Review Article Open Access
The Tripeptide, GHK, Induces Programmed Cell Death in SH-SY5Y Neuroblastoma Cells
Luay E. Matalka1, Ashley Ford2 and M. Tino Unlap3*1UAB Global and Community Leadership Program, University of Alabama at Birmingham
2Department of Biology, University of Alabama at Birmingham
3Department of Clinical and Diagnostic Sciences and Biochemistry and Molecular Genetics, University of Alabama at Birmingham
- Corresponding Author:
- M. Tino Unlap, PhD
Department of Clinical and Diagnostic Sciences
SHPB 476, University of Alabama at Birmingham
Birmingham, AL 35294
Tel: 205-934-7382
E-mail: unlap@uab.edu
Received date: July 02, 2012; Accepted date: August 06, 2012; Published date: August 09, 2012
Citation: Matalka LE, Ford A, Unlap MT (2012) The Tripeptide, GHK, Induces Programmed Cell Death in SH-SY5Y Neuroblastoma Cells. J Biotechnol Biomater 2:144. doi:10.4172/2155-952X.1000144
Copyright: © 2012 Matalka LE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
GHK-Cu is a tripeptide that is found in plasma and increases fibroblast proliferation and is widely used in wound healing creams. Studies show that GHK-Cu stimulates the level of p63, a member of the antitumor suppressor family which includes p53 and p73. Because of its effect on p63, we tested the hypothesis that GHK induces apoptosis in neuroblastoma cells, SH-SY5Y, and that modification of GHK by polyethylene glycol (PEG) conjugation, PEGylation, potentiates its effects. Initial studies on NIH-3T3 fibroblasts and U937 histiocytic lymphoma cells show that GHK-Cu and GHK-PEG stimulate fibroblast proliferation while attenuating U937 cell proliferation. SH-SY5Y cells were treated with GHK or GHK-PEG at 1 and 10 nM for 24 hours followed by cell proliferation, cell viability, cell cytotoxicity and apoptosis assays. Our results showed that 24 hour GHK treatment elevated apoptosis by 1.8 ± 0.17 and 3.3 ± 0.15 fold at 1 and 10 nM, respectively. GHK-PEG treatment, on the other hand, stimulated apoptosis by 3.2 ± 0.80 and 4.9 ± 0.9 fold at 1 and 10 nM, respectively. The treatments, while having no effects on cell cytotoxicity, reduced neuroblastoma cell viability and cell proliferation. Therefore, GHK induces apoptosis in neuroblastoma cells, an affect which was potentiated by PEGylation.
Keywords
Pegylation; Apoptosis; Wound Healing; Neuroblastoma; Cytotoxicity
Introduction
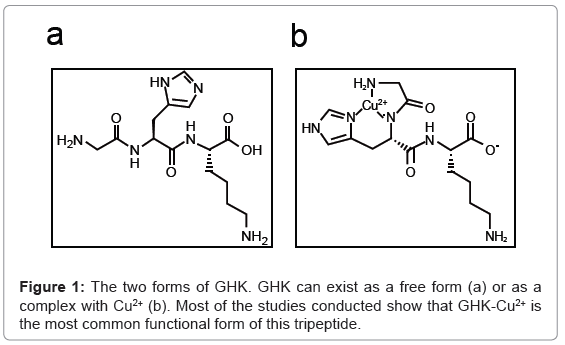
GHK, a glycyl-L-histidyl-L-lysine complex, is a naturally occurring human tripeptide isolated from human plasma but is also present in saliva and urine. It has a high affinity for Copper(II) and so is present in two forms, GHK and GHK-Cu (Figure 1a,1b) [1]. The copper peptide, GHK-Cu, was discovered by Loren Pickart in human plasma albumin in 1973. The potential role of this peptide in wound healing and tissue regeneration was demonstrated in a study which showed that because of the increased level of GHK-Cu in the plasma of younger adults compared to older adults, livers from older patients transplanted into younger patients began to function in the same way as younger liver tissue. This was attributed to GHK-Cu having the ability to modulate copper intake into cells [2,3].
