet Med B 47: 745-751.
- Bagni M, Romano N, Finoia MG, Abelli L, Scapigliati G, et al. (2005) Shortand long-term effects of a dietary yeast beta-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol 18: 311-325.
- Bonaldo A, Thompson KD, Manfrin A, Adams A, Murano E, et al. (2007) The influence of dietary beta-glucans on the adaptive and innate immune responses of European sea bass (Dicentrarchus labrax) vaccinated against vibriosis. Italian J Anim Sci 6: 151-164.
- Cook MT, Hayball PJ, Hutchinson W, Nowak B, Hayball JD (2003) Administration of a commercial immunostimulant preparation, EcoActiva™, as a feed supplement enhances macrophage respiratory burst and the growth rate of snapper (Pagrus auratus, Sparidae (Bloch and Schneider)) in winter. Fish Shellfish Immunol 14: 333-345.
- Wang WS, Hung SW, Lin YH, Tu CY, Wong ML, et al. (2007) The effect of five different glycans on innate immune responses by phagocytes of hybrid tilapia and Japanese eels, Anguilla japonica. J Aquatic Anim Health 19: 49-59.
- Cain KD, Grabowski L, Reilly J, Lytwyn M (2003) Immunomodulatory effects of a bacterial-derived β-1, 3 glucan administered to tilapia (Oreochromis nilotocus L) in a Spirulina-based diet. Aquacult Res 34: 1241-1244.
- Galindo-Villegas J, Masumoto T, Hosokawa H (2002) Immunostimulants and disease resistance in Japanese flounder, Paralichthys olivaceus. In: Proceedings of the 5th Korean-Japan Biannual Joint Meeting, Kunsan University, Kunsan, Korea, p 26.
- Welker T, Lim C, Yildirim-Aksoy M, Shelby R, Klesius PH (2007) Immune response and resistance to stress and Edwardsiella ictaluri challenge in channel catfish, Ictalurus punctatus, fed diets containing commercial wholecell yeast or yeast subcomponents. J World Aquacult Soc 38: 24-35.
- Das BK, Debnath CD, Patnaik P, Swain DK, Kumar K, et al. (2009) Effect of β-glucan on immunity and survival of early stage of Anabas testudineus (Bloch). Fish Shellfish Immunol 27: 678-683.
- Campa-Cordova AI, Hernandez-Saavedra NY, de Philippis R, Ascencio F (2002) Generation of superoxide anion and SOD activity in haemocytes and muscle of American White shrimp (Litopenaeus vannamei) as a response to β-glucan and sulphated polysaccharide. Fish Shellfish Immunol 12: 353-366.
- Anas, A, Lowmann DW, Williams D, Millen S, Pai SS, et al. (2009) Alkali insoluble glucan extracted from Acremonium diospyri is a more potent immunostimulant in the Indian White Shrimp, Fenneropenaeus indicus than soluble glucan. Aquacult Res 40: 1320-1327.
- Bai N, Zhang W, Mai K, Wang X, Xu W, et al. (2010) Effects of discontinous administration of ß-glucan and glycyrrhizin on the growth and immunity of white shrimp Litopenaeus vannamei. Aquaculture 306: 218-224.
- Costa MM, Novoa B, Figueras A (2008) Influence of ß-glucans on the immune responses of carpet shell clam (Ruditapes decussates) and Mediterranean mussel (Mytilus galloprovincialis). Fish Shellfish Immunol 24: 498-505.
- Lin T, Xing J, Jiang J, Tang X, Zhan W (2011) ß-glucan-stimulated activation of six enzymes in the haemocytes of the scallop Chlamys farreri at different water temperatures. Aquaculture 315: 213-221.
- Cook MT, Hayball PJ, Hutchinson W, Nowak B, Hayball JD (2001) The efficacy of a commercial ß-glucan preparation, EcoActivaT, on stimulating respiratory burst activity of head-kidney macrophages from pink snapper (Pagrus auratus), Sparidae. Fish Shellfish Immunol 11: 661-672.
