Research Article Open Access
Trabecular Titanium Induces Osteoblastic Bone Marrow Stem Cells Differentiation
Vincenzo Sollazzo1, Annalisa Palmieri2, Ambra Girardi3, Francesca Farinella2 and Francesco Carinci2*1Orthopedic Clinic, University of Ferrara, Ferrara, Italy
2Department of Maxillofacial Surgery, University of Ferrara, Ferrara, Italy
3Department of Histology, Embryology and Applied Biology, University of Bologna, Bologna, Italy
- Corresponding Author:
- Prof. Francesco Carinci, MD
Deptartment of D.M.C.C.C, University of Ferrara
Corso Giovecca, 203,44100 Ferrara, Italy
Tel/Fax: 0039-0532-455582
E-mail: crc@unife.it
Received date: November 13, 2010; Accepted date: February 07, 2011; Published date: March 21, 2011
Citation: Sollazzo V, Palmieri A, Girardi A, Farinella F, Carinci F (2011) Trabecular Titanium Induces Osteoblastic Bone Marrow Stem Cells Differentiation. J Biotechnol Biomaterial 1:102. doi:10.4172/2155-952X.1000102
Copyright: © 2011 Sollazzo V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Pure titanium and titanium alloys are materials widely used in orthopedics and dental surgery because of their mechanical properties, chemical stability and biocompatibility. Although excellent clinical results have been shown, traditional porous metals have several inherent limitations (low volumetric porosity, relatively high modulus of elasticity and availability as a coating only). With the aim of going over these limits, improving the potentiality of osteointegration, a new highly porous titanium biomaterial (Trabecular Titaniumâ„¢, TT) has been developed. Because the molecular events due to TT and able to alter osteoblast activity to promote bone formation are poorly understood, expression of osteoblastic related genes in mesenchymal stem cells exposed to TT was investigated. The expression levels of bone related genes like RUNX2, SPP1, COL1A1, COL3A1, BGLAP, ALPL, and FOSL1) and mesenchymal stem cells marker (CD105) were analyzed, using real time Reverse Transcription-Polymerase Chain Reaction.TT causes induction of bone related genes osteopontin (SPP1), osteocalcin (BGLAP) alkaline phosphatase (ALPL) and indicating the differentiation effect of this biomaterial on mesenchymal stem cells.The obtained results can be relevant to better understand the molecular mechanism of bone regeneration and as a model for comparing other materials with similar clinical effects.
Keywords
Trabecular titanium; Stem cells; Gene expression; Bone; Differentiation
Introduction
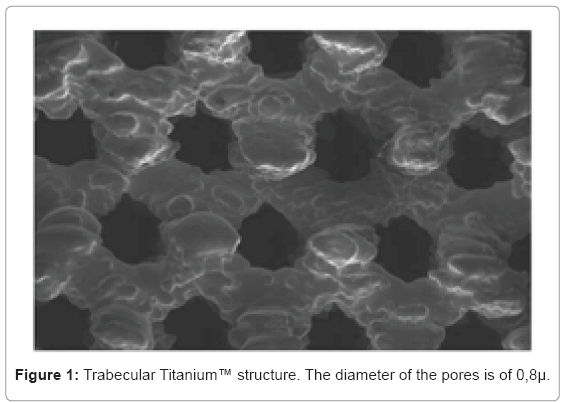
Several features of the implant surface such as composition, topography, roughness and energy level play a relevant role in implant osseointegration [1,2]. Little is known about the structural and chemical surface properties that may influence biological responses [3]. Pure titanium and titanium alloys are widely used in orthopedics and dental surgery because of their mechanical properties, such as chemical stability and biocompatibility [4,5]. In fact, titanium is used to manufacture joint prostheses for partial and total joint replacement. Moreover, titanium is also used to produce plates and screws for the osteosynthesis of fractures and for dental implants [6,7]. Surface roughness has been demonstrated to have positive effects on adsorption molecules, local factor production and proliferation and differentiation of cells [8-10]. Conventional orthopedic implants are composed of stainless steel, cobalt- chromium (CoCr) and titanium alloys [11]. Titanium is the most commonly used metal for uncemented implants because of its excellent biocompatibility, ready availability and high strength because of its low specific gravity [12]. Although good clinical results have been shown, traditional porous materials have several inherent limitations, such as low volumetric porosity, relatively high modulus of elasticity, low frictional characteristics. Moreover, it may be available as mere coating. In order to overcome these limits, and to improve the potentiality of osteointegration and, consequently, the stability of prosthetic implants, a multi-planar, exagonal, highly porous biomaterial (Trabecular Titanium ™) has been developed which imitates the cell structure of the cancellous bone (Lima Corporate, San Daniele del Friuli, Udine). The morphology and dimension of the open pores have been developed to induce osseointegration, its resistance is higher than the cancellous bone and the low rigidity has been studied to foster the transmission of physiological loads from implant to bone.