The copper peptide GHK-Cu has a number of biological functions in humans, including stimulation of wound healing, nerve regeneration and potential hair growth [2]. The diversity of its actions in human is attributed to its ability to modulate the expression of a number of genes [4]. Studies showed that the GHK-Cu sequence was found in collagen and was thought to be released, as a collagen degradation product, in response to tissue injury. Therefore in the late 1980’s, GHK-Cu began to gain wide use as a wound healing agent [5,6]. In one clinical trial which consisted of 120 diabetic patients treated with 2% GHK gel, patients showed increased in the percentage of ulcer closure from 60.8% to 98.5% and decreased in the percentage of infection from 34% to 7%. Furthermore, the GHK gel increased the rate of healing by three fold. These results were also seen in preclinical animal trials [7].
PEGylation is the covalent conjugation of polyethylene glycol (PEG) polymer to a target molecule and enhances that molecule’s delivery to its potential target. The modified molecule may be a protein, peptide, or non-peptide molecule and one or more PEG chains can be attached. The use of PEG for modification of drugs is FDA approved, non-immunogenic, non-antigenic, non-toxic, and increases the solubility of drugs in aqueous solutions. PEGylation has also been shown to increase the bioavailability or the half-life of therapeutics in the plasma [8,9], including anticancer therapeutics [10]. Therefore, PEGylation now plays an important role in drug delivery, especially of peptide and protein based drugs, thus increasing their potential as therapeutic agents [11].
The potential role of this tripeptide in cancer therapeutics was demonstrated in studies which showed that GHK-Cu reversed the expression of certain genes involved in metastatic spread of colon cancer, even at very low concentrations [12]. Studies also showed that a b GHK stimulates the level of p63 in human keratinocytes [13]. p63 is a member of the tumor suppressor family which includes p53 and p73 and while it was discovered much later than the others, p63 appears to be the original member of this family from which p53 and p73 evolved [14-17]. These proteins, particularly p53, are activated in response to stress and induce the apoptosis of damaged cells and arrest cell cycle in cells containing DNA mutations in order to allow them time to correct the mutation. Mutations of these genes lead to abnormally high incidence of cancer. A product of lipid peroxidation, 4-hydroxynonenal, has been shown to induce apoptosis in neuroblastoma cells by elevating the levels of p53, p63 and p73 and p63 staining has been used as a marker for prostatic adenocarcinoma where lack of p63 staining indicates malignant prostatic adenocarcinoma [14,18]. Therefore, because GHK-Cu elevates the levels of p63 and PEGylation has been shown to increase the solubility and half-life of protein based drugs, we proposed and tested the hypothesis that GHK-Cu induces apoptosis in neuroblastoma SH-SY5Y cells and that PEGylation potentiates this effect. The findings of this study are significant for two reasons: 1) GHK is a natural peptide and if it demonstrates anticancer effects it will be an excellent candidate for chemotherapy, and 2) GHK can be modified by PEGylation and once modified it can then be used for both wound healing and chemotherapy.
Materials and Methods
Cell culture and seeding for experiments
NIH-3T3, U937 and neuroblastoma cells, SH-SY5Y, were obtained from ATCC and were maintained in HyQ-DMEM media with 5% Fetal Clone III, (Gibco/BRL), 50 U/ml penicillin, and 100 μg/ml streptomycin in 100 mm Petri dishes. Cells were generally passaged twice a week. Before each experiment, cells were detached by trypsinization, counted using a hemocytometer, seeded in 24 well plates at 10,000 cells per well and incubated for 24 hrs prior to the initiation of each experiment.