- Paulsen SM, Engstad RE, Robertsen B (2001) Enhanced lysozyme production in Atlantic salmon (Salmo salar L) macrophages treated with yeast ß-glucan and bacterial lipopolysaccharide. Fish Shellfish Immunol 11: 23-37.
- Kiseleva MI, Balabanova LA, Rasskazov VA, Zvyagintseeva TN (2008) Effect of 1,3;1,6-ß-D-glucans on developing sea urchin embryos. Mar Biotechnol 10: 466-470.
- Jørgensen JB, Lunde H, Robertsen B (1993) Peritoneal and head kidney cell response to intraperitoneal injected yeast glucan in Atlantic salmon, Salmo salar L. J Fish Dis 16: 313-325.
- Refstie S, Baeverfjord G, Seim RP, Elvebø O (2010) Effects of dietary yeast cell wall ß-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture 305: 109-116
- Jørgensen, JB, Sharp GJE, Secombes CJ, Robertsen B (1993) Effect of a yeast cell wall glucan on the bactericidal activity of rainbow trout macrophages. Fish Shellfish Immunol 3: 267-277.
- Chang CF, Chen HY, Su MS, Liao IC (2000) Immunomodulation of dietary ß-1,3-glucan in the brooders of the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol 10: 505-514.
- Jeney G, Anderson DP (1993) Glucan injection or bath exposure given alone or in combination with bacterin enhance the non-specific defence mechanism in rainbow trout (Oncorhynchus mykiss). Aquaculture 116: 315-329.
- Robertsen B, Rørstad G, Engstad R, Raa J (1990) Enhancement of nonspecific disease resistance in Atlantic salmon, Salmo salar L, by a glucan from Saccharomyces cerevisiae cell walls. J Fish Dis 13: 391-400.
- Engstad RE, Robertsen B, Frivold, E (1992) Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol 2: 287-297.
- Rørstad G, Aasjord P M, Robertsen B (1993) Adjuvant effect of a yeast glucan in vaccines against furunculosis in Atlantic salmon (Salmo salar L). Fish Shellfish Immunol 3: 179-190.
- Aakre R, Wergeland HI, Aasjord PM, Endresen C (1994) Enhanced antibody response in Atlantic salmon (Salmo salar L) to Aeromonas salmonicida cell wall antigens using a bacterin containing ß-1,3-M-glucan as adjuvant. Fish Shellfish Immunol 4: 47-61.
- Dalmo RA, Bøgwald J, Ingebrigtsen K, Seljelid R (1996) The immunomodulatory effect of laminaran [beta(1,3)-D-glucan] on Atlantic salmon, Salmo salar L, anterior kidney leucocytes after intraperitoneal, peroral and peranal administration. J Fish Dis 19: 449-457.
- Thompson KD, Cachos A, Inglis V (1995) Immunomodulating effects of glucans and oxytetracycline in rainbow trout, Oncorhynchus mykiss, on serum lysozyme and protection. In: Shariff M, Subasighe RP, Arthur JR (Eds.), Diseases in Asian Aquaculture Vol. 11. Fish health Section, Asian Fisheries Society, Manila, Philippines, pp. 433-439.
- Barnes ME, Durben DJ, Reeves SG, Sanders R (2006) Dietary yeast culture supplementation improves initial rearing of McConaughy strain rainbow trout. Aquacult Nutr 12: 388-394.
- Pal D, Joardar SN, Roy B (2007) Immunostimulatory effects of a yeast (Saccharomyces cerevisiae) cell wall feed supplement on rohu (Labeo rohita), an Indian major carp. The Israeli J Aquacult - Bamidgeh 59: 175-181.
- Pal D, Joardar SN, Roy B (2007) Yeast cell wall preparation from Saccharomyces cerevisiae enhances immune effector cells of Indian major carp, Catla catla (Hamilton). Indian J Fish 56: 33-38.
- Watanabe AL, Macedo Viegas EM, Goncalves LU (2010) Levels of yeast and its by-products on pacu juveniles feeding. Revista Bras Zoo 39: 447-453.
- Palic D, Andreasen CB, Herolt DM, Menzel BW, Roth JA (2006) Immunomodulatory effects of ß-glucan on neutrophil function in fathead minnows (Pimephales promelas Rafinesque, 1820). Dev Comp Immunol 30: 817-830.