The first step in the osseointegration of a prosthetic implant is the osteoblastic differentiation of mesenchimal stem cells surrounding the implant [13,14]. Since few reports, to our knowledge, analyze the effects of Trabecular Titanium™ on stem cells [15] and none focuses on the genetic effects, the expression of genes related to the osteoblast differentiation was analyzed using cultures of bone marrow derived human mesenchymal stem cells (BM-hMSCs) treated with Trabecular Titanium ™ for seven days, in order to detect the early effects of the common part of BMPs on stem cells.
Materials and Methods
Stem preparation
Bone marrow derived human mesenchymal stem cells (BM-hMSCs) were obtained from healthy adult volunteers (mean age 45 years). Bone Marrow collected in heparinized tubes, was diluted 1:3 with phosphate buffered saline (PBS) (Lonza, Basel, Switzerland) and layered over a Ficoll-Histopaque gradient (1.077g/ml; Sigma, St. Louis, MO). The low-density mononuclear cells were washed twice in PBS, counted and plated at 106/cm2 in cell culture flasks (Falcon BD, Bedford, MA, USA) in Dulbecco's Modified Eagle's Medium (DMEM) (Lonza, Basel, Switzerland) supplemented with 20% heat inactivated fetal bovine serum (FBS) (Lonza, Basel, Switzerland) and antibiotics (100 U/ml penicillin, 100 µg/ml streptomicin) (Sigma Aldrich, Inc., St Louis, Mo, USA), and incubated at 37°C in a humidified atmosphere with 5% CO2. After 1 week, the non-adherent cells were removed by replacing the medium supplemented with 10% FBS. When the cultures were near confluence (after 2 weeks) the cells were recovered, by treatment with 1X trypsin/ EDTA solution (Sigma Aldrich, Inc., St Louis, Mo, USA), for cytometric analysis and functional assays. BM-hMSCs were maintained and subcultured for up to 10-15 passages.
Immunofluorescence
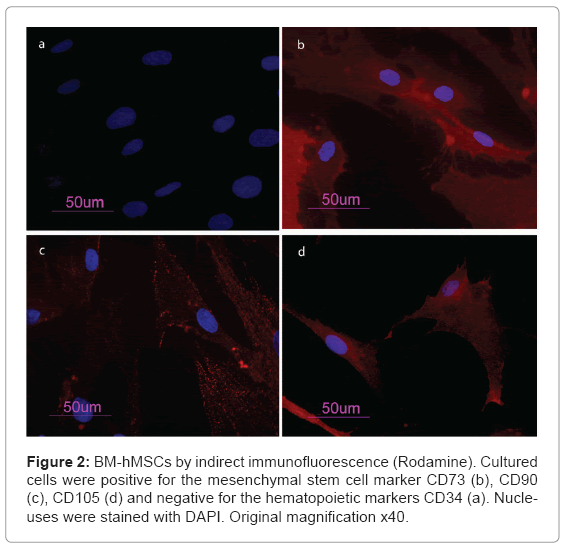
Cells were washed with PBS for three times and fixed with cold methanol for 5 min at room temperature. After washing with PBS, cells were blocked with bovine albumin 3% (Sigma Aldrich, Inc., St Louis, Mo, USA) for 30 min at room temperature. The cells were incubated overnight sequentially at 4°C with primary antibodies raised against CD105 1:200, mouse (BD Biosciences, San Jose, CA, USA), CD73 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA), CD90 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA), CD34 1:200, mouse (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA). They were washed with PBS and incubated for 1 h at room temperature with secondary antibody conjugated-Rodamine goat antimouse 1:200 (Santa Cruz Biotecnology, Inc., Santa Cruz, CA, USA). Subsequently, cells were mounted with the Vectashield Mounting Medium with DAPI (Vector Laboratories, Inc., Burlingame, CA, USA) and observed under a fluorescence microscope (Eclipse TE 2000-E, Nikon Instruments S.p.a., Florence, Italy).
