Review Article Open Access
The Role of Photodynamic Therapy in Non-malignant and Malignant Eye Disorders
Patrycja Nowak-Sliwinska1,2*, Andrea Weiss1 , Michel Sickenberg1,3, Arjan W Griffioen2 and Hubert van den Bergh1*
1Medical Photonics Group, Swiss Federal Institute of Technology (EPFL), Lausanne, Switzerland
2Angiogenesis Laboratory, Department of Medical Oncology, VU University Medical Center, Amsterdam, Netherlands
3Save Sight, Lausanne, Switzerland
- *Corresponding Author:
- Patrycja Nowak-Sliwinska
Institute of Chemical Sciences and Engineering (ISIC)
Swiss Federal Institute of Technology (EPFL)
Lausanne, CH-1015, Switzerland
Tel: +41 21 6935169
Fax: +41 21 6935110
E-mail: patrycja.nowak-sliwinska@epfl.ch
- *Corresponding Author:
- Hubert van den Bergh
Institute of Chemical Sciences and Engineering (ISIC)
Swiss Federal Institute of Technology (EPFL)
Lausanne, CH-1015, Switzerland
Tel: +41 21 6935169
Fax: +41 21 6935110
E-mail: hubert.vandenbergh@epfl.ch
Received date: August 13, 2013; Accepted date: September 20, 2013; Published date: September 23, 2013
Citation: Nowak-Sliwinska P, Weiss A, Sickenberg M, Griffioen AW, van den Bergh H (2013) The Role of Photodynamic Therapy in Non-malignant and Malignant Eye Disorders. J Anal Bioanal Tech S1:007. doi: 10.4172/2155-9872.S1-007
Copyright: © 2013 Nowak-Sliwinska P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Photodynamic therapy (PDT) has represented an important treatment modality in many ocular disorders for more than a decade. The introduction of verteporfin-PDT to the treatment of neovascular age-related macular degeneration and polypoidal choroidal vasculopathy has rescued millions of patients from vision loss and blindness around the world. The most well-known vascular tumors in the eye treated with PDT include retinal capillary hemangioma, choroidal hemangioma, retinal vasoproliferative tumor or Wyburn-Mason syndrome. The discovery of the role of vascular endothelial cell growth factor in age-related macular degeneration and the revolution of its treatment by anti-vascular endothelial cell growth factor agents reduced the need for PDT in age-related macular degeneration; however, the use of PDT in the treatment of PCV is dramatically increasing. Furthermore, clinical results obtained with PDT, in combination with angiogenesis inhibitors, vascular disrupting agents, or/and anti-inflammatory compounds as adjuvant therapies, may well keep PDT as one the main treatment options. This review summarizes the present role and future possible improvements of ocular PDT.
Keywords
Anti-angiogenesis; Inflammation; Ophthalmology; Photodynamic therapy; Polypoidal choroidal vasculopathy; Tumor
Abbreviations
AMD: Age-related Macular Degeneration; AS: Angioid Streaks; bFGF: basic Fibroblast Growth Factor; BMD-MA: benzoporphyrin derivative monoacid ring A; CCH: Circumscribed Choroidal Hemangioma; CD36: Cluster of Differentiation 36; CFT: Choroidal Foveal Thickness; CNV: Choroidal Neovascularization; CSCR: Central Serous Chorioretinopathy; EC: Endothelial Cell; GCL: Ganglion Cell Layer; ICGA: Indocyanine Green Angiography; INL: Inner Nuclear Layer; IPL: Inner Plexiform Layer; IVTA: Intra-vitreal Triamcinolone; MC: Mutifocal Choroidopathy; m-THPC: metatetra( hydroxyphenyl)chlorin; OFL: Optic Fiber Layer; ONL: Outer Nuclear Layer; OPDT: Oscillatory Photodynamic Therapy; OPL: Outer Plexiform Layer; PCV: Polypoidal Choroidal Vasculopathy; PD-1, Programmed Death-1 gene; PIC: Punctate Inner Choroidopathy; RPE: Retinal Pigmented Epithelium; SRF: Sub-Retinal Fluid; SWS: Sturge- Weber Syndrome; TTT: Transpupillary Thermotherapy; VA: Visual Acuity; VDA: Vascular Disrupting Agents; VEGF: Vascular Endothelial Growth Factor; v-PDT: verteporfin-Photodynamic Therapy; vWF: von Willebrand Factor