- Sung HH, Kou GH, Song YL (1994) Vibriosis resistance induced by glucan treatment in tiger shrimp (Penaeus monodon). Fish Pathol 29: 11-17.
- Thanardkit P, Khunrae P, Suphantharika M, Verduyn C (2002) Glucan from brewer's yeast: preparation, analysis and use as a potential immunostimulant in shrimp feed. World J Microbiol Biotechnol 18: 527-539.
- Chotikachinda R, Lapjatupon W, Chaisilapasung S, Sangsue D, Tantikitti C (2008) Effect of inactive yeast cell wall on growth performance, survival rate and immune parameters in Pacific white shrimp, (Litopenaeus vannamei). Songklanakarin J Sci Technol 22: 677-688.
- Verlhac V, Gabaudan J, Obach A, Schüep W, Hole R (1996) Influence of dietary glucan and vitamin C on non-specific and specific immune responses of rainbow trout (Oncorhynchus mykiss). Aquaculture 143: 123-133.
- Oliva-Teles A, Goncalves P (2001) Partial replacement of fishmeal by brewers yeast (Saccaromyces cerevisiae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture 202: 269-278.
- Rodríguez A, Cuesta A, Ortuño J, Esteban MA, Meseguer J (2003) Immunostimulant properties of a cell wall-modified whole Saccharomyces cerevisiae strain administered by diet to seabream (Sparus aurata L). Vet Immunol Immunopathol 96: 183–192.
- Rodríguez A, Esteban MA, Meseguer J (2003) Phagocytosis and peroxidise release by seabream (Sparus aurata L) leucocytes in response to yeast cells. The Anatom Rec Part A 272 A: 415–423.
- Cuesta A, Meseguer J, Esteban MA (2004) Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L). Vet Immunol Immunopathol 101: 203-210.
- Esteban MA, Rodriguez A, Meseguer J (2004) Glucan receptor but not mannose receptor is involved in the phagocytosis of Saccharomyces cerevisiae by seabream (Sparus aurata L) blood leucocytes. Fish Shellfish Immunol 16: 447-451.
- Oliva-Teles A, Guedes MJ, Vachot C, Kaushik, SJ (2006) The effect of nucleic acids on growth, ureagenesis and nitrogen extretion of gilthead sea bream Sparus aurata juveniles. Aquaculture 253: 608-617.
- Ortuño J, Cuesta A, Rodríguez A, Esteban MA, Meseguer J (2002) Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L). Vet Immunol Immunopathol 85: 41–50.
- Salnur S, Gultepe N, Hossu B (2009) Replacement of fish meal by yeast (Saccharomyces cerevisiae): Effects on digestibility and blood parameters of gilthead sea bream (Sparus aurata). J Anim Vet Adv 8: 2557-2561.
- Lara-Flores M, Olvera-Novoa MA, Guzman-Mendez BE, Lopez-Madrid W (2003) Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promotors in Nile tilapia (Oreochromis niloticus). Aquaculture 216: 193-201.
- Zerai DB, Fitzsimmons KM, Collier RJ, Duff GC (2008) Evaluation of brewer`s waste as partial replacement of fish meal protein in Nile tilapia, Oreochromis niloticus, diets. J World Aquacult Soc 39: 556-564.
- Zaki M, Nour AE, Gaber M, Labib E (2010) Brewers dried yeast as a good alternative ingredient in Nile tilapia, (Oreochromis niloticus) feeds. Proc 2010 Intern Conf Agricult Anim 338-342.
- Li P, Gatlin DM (2003) Evaluation of brewers yeast (Saccharomyces cervisiae) as a feed supplement for hybrid striped bass (Morone chrysops×M. saxatilis). Aquaculture 219: 681–692.
- Li P, Gatlin DM (2004) Dietary brewers yeast and the prebiotic GroBiotic™ AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops×M. saxatilis) to Streptococcus iniae infection. Aquaculture 231: 445–456.