Cell culture
BM-hMSCs at fourth passage were grown in medium (Alphamem Sigma Aldrich, Inc., St Louis, Mo, USA) supplemented with 10% fetal calf serum, antibiotics (Penicillin 100 U/ml and Streptomycin 100 micrograms/ml - Sigma Aldrich, Inc., St Louis, Mo, USA) and amminoacids (L-Glutamine - Sigma Aldrich, Inc., St Louis, Mo, USA). The cultures were maintained in a 5% CO2 humidified atmosphere at 37°C. For the assay, cells were collected and seeded at a density of 1x105 cells/ ml in 9 cm2 (3ml) wells by using 0.1% trypsin, 0.02% EDTA in Ca++ - and Mg - free Eagle's buffer for cell release. Cells were grown in one set of wells on sterile Trabecular Titanium™ disks with a pores size of 0,8µ (Figure 1). Another set of wells containing untreated cells was used as control. The medium was changed every 3 days.After seven days, when cultures were sub-confluent, cells were processed for RNA extraction.
RNA processing
Reverse transcription to cDNA was performed directly from cultured cell lysate using the TaqMAn Gene Expression Cells-to-Ct Kit (Ambion Inc., Austin, TX, USA), following the manufacturer's instructions. Briefly, cultured cells were lysed with lysis buffer and RNA released in this solution. Cell lysate were reverse transcribed to cDNA using the RT Enzyme Mix and appropriate RT buffer (Ambion Inc., Austin, TX, USA). Finally the cDNA was amplified by real-time PCR using the included TaqMan Gene Expression Master Mix and the specific assay designed for the investigated genes.
Real time PCR
Expression was quantified using real time RT-PCR. The gene expression levels were normalized to the expression of the housekeeping gene RPL13A and were expressed as fold changes relative to the expression of the untreated BM-hMSCs. Quantification was done with the delta/ delta calculation method [16]. Forward and reverse primers and probes for the selected genes were designed using primer express software (Applied Biosystems, Foster City, CA, USA) and are listed in Table 1. All PCR reactions were performed in a 20 µl volume using the ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA). Each reaction contained 10 µl 2X TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA, USA), 400 nM concentration of each primer and 200 nM of the probe, and cDNA. The amplification profile was initiated by 10-minute incubation at 95°C, followed by two-step amplification of 15 seconds at 95°C and 60 seconds at 60°C for 40 cycles. All experiments were performed including non-template controls to exclude reagents contamination. PCRs were performed with two biological replicates. The experiments were performed in triplicate.