Introduction
Photodynamic therapy (PDT) has been an effective treatment for choroidal neovascularization (CNV), secondary to pathologic myopia and neovascular forms of age-related macular degeneration (AMD), since the late 1990s [1]. Verteporfin-PDT (v-PDT), was in fact, the first therapy approved by the Food and Drug Administration (FDA) in 2000, for the treatment of subfoveal lesions, and at the time of its development, it was the only available treatment to prevent major progression of vision loss and eventual blindness [2], with negligible side effects. Verteporfin or benzoporphyrin derivative monoacid ring A (BPD-MA) is a secondgeneration lipophilic/amphiphilic photosensitizer. It is applied as a liposomal formulation named Visudyne® (Novartis Pharmaceuticals), which is a mixture of the two regioisomers, each a racemic mixture of two enantiomers. Other photosensitizers have not made it to market approval for the treatment of ocular disorders, although this field is moving toward the discovery of improved compounds.
Although PDT was an effective therapy for several of these indications, it was soon realized that the long term efficacy of PDT was limited by the occurrence of secondary tissue reactions, such as hypoxia and inflammation, leading to angiogenesis and the necessity for retreatment, and that consequently, more effective treatments were still needed [3-5]. The discovery of the prominent role of VEGF following PDT led to the advent of anti-VEGF therapy for neovascular eye diseases. Since long-term beneficial effects are still difficult to achieve with anti-VEGF monotherapy [6], the investigation of new therapies involving PDT and based on its combination with angiostatic and anti-inflammatory strategies, continues to be developed and promising clinical results have been obtained. This review discusses the clinical efficiency of v-PDT in both cancerous and non-malignant ocular disorders. Moreover, we present recent strategies aimed at improving the outcome of PDT in the clinic through combination with other therapies.
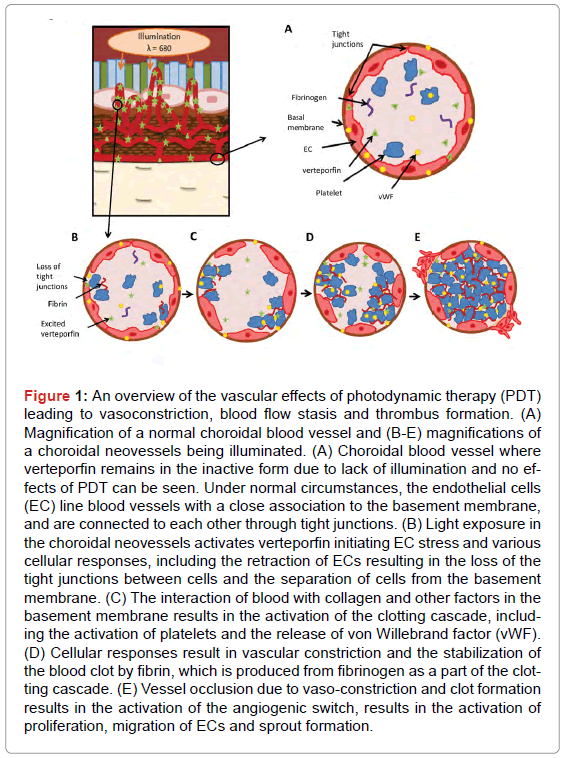
PDT is a form of minimally invasive therapy based, in the present case, on the systemic administration of a photosensitizer and its local activation with light. Irradiation of the photosensitizer with a specific wavelength of visible or near infrared light excites the photosensitizer first to its excited singlet state, from which it can undergo intersystem crossing to a metastable triplet state. Once in the triplet state, the photosensitizer can then interact with environmental molecular oxygen through three possible reactions: (i) energy transfer with triplet ground state oxygen to produce singlet molecular oxygen, which can then react with the photosensitizer (bleaching it) or oxidize a nearby biomolecular target; (ii) a hydrogen exchange with a substrate molecule which can react with O2, forming peroxide radicals as intermediary molecules in the process of oxidizing the substrate