- Li P, Gatlin DM (2005) Evaluation of the prebiotic GroBiotic®-A and brewers yeast as dietary supplements for sub-adult hybrid striped bass (Morone chrysops×M. saxatilis) challenged in situ with Mycobacterium marinum. Aquaculture 248: 197-2005.
- Ozòrio ROA, Turini BGS, Moro GV, Oliveira LST, Portz L, et al. (2010) Growth, nitrogen gain and indispensable amino acid retention of pacu (Piaractus mesopotamicus, Holmberg 1887) fed different brewers yeast (Saccharomyces cerevisiae) levels. Aquacult Nutr 16: 276-283.
- Abdel-Tawwab M, Mousa MAA, Mohammed MA (2010) Use of live baker`s yeast, Saccharomyces cerevisiae, in practical diet to enhance the growth performance of galilee tilapia, Sarotherodon galilaeus (L), and its resistance to environmental copper toxicity. J World Aquacult Soc 41: 214-223.
- Vadstein O, Øie G, Olsen Y, Salvesen I, Skjermo J, et al. (1993) A strategy to obtain microbial control during larval development of marine fish. In: Reinertsen H, Dahle LA, Jørgensen L, Tvinnereim K (Eds.), Fish Farming Technology AA Balkema Publishers, Rotterdam, pp. 69-75.
- Skjermo J, Berg Ø (2004) High-M alginate immunostimulation of Atlantic halibut (Hippoglossus hippoglossus) larvae using Artemia for delivery, increases resistance against vibriosis. Aquaculture 238: 107-113.
- Gabrielsen BO, Austreng E (1998) Growth, product quality and immune status of Atlantic salmon, Salmo salar L., fed wet feed with alginate. Aquacult Res 29: 397-401.
- Conceicao LEC, Skjermo J, Skjåk-Bræk G, Verreth JAJ (2001) Effect of an immunostimulating alginate on protein turnover of turbot (Scophthalmus maximus L.) larvae. Fish Physiol Biochem 24: 207–212.
- Gioacchini G, Lombardo F, Avella MA, Olivotto I, Carnevali O (2010) Welfare improvements using algínico acid in rainbow trout (Oncorhynchus mykiss) juveniles. Chem Ecol 26: 111-121.
- Wang W, Wang D (1997) Enhancement of the resistance of tilapia and grass carp to experimental Aeromonas hydrophila and Edwardsiella tarda infections by several polysaccharides. Comp Immunol Microbiol Inf Dis 20: 261-270.
- Sahoo PK, Mukherjee SC (2001) Effect of dietary β-1, 3 glucan on immune responses and disease resistance of healthy and aflatoxin B1-induced immunocompromised rohu (Labeo rohita Hamilton). Fish Shellfish Immunol 11: 683–695.
- Misra CK, Das BK, Mukherjee SC, Pattnaik P (2006) Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture 255: 82-94.
- Misra CK, Das BK, Mukherjee SC, Pattnaik P (2006) Effect of multiple injections of β-glucan on non-specific immune response and disease resistance in Labeo rohita fingerlings. Fish Shellfish Immunol 20: 305-319.
- Kamilya D, Maiti TK, Joardar SN, Mal BC (2006) Adjuvant effect of mushroom glucan and bovine lactoferrin upon Aeromonas hydrophila vaccination in catla, Catla catla (Hamilton). J Fish Dis 29: 331-337.
- Selvaraj V, Sampath K, Sekar V (2005) Use of glucan from Saccharomyces cerevisiae as an immunostimulant in carp: Impact on hematology, phagocyte function, and infection with Aeromonas hydrophila. The Israeli J Aquacult – Bamidgeh 57: 39-48.
- Selvaraj V, Sampath K, Sekar V (2005) Administration of yeast glucan enhanced survival and some non-specific and specific immune parameters in carp (Cyprinus carpio) infected with Aeromonas hydrophila. Fish Shellfish Immunol 19: 293-306.
- Selvaraj V, Sampath K, Sekar V (2006) Adjuvant and immunostimulatory effects of β-glucan administration in combination with lipopolysaccharide enhances survival and some immune parameters in carp challenge with Aeromonas hydrophila. Vet Immunol Immunopathol 114: 15-24.