| Gene symbol | Gene name | Primer sequence (5’>3’) | Probe sequence (5’>3’) |
|---|---|---|---|
| SPP1 | osteopontin | F-GCCAGTTGCAGCCTTCTCA
R-AAAAGCAAATCACTGCAATTCTCA |
CCAAACGCCGACCAAGGAAAACTCAC |
| COL1A1 | collagen type I alpha1 | F-TAGGGTCTAGACATGTTCAGCTTTGT R-GTGATTGGTGGGATGTCTTCGT |
CCTCTTAGCGGCCACCGCCCT |
| RUNX2 | runt-related transcription factor 2 | F-TCTACCACCCCGCTGTCTTC R-TGGCAGTGTCATCATCTGAAATG |
ACTGGGCTTCCTGCCATCACCGA |
| ALPL | alkaline phospatasi | F-CCGTGGCAACTCTATCTTTGG R-CAGGCCCATTGCCATACAG |
CCATGCTGAGTGACACAGACAAGAAGCC |
| COL3A1 | collagen, type III, alpha 1 | F-CCCACTATTATTTTGGCACAACAG R-AACGGATCCTGAGTCACAGACA |
ATGTTCCCATCTTGGTCAGTCCTATGCG |
| BGLAP | osteocalcin | F-CCCTCCTGCTTGGACACAAA R-CACACTCCTCGCCCTATTGG |
CCTTTGCTGGACTCTGCACCGCTG |
| CD105 | endoglin | F-TCATCACCACAGCGGAAAAA
R-GGTAGAGGCCCAGCTGGAA |
TGCACTGCCTCAACATGGACAGCCT |
| FOSL1 | FOS-like antigen 1 | F-CGCGAGCGGAACAAGCT
R-GCAGCCCAGATTTCTCATCTTC |
ACTTCCTGCAGGCGGAGACTGACAAAC |
| RPL13A | ribosomal protein L13 | F-AAAGCGGATGGTGGTTCCT
R-GCCCCAGATAGGCAAACTTTC |
CTGCCCTCAAGGTCGTGCGTCTG |
Table 1: Primer and probes used in real time PCR.
Results
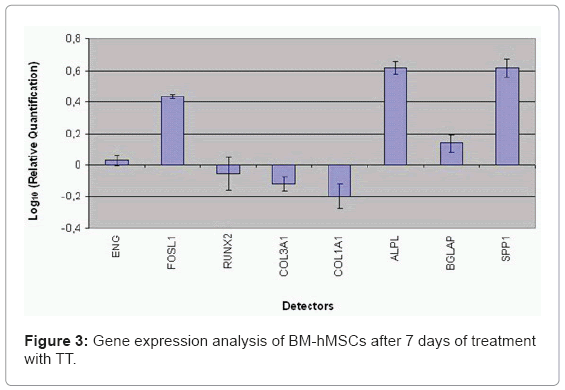
BM-hMSCs cell surfaces were positive for mesenchymal stem cell marker, CD105, CD90 and CD73 and negative for markers of haematopoietic origin, CD34 (Figure 2). Transcriptional expressions of several osteoblast-related genes (RUNX2, SPP1, COLIA1, COL3A1, BGLAP, ALPL and FOSL1) and mesenchymal stem cells marker (ENG) were examined after 7 days of supplement treatment with Trabecular Titanium™. Quantitative real-time RT-PCR of FOSL1, ALPL, SPP1, BGLAP showed a significant induction after treatment with Trabecular Titanium™. However, Trabecular Titanium™ treatment did not significantly affect the mRNA expression of RUNX2 (slightly decreased) and the stem cell marker ENG (slightly increased) that were similar in both treated and untreated BM-hMSCs. COL1A1 and COL3A1 were decreased in the presence of Trabecular Titanium™ at day 7 (Figure 3).
Discussion
Titanium and titanium alloys are widely used as implant materials due to their excellent biocompatibility and mechanical properties. The goal of prosthetic surgery is to have the best possible osseointegration to ensure stability and long-term survival rate of the implants [17]. Implant osseointegration at the tissue-prosthesis interface contributes to determining the success or the failure of the device. Growing on the implant surface, the osteoblasts are responsible for the tissue response on the biomaterial surface [18] whose modality and quality depend on the surface morphology.