GHK and GHK-PEG induce fibroblast proliferation
Before testing the effects of GHK-Cu and GHK-PEG on malignant cells, their ability to induce NIH3T3 cell proliferation was assessed. Cells were cultured in 24 well plates and treated with GHK or GHKPEG at 1, 5 and 10 nM for 24, 48 or 72 hr. Following treatment with GHK or GHK-PEG, media was aspirated from each well and 2 ml of trypsin was added to each well. Following complete detachment of cells from the wells, 2ml of media was added to neutralize the trypsin and an aliquot was used to determine cell number using a hemocytometer. A 20 μl aliquot of trypsinized cells was transferred to a microcentrifuge tube containing an equal amount of 0.4% trypan blue dye, mixed and loaded onto a hemocytometer. The number of cells in five of the central 25 squares was counted and multiplied by 100,000 to obtain number of cells per ml of media. The rate of cell proliferation was expressed as fold control and compared between treated and non-treated cells using analysis of variance (ANOVA). n=3; *p≤0.05
GHK and GHK-PEG attenuate U937 cell proliferation
The effect of GHK and GHK-PEG on malignant cell proliferation was first tested on U937 histiocytic lymphoma cells. Cells were cultured in 24 well plates and treated with GHK or GHK-PEG at 1 or 10 nM for 24 h. Following treatment with GHK or GHK-PEG, the number of cells per ml of media was assessed as previously described and the rate of cell proliferation was expressed as fold control and compared between treated and non-treated cells using analysis of variance (ANOVA). n=3; *p≤0.05.
The effect of GHK or GHK-PEG on SH-SY5Y neuroblastoma cell proliferation
SH-SY5Y cells were cultured, treated, and the number of cells per ml of media was assessed as previously described. The rate of cell proliferation was compared between treated and non-treated cells using analysis of variance (ANOVA). n=3; *p≤0.05.
The effect of GHK or GHK-PEG on cytotoxicity, cell viability, and apoptosis in neuroblastoma cells
For apoptosis assay, neuroblastoma cells, SH-SY5Y, were cultured in 96 well plates and treated with GHK or GHK-PEG at 1 and 10 nM for 24 hrs and assayed using the Apo-ToxGlo Triplex Assay by Promega. This assay measured the levels of caspase 3 and caspase 7 in response to treatments. In brief, 20 μl of Viability/Cytotoxicity Reagent containing both GF-AFC was added to each well, followed by 30 sec mixing at room temperature and incubation at 37°C for 30 min. Fluorescence was measured at 400/505 for cell viability and 485/520 for cytotoxicity. Caspase-Glo 3/7, 100 μl, was added to each well, mixed for 30 sec and incubated at room temperature for 30 min. The luminescence was measured using a microplate reader to assess apoptosis. The results are expressed as cells/ml versus treatment for cell viability and fold control versus treatment for cytotoxicity and apoptosis.The results were compared between treated and non-treated sample susing ANOVA. n=3; *p≤0.05.
Results and Discussion
GHK and GHK-PEG induce fibroblast proliferation
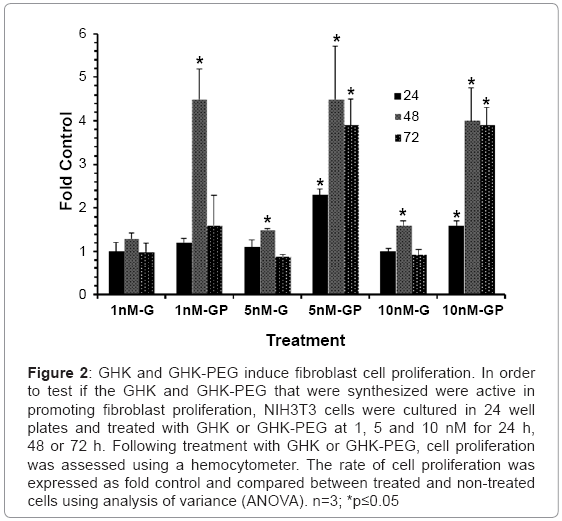
Prior to assessing the effects of GHK and GHK-PEG on apoptosis in SH-SY5Y neuroblastoma cells, it was necessary to ensure that both peptides stimulated fibroblast cell proliferation as previously demonstrated by a number of studies. The results (Figure 2) show that GHK stimulates fibroblast proliferation at 5 and 10 nM after 48 hrs of treatment while GHK-PEG stimulates fibroblast proliferation at 1 nM after 24 hrs of treatment and at 5 and 10 nM after 24, 48, and 72 hrs of treatment.