molecule; or (iii) an electron transfer with a substrate molecule, generating radical ions which, in combination with O2, can cause other oxidizing reactive intermediates, such as superoxide radical anion O2-,H2O2, or a hydroxyl [7]. Visudyne® is thought to primarily act through the first mechanism, which is referred to as a type II reaction. These cytotoxic oxygen species can ultimately lead to cell and tissue damage [7], and in the case of vascular targeted PDT, can cause vaso-occlusion, as schematically shown in Figure 1. Singlet oxygen can damage the endothelial cell (EC) luminal surface (Figure 1A) mitochondria and to various cyctoskeletal components, including the cytoplasmic microtubules and cytoskeletal proteins. Cytoskeletal damage causes cells to undergo cell shape changes, losing inter EC tight junctions, which destroys the continuity of the EC lining, exposes the basement membrane [8,9], and activates von Willebrand factor (vWF) and the platelets. When the activated platelets get into contact with collagen in the basement membrane, they bind to the collagen and begin to aggregate (Figure 1B). Damaged and activated platelets release vasoactive eicosanoids, such as thromboxane and other factors, which in turn activate more platelets. The adhesion of polymorphonuclear leukocytes following EC damage has also been associated with increased vascular permeability and edema [10], as well as the release of additional thromboxane and leukotriene B4 [11,12]. The release of these factors, particularly thromboxane, alters the equilibrium between platelet pro-aggregatory and vasoconstricting regulators (thromboxane) and anti-aggregatory vasodilating molecules (prostacyclin) [13,14], inducing smooth muscle constriction and additional platelet aggregation (Figure 1C). Vasoconstriction and platelet aggregations results in the formation of a thrombus, which is eventually stabilized to form a blood clot (Figure 1D).
Figure 1: An overview of the vascular effects of photodynamic therapy (PDT) leading to vasoconstriction, blood flow stasis and thrombus formation. (A) Magnification of a normal choroidal blood vessel and (B-E) magnifications of a choroidal neovessels being illuminated. (A) Choroidal blood vessel where verteporfin remains in the inactive form due to lack of illumination and no effects of PDT can be seen. Under normal circumstances, the endothelial cells (EC) line blood vessels with a close association to the basement membrane, and are connected to each other through tight junctions. (B) Light exposure in the choroidal neovessels activates verteporfin initiating EC stress and various cellular responses, including the retraction of ECs resulting in the loss of the tight junctions between cells and the separation of cells from the basement membrane. (C) The interaction of blood with collagen and other factors in the basement membrane results in the activation of the clotting cascade, including the activation of platelets and the release of von Willebrand factor (vWF). (D) Cellular responses result in vascular constriction and the stabilization of the blood clot by fibrin, which is produced from fibrinogen as a part of the clotting cascade. (E) Vessel occlusion due to vaso-constriction and clot formation results in the activation of the angiogenic switch, results in the activation of proliferation, migration of ECs and sprout formation.