Generally, it is possible to distinguish the macro-, mini-, microand nano-design of prostheses. The macrodesign is the shape of the prosthesis. The minidesign is related to the featured dimensions of the prosthesis, like indentations, threads and fins. The microdesign is the shape of the implant surface, due to surface treatments like machining, acid etching and sand-blasting procedures that determine the surface roughness, characterized by a cellular dimension [19]. Finally, the nanodesign is determined by the molecular composition of the surface. The porous Titanium surface is rough and it contains many pores and grooves of different sizes regularly distributed over the whole surface.
The osteoblasts are sensitive to surface roughness and material composition [20]. It was shown that osteoblast-like cells (MG63) grown on rough titanium surfaces exhibited a reduced cell proliferation rate but increased alkaline phosphatase-specific activity and osteocalcin production [9,21,22]. Titanium promotes protein adsorption, the crucial factor for the initial cell adhesion on artificial surfaces. Molecules involved in cell adhesion include extracellular matrix proteins, transmembrane receptors and intracellular cytoskeletal components [20].
Mesenchymal stem cells are defined as self-renewable, multipotent progenitors cells with the ability to differentiate, under adequate stimuli, in several mesenchymal lineages, including osteoblasts [23]. The aim of this study was to verify the early effects of Trabecular Titanium ™ on osteogenesis. Therefore we treated BM-hMSCs cultures with Trabecular Titanium™ disks. In order to examinein further detail how Trabecular Titanium™ acts on BM-hMSCs, changes in expression of bone related marker genes (RUNX2, SPP1, COLIA1, COL3A1, BGLAP, ALPL and FOSL1) and mesenchymal stem cells marker (ENG) were investigated by real-time RT-PCR.
In our study, mesenchymal stem cells from human bone marrow were isolated and characterized by morphology and immunophenotype. Isolated BM-hMSCs showed fibroblast-like morphology and were positive for MSC surface molecules (CD90, CD105, CD73) and negative for markers of haematopoietic progenitors (CD34). After 7 days of treatment with Trabecular Titanium™ the expression levels of osteodifferentiation genes were measured by relative quantification methods using real-time RT-PCR.Two osteoblast-specific genes, SPP1 and BGLAP, that are generally expressed by osteoblast in the early stage of their differentiation [24], were up-regulate in treated BM-hMSCs.
ENG (CD105) mRNA expression, a surface markers used to define a bone marrow stromal cell population capable of multilineage differentiation [25], was similar in treated BM-hMSCs respect to control. There is an inverse correlation between CD105 expression and the differentiation status of MSC [26]. This gene is a receptor for TGF-β1 and -β3 [27] and modulates TGF-β signalling by interacting with related molecules, such as TGF-β1, -β3, BMP-2, -7, and activin A. It is speculated that these members of the TFG-β superfamily are mediators of cell proliferation and differentiation and play regulatory roles in cartilage and bone formation [28]. The disappearance of the CD105 antigen during osteogenesis suggests that this protein, like others in the TFG-β superfamily, is involved in the regulation of osteogenesis [29].
Expression of RUNX2 didn't have significant change in treated cells if compared to control after 7 days of treatment with Trabecular Titanium ™. RUNX2 is the most specific osteoblast transcription factor and is a prerequisite for osteoblast differentiation and consequently mineralization. This result is comparable with data reported by Kim et al. [30]. They showed that there was no BMP-2-mediated up-regulation of RUNX2 mRNA expression at days 3 or 7, but BMP-2 treatment induced a significant and time dependent increase in SP7 mRNA expression [30].