Figure 2: GHK and GHK-PEG induce fibroblast cell proliferation. In order to test if the GHK and GHK-PEG that were synthesized were active in promoting fibroblast proliferation, NIH3T3 cells were cultured in 24 well plates and treated with GHK or GHK-PEG at 1, 5 and 10 nM for 24 h, 48 or 72 h. Following treatment with GHK or GHK-PEG, cell proliferation was assessed using a hemocytometer. The rate of cell proliferation was expressed as fold control and compared between treated and non-treated cells using analysis of variance (ANOVA). n=3; *p≤0.05
GHK and GHK-PEG attenuate U937 cell and SH-Sy5y cell proliferation
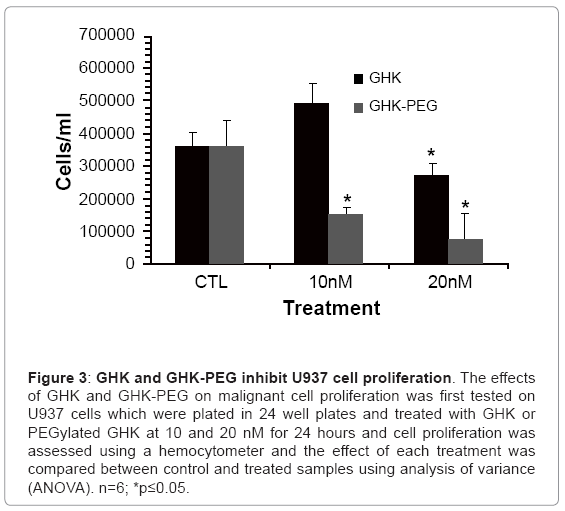
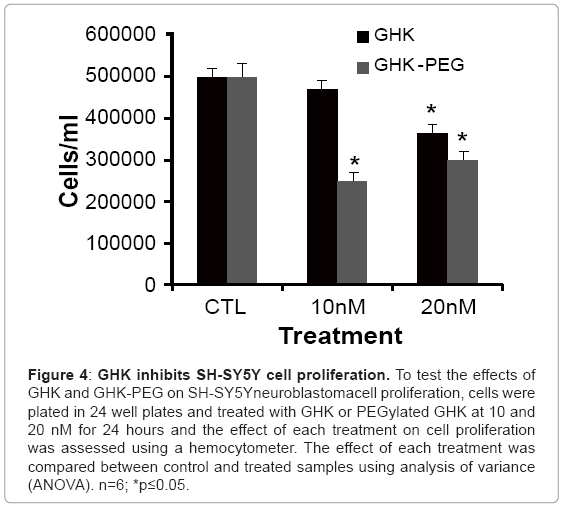
After establishing that the two tripeptides induced fibroblast cell proliferation, studies were performed in order to assess the effects of these tripeptides on malignant cell proliferation. Because of its availability, the effect of GHK and GHK-PEG were first tested in histiocytic lymphomacells (U937). Our results show that after 24 hr treatment, GHK attenuates U937 cell proliferation at 20 nM while GHK-PEG attenuates cell proliferation at 10 and 20 nM (Figure 3). Once the effects of the tripeptides were confirmed in U937 cells, the experiments were repeated in SH-SY5Y neuroblastoma cells and similar results were obtained (Figure 4). This was a significant finding in that PEGylation is routinely used to increase the solubility and efficacy of drugs, especially anticancer drugs [8,9,11].