- Moqsood S, Samoon MH, Singh P (2009) Immunomodulatory and growth promoting effect of dietary levamisole in Cyprinus carpio fingerlings against the challenge of Aeromonas hydrophila. Tur J Fish Aqua Sci 9: 111-120.
- Gopalakannan A, Arul V (2010) Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole yeast. Aquacult Res 41: 884-892.
- Kumari J, Sahoo PK (2006) Dietary immunostimulants influence specific immune response and resistance of healthy and immunocompromised Asian catfish Clarias batrachus to Aeromonas hydrophila infection. Dis Aqua Org 70: 63-70.
- Samuel M, Lam TJ, Sin YM (1996) Effect of laminaran [β (1-3)-D-glucan] on the protective immunity of blue gourami, Trichogaster trichopterus against Aeromonas hydrophila. Fish Shellfish Immunol 6: 443-454.
- Kamilya D, Joardar SN, Mal BC, Maiti TK (2008) Effects of a glucan from the edible mushroom (Pleurotus florida) as an immunostimulant in farmed Indian major carp (Catla catia). Israeli J Aquacult - Bamidgeh 60: 37-45.
- Rodriguez I, Chammoro R, Novoa B, Figueras A (2009) β-glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio). Fish Shellfish Immunol 27: 369-373.
- Abdel-Tawwab M, Abdel-Rahman AM, Ismael NEM (2008) Evaluation of commercial live bakers`yeast, Saccharomyces cerevisiae as growth and immunity promoter for fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 280, 185-189.
- Reque VR, de Moraes JRE, de Andrade Belo MA, de Moraes FR (2010) Inflammation induced by inactivated Aeromonas hydrophila in Nile tilapia fed diets supplemented with Saccharomyces cerevisiae. Aquaculture 300: 37-42.
- Abdel-Tawwab M, Ahmad MH, Seden MEA, Sakr SFM (2010) Use of green tea, Camellia sinensis L., in practical diet for growth and protection of Nile tilapia, Oreochromis niloticus (L.), against Aeromonas hydrophila infection. J World Aquacult Soc 41: 208-213.
- Reyes-Becerril M, Tovar-Ramirez D, Ascenico-valle F, Civera-Cerecedo R, Gracia-lopez V, et al. (2010) Effects of dietary supplementation with probiotic live yeast Debaryomyces hansenii on the immune and antioxidant systems of leooard grouper Mycteroperca rosacea infected with Aeromonas hydrophila. Aquacult Res early view.
- Siwicki AK, Zakes Z, Terech-Majewska E, Kazun K, Lepa A, et al. (2010) Dietary Macrogard reduces Aeromonas hydrophila mortality in tench (Tinca tinca) through the activation of cellular and humoral defence mechanisms. Rev Fish Biol Fish 20: 435-439.
- Türnau D, Schmidt H, Kürzinger H, Böhm KH (2000) Potency testing of β-glucan immunostimulating effect in food for ornamental fish. Bull Eur Ass Fish Pathol 20: 143-147.
- Anderson DP, Siwick AK (1992) Duration of protection against Aeromonas salmonicida in brook trout immunostimulated with glucan or chitosan by injection or immersion. Prog Fish Cult 56: 258-261.
- Siwicki AK, Anderson DP, Rumsey GL (1994) Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol 41: 125–139.
- Dalmo RA, Martinsen B, Horsberg TE, Ramstad A, Seljelid R, (1998) Prophylactic effect of β (1,3-D-glucan (laminaran) against experimental Aeromonas salmonicida and Vibrio salmonicida infections. J Fish Dis 21: 459- 462.
- Duncan PL, Klesius, PH (1996) Dietary immunostimulants enhance nonspecific immune response in channel catfish but not resistance to Edwardsiella ictaluri. J Aquatic Anim Health 8: 241-248.
- Sahoo PK, Mukherjee SC (2002) The effect of dietary immunomodulation upon Edwardsiella tarda vaccination in healthy and immunocompromised Indian major carp (Labeo rohita). Fish Shellfish Immunol 12: 1–16.