Clinical Applications of PDT
Neovascularization-based non-malignant disorders
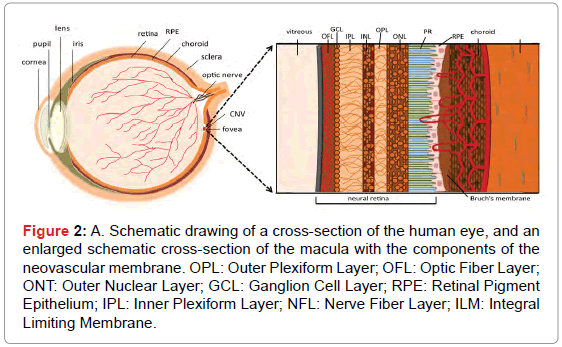
Choroidal neovascularization (CNV): CNV is the growth of blood vessels in the choroidal layer of eye, located between the retina and the sclera [7]. CNV can develop as a consequence of many disorders, such as pathologic myopia, inflammation, angioid streaks, trauma or choroidal rupture, however, it is most frequently encountered as a complication in patients suffering from AMD (Figure 2) [15]. The central part of the macula, called the fovea, is responsible for the highresolution central field of vision. The development of CNV in the fovea of the eye can, therefore, lead to severe vision loss. The maintenance of normal macular function is dependent on the integrity of the microstructure of the different layers in this tissue, which include the choroid, Bruch’s membrane, the retinal pigmented epithelium (RPE) containing the photoreceptors, and the neural retina. A schematic cross-section of the retina’s “layered” structure within the macula and the conditions associated with pathologic CNV are shown in Figure 2. CNV is due to the activation of angiogenesis in the choroid, resulting in neo-angiogenesis and vascular sprouting from the choroid into Bruch’s membrane. Neovessels are frequently restricted to the area below the RPE and near Bruch’s membrane; however, they are sometimes capable of perforating the RPE [16]. This process is believed to be stimulated by the production of inflammatory and pro-angiogenic factors by the RPE cells, as a response to inflammation and oxidative stress in the RPE and Bruch’s membrane [17].
Figure 2: A. Schematic drawing of a cross-section of the human eye, and an enlarged schematic cross-section of the macula with the components of the neovascular membrane. OPL: Outer Plexiform Layer; OFL: Optic Fiber Layer; ONT: Outer Nuclear Layer; GCL: Ganglion Cell Layer; RPE: Retinal Pigment Epithelium; IPL: Inner Plexiform Layer; NFL: Nerve Fiber Layer; ILM: Integral Limiting Membrane.
Age-related macular degeneration (AMD): Exudative or wet AMD associated with CNV is the leading cause of vision loss in the elderly populations of developed countries [18]. AMD is characterized by the development of CNV in the fovea, leading to the gradual destruction of the high-resolution central field of vision, a process that is strongly correlated with aging. The wet or exudative form of AMD is the more severe form and frequently results in rapid vision loss; patients can lose as much as one letter a month on the ETDRS (Early Treatment Diabetic Retinopathy Study) vision chart without treatment. Exudative AMD is characterized by abnormally leaky CNV, which result in retinal edema. In this condition, newly formed blood vessels are pathological and functionally abnormal, leaking lipids, fluid and blood into the retina, causing the edema and retinal thickening, which is often associated with vision impairment. If this leakage persists for longer periods of time, it can lead to the formation of scar tissue and to a scotoma, a dark region in the field of vision. The early and late dry form of AMD, also frequently referred to as central geographic atrophy, accounts for approximately 90% of all AMD cases [19]. This dry form of AMD is associated with cell death and retinal thinning, however, it is generally not associated with rapid vision loss, as in the case of the wet form of the disease. The history of treatments for CNV associated with AMD has been extensively reviewed [5,19].
Various chlorin-derivatives were developed and tested for AMD treatment, e.g. tin ethyl etiopurpurin (SnET2/Rostaporfin/Purlytin®) and N-aspartyl chlorin e6 (NPe6/Talaporfin). SnET2 passed phase III trials for the treatment of AMD [20], but has not been approved by the FDA, due to a requirement of further efficacy and safety assessments. NPe6-PDT was shown to selectively localize in experimental CNV of Macaca monkeys, leading to CNV occlusion [21]. Moreover, lutetium texaphyrin (Lu-Tex/Optrin®), a texaphiryn complex was tested in the laser-injured eyes of New Zealand white rabbits [22], and in phase I/II clinical trials for AMD [23].