Figure 3: GHK and GHK-PEG inhibit U937 cell proliferation. The effects of GHK and GHK-PEG on malignant cell proliferation was first tested on U937 cells which were plated in 24 well plates and treated with GHK or PEGylated GHK at 10 and 20 nM for 24 hours and cell proliferation was assessed using a hemocytometer and the effect of each treatment was compared between control and treated samples using analysis of variance (ANOVA). n=6; *p≤0.05.
Figure 4: GHK inhibits SH-SY5Y cell proliferation. To test the effects of GHK and GHK-PEG on SH-SY5Yneuroblastomacell proliferation, cells were plated in 24 well plates and treated with GHK or PEGylated GHK at 10 and 20 nM for 24 hours and the effect of each treatment on cell proliferation was assessed using a hemocytometer. The effect of each treatment was compared between control and treated samples using analysis of variance (ANOVA). n=6; *p≤0.05.
GHK and GHK-PEG induced apoptosis and slightly attenuate cell viability and cytotoxicity in neuroblastoma cells
Once GHK and GHK-PEG demonstrated antiproliferative effects in neuroblastoma cells, the effects of these tripeptides on cell viability, cytotoxicity and apoptosis were assessed. These are three critical parameters in assessing the effects of treatments on cancer cells. Viability and cytotoxicity are inversely proportional such that if the viability of cells is reduced, cytotoxicity is usually stimulated and programmed cell death, apoptosis, is either induced or not induced. If apoptosis is induced the compound of interest is an excellent anticancer candidate. If apoptosis is not induced, this is primary necrosis and the compound of interest is not a good candidate for cancer therapeutics.
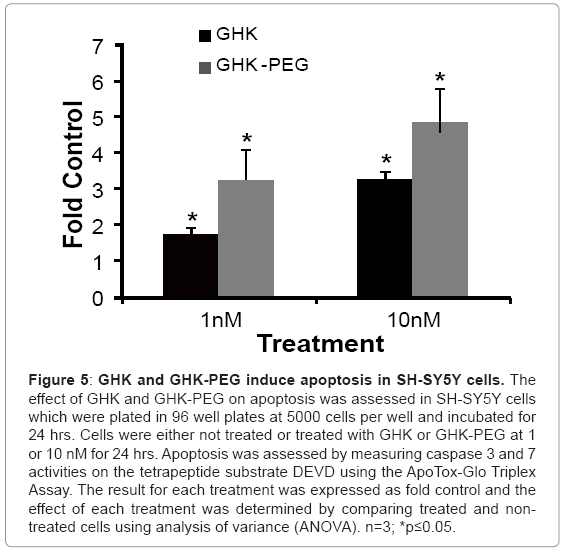
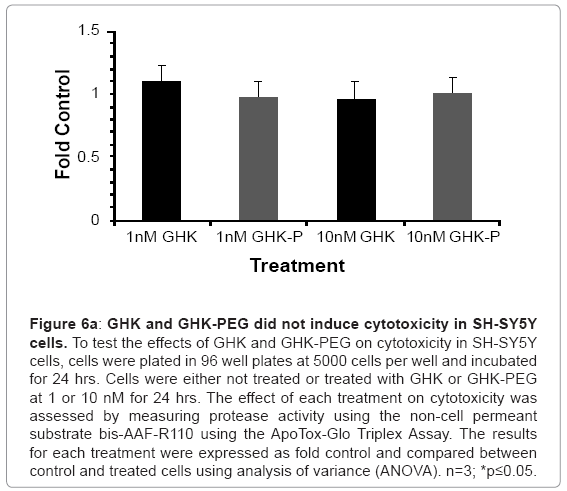
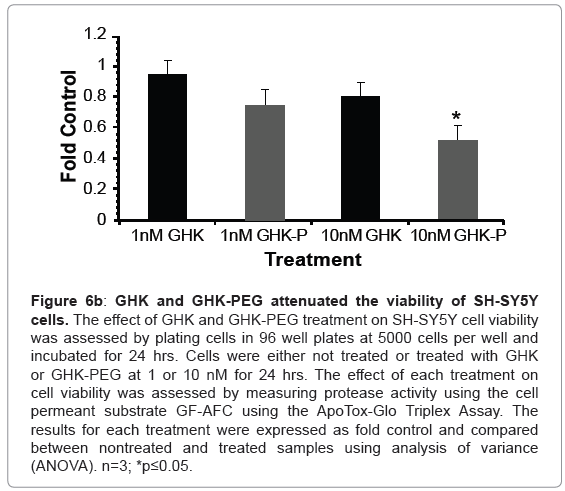
Our results show that GHK and GHK-PEG induce apoptosis in SH-SY5Y neuroblastoma cells at 1 and 10 nM with GHK-PEG demonstrating greater effects (Figure 5). Our studies also demonstrate that both tripeptides attenuate cell viability in SH-SY5Y cells with GHK demonstrating greater effects (Figure 6b). The cytotoxicity results, on the other hand, show that neither GHK nor GHK-PEG stimulates cytotoxicity in these neuroblastoma cells (Figure 6a). How viability can be attenuated and apoptosis induced without cytotoxicity can be explained based on the length of time that it takes a compound to induce apoptosis. If cells are treated with