- Matsuyama H, Mangindaan REP, Yano T (1992) Protective effect of schizophyllan and scleroglucan against Streptococcus sp. infection in yellowtail (Seriola quinqueradiata). Aquaculture 101: 197-203.
- Couso N, Castro R, Magarinos B, Obach A, Lamas J (2003) Effect of oral administration of glucans on the resistance of gilthead seabream to pasteurellosis. Aquaculture 219: 99-109.
- Chang J, Zhang W, Mai K, Liufu Z, Ai Q (2011) Effects of dietary β-glucan and glycyrrhizin on non-specific immunity and disease resistance of white shrimp, Litopenaeus vannamei (Boone) challenged with Vibrio alginolyticus. Aquacult Res 42: 1101-1109.
- Misra CK, Das BK, Pradhan J, Pattnaik P, Sethi S, et al. (2004) Changes in lysosomal enzyme activity and protection against Vibrio infection in Macrobrachium rosenbergii (De Man) post larvae after bath immunostimulation with β-glucan. Fish Shellfish Immunol 17: 389-395.
- Pedersen GM, Gildberg A, Olsen RL (2004) Effects of including cationic proteins from cod milt in the feed to Atlantic cod (Gadus morhua) fry during a challenge trial with Vibrio anguillarum. Aquaculture 233: 31-43.
- Marques A, Dhont J, Sorgeloos P, Bossier P (2006) Immunostimulatory nature of β-glucans and baker’s yeast in gnotobiotic Artemia challenge tests. Fish Shellfish Immunol 20: 682-692.
- Soltanian S, Thai TQ, Dhont J, Sorgeloos P, Bossier P (2007) The protective effect against Vibrio campbellii in Artemia nauplii by pure β-glucan and isogenic yeast cells differing in β-glucan and chitin content operated with a source-dependent time lag. Fish Shellfish Immunol 23: 1003-1004.
- Soltanian S, Francois JM, Dhont J, Arnouts S, Sorgeloos P, et al. (2007) Enhanched disease resistance in Artemia by application of commercial β-glucans sources and chitin in a gnotobiotic Artemia challenge test. Fish Shellfish Immunol 23: 1304-1314.
- Figueras A, Santarém MM, Novoa B (1998) Influence of the sequence of administration of β-glucan and a Vibrio damsela vaccine on immune response of turbot (Scophthalmus maximus L.). Vet Immunol Immunopathol 64: 59-68.
- Ai Q, Mai K, Zhang L, Tan B, Zhang W (2007) Effects of dietary β-1,3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol 22: 394–402.
- Burgents JE, Burnett KG, Burnet LE (2004) Disease resistance of pacific white shrimp, Litopenaeus vannamei, following the dietary administration of a yeast culture supplement. Aquaculture 231: 1-8.
- Tukmechi A, Andani HRR, Manaffar R, Sheikhzadeh N (2011) Dietary administration of beta-mercapto-ethanol treated Saccharomyces cerevisiae enhanced the growth, innate immune response and disease resistance of the rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol 30: 923-928.
- Romalde JL, Luzardo-Alaverez A, Ravelo C, Toranzo AE, Blanco-Mendez J (2004) Oral immunization using alginate microparticles as a useful strategy for booster vaccination against fish lactococcosis. Aquaculture 236: 119-129.
- Chiu ST, Tsai RT, Hsu JP, Liu CH, Cheng W (2008) Dietary sodium alginate administration to enhance the non-specific immune responses, and disease resistance of the juvenile grouper Epinephelus fuscoguttatus. Aquaculture 277: 66-72.
- Jaramillo F, Gatlin DM (2004) Comparison of purified and practical diets supplemented with or without beta-glucan and selenium on resistance of hybrid striped bass Morone chrysops female x M. saxatilis male to Streptococcus iniae infection. J World Aquacult Soc 35: 245-252.
- Whittington R, Lim C, Klesius, PH (2005) Effect of dietary β-glucan levels on the growth response and efficacy of Streptococcus iniae vaccine in Nile tilapia, Oreochromis niloticus. Aquaculture 248: 217-225.