Verteporfin-PDT is one of the few therapies, which has been shown to effectively slow the progression of the wet form of AMD, and even to stabilize visual acuity (VA) over many years. V-PDT was approved by the FDA in 2000 as an effective treatment strategy for exudative AMD. This treatment involves the intravenous administration of Visudyne®, followed by the local activation of this photosensitizer using light with a wavelength of 689 nm. The “standard” protocol of local light delivery (light dose of 50 J/cm2, irradiance of 600 mW/cm2 over 83 sec, starting 15 minutes after the beginning of the i.v. infusion) produces a vasoocclusive effect. It is applied homogenously in a round spot that covers the lesion, plus a burden of several hundreds of microns. Clinical trials involving v-PDT in CNV secondary to AMD are presented in Table 1. Standard fluence v-PDT was used for the first time in the TAP (Treatment of Age-related macular degeneration with Photodynamic therapy) trial, which was composed of 2 simultaneous double-masked, placebo-controlled, randomized arms, with patients suffering subfoveal CNV secondary to AMD. The 609 patients enrolled in this study were re-treated every 3 months, if judged necessary based on fluorescein angiograms showing recurrence or persistence of leakage. 12 months after the first treatment, PDT-treated patients showed an improvement in VA of 1.3 lines on the ETDRS chart, as compared to the treatment group who were administered a sham-treatment [24]. An open-label extension of this study for an additional 3 years showed that VA could be maintained at the level observed at the one year checkpoint, resulting in the recommendation that v-PDT treatment be continued beyond 24 months, if fluorescein leakage from CNV is still observed at this time point [3].
| Study | Treatment regiment | Follow up period (months) | Results | Ref |
|---|---|---|---|---|
| TAP | *v-PDT | 24 | V-PDT reduced the risk of moderate or severe vision loss in patients with CNV due to AMD. | [3] |
| VIP | *v-PDT | 24 | In the v-PDT group, VA improvement by at least 5 letters was observed in patients with CNV due to pathologic myopia. | [42] |
| Lam et al. | *v-PDT (Asian patients) | 24 | Median VA improvement was 1.7 lines, but the mean number of PDT retreatments required in the first 2 years was 2.3-lower than in the VIP study. | [43] |
| VISION | pegaptanib sodium (0.3 mg) | 12 | 70% of patients lost fewer than 15 letters of visual acuity, as compared with 55% among the controls. Improvement in mean visual acuity was not observed. | [115] |
| ANCHOR | ranibizumab (0.3 mg or 0.5 mg) vs. *v-PDT |
12 | Ranibizumab was clearly superior to PDT with Respect to both visual acuity (VA) and anatomic (lesion size and CNV leakage) efficacy outcomes. | [41] |
| FOCUS | ranibizumab 0.5 mg monthly or sham injections monthly followed by *v-PDT at day zero | 24 | Combination therapy was more effective than v-PDT alone and had a low rate of adverse events. | [116] |
| PROTECT | same-day *v-PDT and ranibizumab 0.5 mg in CNV AMD | 12 | Improved VA, lesions were stabilized with minimal treatment required after month 3. | [117] |
| MONT BLANC | *v-PDT, ranibizumab 0.5 mg In CNV AMD |
12 | VA improvements in the combination group are non-inferior to a ranibizumab alone with three ranibizumab doses followed by injections on a monthly regimen. | [118] |
| DENALI | *v-PDT+ranibizumab 0.5 mg, **reduced fluence v-PDT +ranibizumab 0.5 mg, ranibizumab 0.5 mg In CNV AMD |
12 | DENALI did not demonstrate non-inferior visual acuity gain for v-PDT combination therapy compared with ranibizumab monthly monotherapy. | [120] |
| EVEREST | *v-PDT+ranibizumab 0.5 mg in PCV | 12 | Complete regression of polypoidal lesions in combination therapy group. | [119] |
| RADICAL | **v-PDT+within 2 h ranibizumab (0.5mg)+dexamethasone (0.5 mg); vs. ***v-PDT + ranibizumab (0.5 mg) + dexamethasone (0.5 mg); vs. ranibizumab only (0.5 mg) |
24 | Cumulative retreatment rates were lower in all combination groups compared with the ranibizumab monotherapy group. Mean VA change from baseline was not statistically different among the treatment groups. | [126] |
| VALIO | *v-PDT (light applied 30 minutes after the start of verteporfin infusion) |