a slow-acting apoptosis inducing compound for a short period of time, changes in viability and cytotoxicity may not be measurable even though apoptosis may be. On the other hand, if cells are treated with a fast-acting apoptosis inducing compound for a long period of time viability and apoptosis may be evident but cytotoxicity will be underestimated. It appears that the latter case applies for our studies so that in order to observe all three events, treatment with GHK and GHK-PEG must be carried out for less than 24 hrs.
Figure 5: GHK and GHK-PEG induce apoptosis in SH-SY5Y cells. The effect of GHK and GHK-PEG on apoptosis was assessed in SH-SY5Y cells which were plated in 96 well plates at 5000 cells per well and incubated for 24 hrs. Cells were either not treated or treated with GHK or GHK-PEG at 1 or 10 nM for 24 hrs. Apoptosis was assessed by measuring caspase 3 and 7 activities on the tetrapeptide substrate DEVD using the ApoTox-Glo Triplex Assay. The result for each treatment was expressed as fold control and the effect of each treatment was determined by comparing treated and nontreated cells using analysis of variance (ANOVA). n=3; *p≤0.05.
Figure 6a: GHK and GHK-PEG did not induce cytotoxicity in SH-SY5Y cells. To test the effects of GHK and GHK-PEG on cytotoxicity in SH-SY5Y cells, cells were plated in 96 well plates at 5000 cells per well and incubated for 24 hrs. Cells were either not treated or treated with GHK or GHK-PEG at 1 or 10 nM for 24 hrs. The effect of each treatment on cytotoxicity was assessed by measuring protease activity using the non cell permeant substrate bis-AAF-R110 using the ApoTox-Glo Triplex Assay. The results for each treatment were expressed as fold control and compared between control and treated cells using analysis of variance (ANOVA). n=3; *p≤0.05.
Figure 6b: GHK and GHK-PEG attenuated the viability of SH-SY5Y cells. The effect of GHK and GHK-PEG treatment on SH-SY5Y cell viability was assessed by plating cells in 96 well plates at 5000 cells per well and incubated for 24 hrs. Cells were either not treated or treated with GHK or GHK-PEG at 1 or 10 nM for 24 hrs. The effect of each treatment on cell viability was assessed by measuring protease activity using the cell permeant substrate GF-AFC using the ApoTox-Glo Triplex Assay. The results for each treatment were expressed as fold control and compared between nontreated and treated samples using analysis of variance (ANOVA). n=3; *p≤0.05.