- Russo R, Yanong RPE, Mitchell H (2006) Dietary beta-glucans and nucleotides enhance resistance of red-tail black shark (Epalzeorhynchos bicolor, fam. Cyprinidae) to Streptococcus iniae infection. J World Aquacult Soc 37: 298- 306.
- Chang CF, Su MS, Chen HY, Lo CF, Kou GH (1999) Effect of dietary β-1,3- glucan on resistance to white spot syndrome virus (WSSV) in postlarval and juvenile Penaeus monodon. Dis Aqua Org 36: 163-168.
- Chang CF, Su MS, Chen HY, Liao IC (2003) Dietary β-1,3-glucan effectively improves immunity and survival of Penaeus monodon challenged with white spot syndrome virus. Fish Shellfish Immunol 15: 297-310.
- Sajeevan TP, Lowman DW, Williams DL, Selven S, Anas A, et al. (2009) Marine yeast diet confers better protection than its cell wall component (1- 3)- β-D-glucan as an immunostimulant in Fenneropenaeus indicus. Aquacult Res 40: 1723-1730.
- Sukumaran V, Lowman DW, Sajeevan T, Philip R (2010) Marine yeast glucans confer better protection than that of baker’s yeast in Penaeus monodon against white spot syndrome virus infection. Aquacult Res 41: 1799-1805.
- Jenkins CS, Barker DE (2004) The efficacy of oral immunostimulants in enhancing resistance against microsporidiosis in juvenile Atlantic cod (Gadus morhua L.). Aquacult Ass Can 9: 97-100.
- Guselle NJ, markham RFJ, Speare DJ (2006) Intraperitoneal administration of B-1,3/1,6-glucan to rainbow trout, Oncorhynchus mykiss (Walbaum), protects against Loma salmonae. J Fish Dis 29: 375-381.
- Ramadan A, Atef M (1991) Effect of the biogenic performance enhancer (Ascogen “S”) on growth rate of tilapia fish. Acta Vet Scand 87: S304–S306.
- Li P, Lewis DH, Gatlin DM (2004) A Dietary oligonucleotide from yeast RNA influences immune responses and resistance of hybrid striped bass (Morone chrysops × Morone saxatilis) to Streptococcus iniae infection. Fish Shellfish Immunol 16: 561–569.
- Li P, Wang X, Gatlin DM (2004) Excessive dietary levamisole suppresses growth performance of hybrid striped bass (Morone chrysops × M. saxatilis), and elevated levamisole In vitro impairs macrophage function. Aquacult Res 35: 1380–1383.
- Ramadan A, Afifi NA, Moustafa M, Samy AM (1994) The effect of ascogen on the immune response of tilapia fish to Aeromonas hydrophila vaccine. Fish Shellfish Immunol 5: 159–165.
- Lionardi M, Sandino AM, Klempau A (2003) Effect of a nucleotide-enriched diet on the immune system, plasma cortisol levels and resistance to infectious pancreatic necrosis (IPN) in juvenile rainbow trout (Oncorhynchus mykiss). Bull Eur Ass Fish Pathol 23: 52–59.
- Sahu S, Das BK, Mishra BK, Pradhan J, Sarangi N (2007) Effect of Allium sativum on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. J Appl Ichthyol 23: 80-86.
- Sakai M, Taniguchi K, Mamoto K, Ogawa H, Tabata M (2001) Immunostimulant effects of nucleotide isolated from yeast RNA on carp, Cyprinus carpio L. J Fish Dis 24: 433–438.
- Li P, Lawrence A, Castille FI, Gatlin DM (2007) Preliminary evaluation of a purified nucleotide mixture as a dietary supplement for pacific white shrimp Litopenaeus vannamei (Boone). Aquacult Res 38: 887-890.
- Li P, Gatlin DM, Neill WH (2007) Dietary supplementation of a purified nucleotide mixture transiently enhanced growth and feed utilization of juvenile red drum, Sciaenops ocellatus. J World Aquacult Soc 38: 281-286.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 31173
- [From(publication date):
January-2012 - Nov 16, 2025] - Breakdown by view type
- HTML page views : 25148
- PDF downloads : 6025