12 | Improved VA and angiographic outcomes with an acceptable safety profile compared with standard light application 15 minutes after the start of the infusion | [152] |
| TRIPLE THERAPY |
**v-PDT, 16 h later, dexamethasone (800 μg) and bevacizumab (1.5 mg) | 9 | Less than 25% of the patients treated with this regimen required additional treatment. | [127] |
| *v-PDT+immediate injection of bevacizumab (1.25 mg)+TA (4 mg)+bevacizumab (1.25 mg) every 3 months | 6 | Short-term results of this study (at 6 months) showed low rate of retreatments, sustained CNV closure efficacy and visual acuity improvement. | [128] | |
| Same-day **v-PDT + dexamethasone (200 μg) + bevacizumab (1.5 mg) |
13.7 | VA was maintained, decreased macular thickness in treatment-naïve or previously treated with anti-VEGF. The mean number of repeat triple therapy treatments was 0.3 in both mentioned groups. |
[129] |
CFT: Choroidal foveal thickness; PCV: polyploidal choroidal vasculopathy;
*standard fluence v-PDT: 50 J/cm2, 600 mW/cm2;
**reduced fluence v-PDT: 42 J/cm2, 300 mW/cm2;
***quarter fluence: 15 J/cm2, 180 mW/cm2;
**** low fluence: 25 J/cm2, 300 mW/cm2;
TA: Triamcinolone Acetonide;
VA: Visual Acuity;
v-PDT: verteporfin-photodynamic therapy
Table 1: Summary of clinical trials with v-PDT for choroidal neovascularization.
Reduced fluence v-PDT (42 J/cm2, 300 mw/cm2) protocols were later proposed due to retinal inflammation and normal choriocapillary closure observed after PDT in the study described above, as well as to investigate the potential for increased treatment selectivity. The VIM (Verteporfin In subfoveal minimally classic CNV) trial employed both standard and reduced fluence v-PDT protocols, and showed stabilization of vision in both treatment groups versus the placebo group. This study, however, did not demonstrate a treatment benefit in terms of visual outcome for the reduced fluence v-PDT protocol. It also showed that re-treatment with v-PDT was necessary due to reperfusion of PDT-occluded vessels [25], as well as the induction of angiogenesis [26-28].
Both the reperfusion of blood vessels and the activation of angiogenesis are likely to result from a combination of factors, some of which may include PDT-induced inflammation [29,30], PDT-induced enhanced expression of vascular endothelial growth factor-A (VEGF-A) [27,31] or other growth factors and PDT-induced tissue hypoxia [32]. Even prior to v-PDT, CNV associated with AMD is characterized by elevated levels of certain angiogenic cytokines, including VEGFs, fibroblast growth factors (bFGFs) and angiopoietins [33-35]. Findings indicating that PDT induces an increase in the expression of some of these cytokines have led to clinical trials for AMD which exclude the use of v-PDT treatment, instead targeting these cytokines with topically applied or intravitreously injected compounds that block some of these cytokines [35].
Thus, recently, the use of v-PDT in the treatment of AMD has been replaced to a large extent by anti-VEGF targeted therapy, using monthly injections of ranibizumab or bevacizumab, or bimonthly administration of Aflibercept [5,36]. The choice between these closely related compounds is ophthalmologist and country dependent. The CATT trial (patients in USA) revealed a significant improvement of vision in terms of VA with either drugs, ranibizumab or bevacizumab, when administrated once monthly [37]. At year 2, treatment as needed resulted in less of a gain in VA than after 1 year of monthly treatments. The results of the IVAN trial (similar to CATT, but conducted in the UK), compared fixed monthly dosing with discontinuous (upon need) dosing, was inconclusive after one year [38]. Phase III trials (VIEW 1 and 2) of Aflibercept given at 2 mg every second month after three initial monthly doses showed it to be non-inferior to the recommended regimen of ranibizumab (0.5 mg every month) in terms of the primary endpoint [39].
In order to minimize the side effects accompanying regular intravitreal injections [37], anti-VEGF strategy can be combined with other treatment modalities, such as radiotherapy (epimacular radiotherapy stereotactic radiation therapy, proton therapy, brachytherapy or stereotactic radiation therapy) [40] or PDT.