Alkaline phosphatase regulates mineralization of bone matrix. Several studies demonstrated that the potency of individual substances to induce alkaline phosphatase varies in a species-dependent manner. Glucocorticoids such as dexamethasone are potent inducers in human and rat stromal cells, but they have no effect on alkaline phosphatase activity in mouse stromal cells [31,32]. The Trabecular Titanium™ induced ALPL mRNA production. Trabecular Titanium™ also modulates the expression of FOSL1 that encodes for Fra-1, a component of the dimeric transcription factor activator protein-1 (Ap-1), which is composed mainly of Fos (c-Fos, FosB, Fra-1 and Fra-2) and Jun proteins (c-Jun, JunB and JunD). Ap-1 sites are present in the promoters of many developmentally regulated osteoblast genes, including alkaline phosphatase, collagen I, osteocalcin (OC). McCabe et al. [33] demonstrated that differential expression of Fos and Jun family members could play a role in the developmental regulation of bone-specific gene expression and, as a result, may be functionally significant for osteoblast differentiation. Studying the effect of new anabolic agents that stimulate bone formation, Kim et al. [34] found that this gene is activated in the late stage of differentiation, during the calcium deposition. In our study FOSL1 was up-regulated thus demonstrating that Trabecular Titanium ™ induces osteoblast differentiation in BM-hMSCs.
Trabecular Titanium™ also modulates the expression of genes encoding for collagenic extracellular matrix proteins like collagen type 1α1 (COL1A1) and collagen type 3α1 (COL3A1). COL1A1, and COL3A1 were significantly down expressed if compared to the control when exposed to Trabecular Titanium™, probably because these genes are activated in the late stage of differentiation and are related to extracellular matrix synthesis. In conclusion, the present study shows the effect Trabecular Titanium™ on BM-hMSCs differentiation. Trabecular Titanium™ appears to induce osteogenesis on human stem cells but RUNX2 is not immediately activated. In fact, Trabecular Titanium™ in the early stages of stimulation seems to induce osteoblastic differentiation of BM-hMSCs mainly through the upregulation of FOSL1, SPP1 and ALPL. The results obtained showed that Trabecular Titanium™ participates in the initial process of differentiation of BM-hMSCs in osteoblasts. In future studies, more investigations with different time points would be useful in order to get a global comprehension of the molecular events related to Trabecular Titanium™ action.
Acknowledgements
This work was supported by FAR from the University of Ferrara (FC), Ferrara, Italy, and from Regione Emilia Romagna, Programma di Ricerca Regione Università, 2007-2009, Area 1B: Patologia osteoarticolare: ricerca pre-clinica e applicazioni cliniche della medicina rigenerativa, Unità Operativa n. 14.
References
- Wennenberg A, Albrektsson T, Andersson B (1995) An animal study of c.p. titanium screws with different surface topographies. Journal of Materials Science: Materials in Medicine 6: 302-309.
- Li LH, Kong YM, Kim HW, Kim YW, Kim HE, et al. (2004) Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 25: 2867-2875.
- Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, et al. (1996) Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials 17: 605-616.
- Carinci F, Volinia S, Pezzetti F, Francioso F, Tosi L, et al. (2003) Titanium-cell interaction: analysis of gene expression profiling. J Biomed Mater Res B Appl Biomater 66: 341-346.
- Keshmiri M, Troczynski T (2003) Apatite formation on TiO2 anatase microspheres. Journal of Non-Crystalline Solids 324: 289-294.
- Buser D (2001) Titanium for dental applications. II. Implants with roughened surfaces. In: Brunette DM, Tengvall P, Textor M, Thomson P, editors. Titanium in Medicine, Springer, Berlin, 876-888.
- Gapski R, Wang HL, Mascarenhas P, Lang NP (2003) Critical review of immediate implant loading. Clin Oral Implants Res 14: 515-527.
- Schwartz Z, Martin JY, Dean DD, Simpson J, Cochran DL, et al.(1996) Effect of titanium surface roughness on chondrocyte proliferation, matrix production, and differentiation depends on the state of cell maturation. J Biomed Mater Res 30: 145-155.
- Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, et al. (1995) Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res 29: 389- 401.
- Gotfredsen K, Berglundh T, Lindhe J (2000) Anchorage of titanium implants with different surface characteristics: an experimental study in rabbits. Clin Implant Dent Relat Res 2: 120-128.
- Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ (2006) Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 27: 4671-4681.
- Matsuno H, Yokoyama A, Watari F, Uo M, Kawasaki T (2001) Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 22: 1253-1262.
- Clem WC, Chowdhury S, Catledge SA, Weimer JJ, Shaikh FM, et al. (2008) Mesenchymal stem cell interaction with ultra-smooth nanostructured diamond for wear-resistant orthopaedic implants. Biomaterials 29: 3461-3468.
- Wang CY, Zhao BH, Ai HJ, Wang YW (2008) Comparison of biological characteristics of mesenchymal stem cells grown on two different titanium implant surfaces. Biomed Mater 3: 015004.
- Gastaldi G, Asti A, Scaffino MF, Visai L, Saino E, et al.( 2010) Human adiposederived stem cells (hASCs) proliferate and differentiate in osteoblast-like cells on trabecular titanium scaffolds. J Biomed Mater Res A 94: 790-799.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408.
- Puleo DA, Thomas MV (2006) Implant surfaces. Dent Clin North Am 50: 323- 338.
- Meyer U, Buchter A, Wiesmann HP, Joos U, Jones DB (2005) Basic reactions of osteoblasts on structured material surfaces. Eur Cell Mater 9: 39-49.
- Sollazzo V, Palmieri A, Pezzetti F, Scarano A, Martinelli M, et al. (2008) Genetic effect of anatase on osteoblast-like cells. J Biomed Mater Res B Appl Biomater 85: 29-36.
- Bachle M, Butz F, Hubner U, Bakalinis E, Kohal RJ (2007) Behavior of CAL72 osteoblast-like cells cultured on zirconia ceramics with different surface topographies. Clin Oral Implants Res 18: 53-59.
- Boyan BD, Batzer R, Kieswetter K, Liu Y, Cochran DL, et al. (1998) Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1 alpha, 25-(OH)2D3. J Biomed Mater Res 39: 77-85.
- Kieswetter K, Schwartz Z, Dean DD, Boyan BD (1996) The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med 7: 329-345.
- Alhadlaq A, Mao JJ (2004) Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev 13: 436-448.
- Lian JB SG, Aubin JE. (2003) Bone formation: maturation and functional activities of osteoblast lineage cells. In: MJ F, editor. Primer on the metabolic bone diseases and disorders on mineral metabolism. The American Society for Bone and Mineral Research, Washington D.C, 13-28.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143- 147.
- Jin HJ, Park SK, Oh W, Yang YS, Kim SW, et al. (2009) Down-regulation of CD105 is associated with multi-lineage differentiation in human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun 381: 676-681.
- Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J (1999) The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun 265: 134-139.
- Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, et al. (2001) Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem 81: 368-377.
- Haynesworth SE, Baber MA, Caplan AI (1992) Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone 13: 69-80.
- Kim IS, Song YM, Cho TH, Park YD, Lee KB, et al. (2008) In vitro response of primary human bone marrow stromal cells to recombinant human bone morphogenic protein-2 in the early and late stages of osteoblast differentiation. Dev Growth Differ 50: 553-564.
- Leboy PS, Beresford JN, Devlin C, Owen ME (1991) Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol 146: 370-378.
- Beresford JN, Joyner CJ, Devlin C, Triffitt JT (1994) The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol 39: 941-947.
- McCabe LR, Banerjee C, Kundu R, Harrison RJ, Dobner PR, et al.(1996) Developmental expression and activities of specific fos and jun proteins are functionally related to osteoblast maturation: role of Fra-2 and Jun D during differentiation. Endocrinology 137: 4398-4408.
- Kim JM, Lee SU, Kim YS, Min YK, Kim SH (2008) Baicalein stimulates osteoblast differentiation via coordinating activation of MAP kinases and transcription factors. J Cell Biochem 104: 1906-1917.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15423
- [From(publication date):
March-2011 - Nov 16, 2025] - Breakdown by view type
- HTML page views : 10681
- PDF downloads : 4742