Summary and Conclusion
Our studies show that GHK inhibits the proliferation of U937 cells and SH-SY5Y neuroblastoma cells. Our studies also show that GHK induces apoptosis of SH-SY5Y neuroblastoma cells. The effect of GHK on the neuroblastomacell line is potentiated by the conjugation of polyethylene glycol to GHK.
Acknowledgements
We would like to thank the UAB Global and Community Leadership Program and the Department of Biology for their support of Luay Matalka and Ashley Ford, respectively. We would also like to thank the Department of Clinical and Diagnostic Sciences Program for the use of the laboratory facilities where this research was conducted.
References
- Pickart L, Freedman JH, Loker WJ, Peisach J, Perkins CM, et al. (1980) Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature 288: 715-717.
- Pilgeram L (2010) Control of fibrinogen biosynthesis: role of the FFA/albumin ratio. Cardiovasc Eng 10: 78-83.
- Pilgeram LO, Pickart LR (1968) Control of fibrinogen biosynthesis: the role of free fatty acid. J Atheroscler Res 8: 155-166.
- Lamb J. The Connectivity Map: a new tool for biomedical research. Nat Rev Cancer 2007 7: 54-60.
- Maquart FX, Pickart L, Laurent M, Gillery P, Monboisse JC, et al. (1988) Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett 238: 343-346.
- Wegrowxski Y, Maquart FX, Borel JP (1992) Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Life Sci 51: 1049-1056.
- Mulder GD, Patt LM, Sanders L, Rosenstock J, Altman MI, et al. (1994) Enhanced healing of ulcers in patients with diabetes by topical treatment with glycyl-l-histidyl-l-lysine copper. Wound Repair Regen 2: 259-269.
- Anabousi S, Kleemann E, Bakowsky U, Kissel T, Schmehl T, et al. (2006) Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. J Nanosci Nanotechnol 6:3010-3016.
- Molineux G (2002) Pegylation: engineering improved pharmaceuticals for enhanced therapy. Cancer Treat Rev 28 Suppl A: 13-16.
- Ichikawa K, Hikita T, Maeda N, Takeuchi Y, Namba Y, et al. (2004) PEGylation of liposome decreases the susceptibility of liposomal drug in cancer photodynamic therapy. Biol Pharm Bull 27: 443-444.
- Veronese FM, Pasut G (2005) PEGylation, successful approach to drug delivery. Drug Discov Today10: 1451-1458.
- Hong Y, Downey T, Eu KW, Koh PK, Cheah PY (2010) A 'metastasis-prone' signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin Exp Metastasis 27: 83-90.
- Kang YA, Choi HR, Na JI, Huh CH, Kim MJ, et al. (2009) Copper-GHK increases integrin expression and p63 positivity by keratinocytes. Arch Dermatol Res 301: 301-306.
- Laurora S, Tamagno E, Briatore F, Bardini P, Pizzimenti S, et al. (2005) 4-Hydroxynonenal modulation of p53 family gene expression in the SK-N-BE neuroblastoma cell line. Free Radic Biol Med 38: 215-225.
- Murray-Zmijewski F, Lane DP, Bourdon JC (2006)p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ 13: 962-9
- Nahor I, Abramovitch S, Engeland K, Werner H (2005)The p53-family members p63 and p73 inhibit insulin-like growth factor-I receptor gene expression in colon cancer cells. Growth Horm IGF Res 15: 388-396 .
- Gwosdz C, Balz V, Scheckenbach K, Bier H (2005) p53, p63 and p73 expression in squamous cell carcinomas of the head and neck and their response to cisplatin exposure. Adv Otorhinolaryngo 62: 58-71.
- Baydar DE, Kulac I, Gurel B, and De Marzo A (2011)A case of prostatic adenocarcinoma with aberrant p63 expression: presentation with detailed immunohistochemical study and FISH analysis. Int J Surg Pathol 19: 131-136.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 21151
- [From(publication date):
August-2012 - Nov 30, 2025] - Breakdown by view type
- HTML page views : 16144
- PDF downloads : 5007