The 2-year results of the anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Agerelated Macular Degeneration (ANCHOR) phase III trial indicated that ranibizumab treatment resulted in greater clinical benefits than v-PDT. In this study, patients receiving ranibizumab had a statistically significantly improvement in VA as compared to v-PDT treated patients, with minimal adverse events [41]. Patients receiving ranibizumab had a slightly increased incidence of non-ocular hemorrhage as compared to v-PDT (8.8% for 0.3 mg and 9.3% for 0.5 mg versus 4.9% for v-PDT). Also an increased percentage of patients experienced enhanced immunoreactivity in the high dose ranibizumab group. It should be noted that the VA outcomes of ranibizumab treated patients in the ANCHOR study were better than those in a similar study (MARINA trial) for patients with minimally classic or occult AMD with no classic CNV lesions. This may be due to the fact that the average size of the CNV lesions was smaller in the ANCHOR study, or that predominantly classic lesions are normally more aggressive resulting in more rapid vision loss, possibly causing irreversible damage to the photoreceptors. The addition of v-PDT to ranibizumab therapy may be beneficial in certain conditions, for example with larger or predominantly classic lesions. Additionally, it may be advantageous to use v-PDT instead of ranibizumab in patients who are at increased risk of hemorrhages or other side effects associated with anti-VEGF therapies.
Pathologic myopia: Myopic CNV is another eye disorder for which v-PDT has shown clinical efficacy. This pathology is often associated with socioeconomic setbacks and varies among the populations of different countries and continents. The VIP (Verteporfin in photodynamic therapy) study was a multicenter, double-masked, placebo-controlled, randomized clinical trial in 28 ophthalmology centers in Northern America and Europe. The study was completed by 77 of 81 patients (95%) in the verteporfin group and 36 of 39 patients (92%) in the placebo group. The final examination at the 24-month check-point indicated a benefit in the verteporfin treatment group based on the overall improvement in VA. The v-PDT treatment group showed an average improvement in VA of at least 5 letters (equivalent to at least 1 line) in 40% of the verteporfin-treated cases (32 patients) compared to only 13% of cases in the placebo-treated group (5 patients). Additionally, an improvement of at least 15 letters (at least 3 lines) was seen in 10 of the v-PDT-treated cases (12%), while this level of improvement was not reached by any of the patients in the placebotreated group. The mean number of retreatments needed was 5, and no additional adverse effects of photosensitivity were associated with the v-PDT treatment group [42]. A similar prospective interventional study was performed by Lam et al. [43] in Asian patients. In this study, the 24 month follow-up examination showed a median VA improvement of 1.7 lines; however, the mean number of PDT retreatments required in the first 2 years was 2.3, being significantly lower than in the VIP study. As of 2009, anti-VEGF therapy became the first line therapy for pathological myopia due to the limited medium-term efficacy of other therapies, including v-PDT [44].
Idiopathic CNV: Idiopathic CNV is characterized by lesions, which are smaller, less aggressive in growth rate, and present at a younger age as compared to CNV due to AMD. The v-PDT protocol used in the treatment of classic CNV in AMD was also found to be effective in the treatment of idiopathic CNV in phase I and II clinical trials [45]. Patients treated with v-PDT experienced no deterioration in VA and the majority of patients even gained at least 1 line of vision. A reduction in the size of the leakage area from classic CNV was observed in all patients already 1 week after v-PDT, and a complete disappearance of leakage from classic CNV was observed in almost half of these patients. Other studies have also reported a retrospective improvement in VA. One such study in Asian patients (17 cases) showed that after 12 months, 94% of patients had improved or showed stable vision versus baseline. The mean number of retreatments over 12 months was 1.8 and clinically important complications related to the use of v-PDT were not encountered [46].
Polypoidal choroidal vasculopathy (PCV): A unique form of CNV is polypoidal choroidal vasculopathy (PCV), a feature of which is a branching vascular network arising from the inner choroid that terminates in polypoidal lesions [47]. Another important characteristic of this condition is the presence of degenerated small arterioles and capillaries with a thickened basement membrane. Historically, with the use of indocyanine green angiography (ICGA) atypical cases of AMD had been found with a vascular network that terminates in polypoidal lesions. The polypoidal lesions range from small, detectable only by means of angiography, to large with perfuse and branching inner choroidal vessels [48]. While the branching vascular network in PCV may be quiescent in some cases, it can sometimes cause leakage and exudation. The reason for choroidal hyper permeability in PCV, as detected by indocyanine angiography, remains unknown.
The genetics of PCV and AMD has been extensively studied [49-53]. The association between AMD, PCV and three SNPs in threr following gene regions, rs1061170 (CFH), rs10490924 (ARMS2), and rs11200638 (HTRA1), were verified both in Caucasians and Japanese patients [54,55]. There is a consistent association of the ARMS2/ HTRA1 locus with both neovascular AMD and PCV, suggesting the two diseases at least in part share molecular mechanisms [53]. HTRA1 was found to be associated with both disorders, being about twofold stronger in AMD than in PCV [56] in Asian patients. Another susceptibility gene in PCV is the elastin gene (ELN), which is shown to be more associated with PCV than with AMD [57]. Honda et al. [52] reported on positive association of cluster of differentiation 36 (CD36) gene, and the response to PDT in PCV. CD36 is expressed higher in the macula than in the peripheral retina [58], and accelerates the uptake of oxidized low-density lipoprotein, it might be associated with verteporfin-PDT outcome, since verteporfin is known to bind serum low-density lipoprotein. For a detailed description of PCV and its treatment options, refer [59].
Similar to AMD, the increased expression of pro-angiogenic growth factors, such as VEGF-A, has been observed in histological examination of PCV. However, VEGF targeted therapy has only shown limited efficacy in the treatment of this disease [60]. Although exudative changes regressed with concomitant improvements in VA, complete regression of polypoidal lesions was only noted in 25% of the treated eyes [61]. Interestingly, PCV can be treated much more efficiently using v-PDT. After 12 months of follow-up, complete regression of the polypoidal lesions was seen in 95% of eyes with stable or improved VA [62-64]. Table 2 summarizes the clinical trials using v-PDT to treat PCV (with or without other therapies). A prospective, non-randomized study was conducted involving 42 eyes with newly diagnosed symptomatic PCV treated exclusively with v-PDT and followed-up for a 3-year period [65]. Recent studies reported, however, that in the eyes that were successfully treated with v-PDT, significant recurrence of the polypoidal lesions was reported after 24 months [66]. Beside the characteristic polypoidal dilatations, CNV-like vessels can develop with exudation. Unexpected subretinal hemorrhages after PDT are another serious problem in patients with PCV [67]. This prompted the PDT field to search for efficient strategies to improve the efficiency and selectivity of v-PDT and reduce the treatment burdens. Spaide et al. [68] reported on 16 patients with subfoveal PCV treated with v-PDT. Visual acuity improved in 9 (56.3%) patients, remained the same in 5 (31.3%), and decreased in 2 (12.5%) patients. There were no reported long lasting complications after the treatment. The mean change in visual acuity was an improvement of nearly 2.4 lines. Promising results were also found in other studies in Japanese patients at 1 year after standard fluence v-PDT [69] or reduced-fluence v-PDT [70,71]. In these studies, PCV showed a significantly lower PDT frequency and greater improvement in the visual acuity than in the case of AMD, i.e. fewer v-PDT treatments per year were needed to obtain optimal results in the case of PCV, as compared to patients with wet AMD. The recurrence period of PCV after PDT was clearly significantly longer for PCV patients than in the case of AMD.
| Treatment regiment | Follow up period (months) | Results | Ref |
|---|---|---|---|
| *v-PDT | 12 | VA improved (15 letters or more) in AMD and PCV by 6% and 25%, respectively. Fluorescein leakage was suppressed in 86% of PCV and 61% of AMD eyes, respectively. | [153] |
| *v-PDT | 24 | VA preserved or improved in 79% of eyes. Recurrence of polypoidal lesions in 64% of eyes. An abnormal branching vascular network persisted in all subjects. | [72] |
| v-PDT | 19.2 | Regression of the polypoidal lesions observed in 94% of eyes. The branching vascular network remained in all eyes. | [154] |
| *v-PDT | 12 | Significantly better response to v-PDT in terms of VA improvement and effect durability. | [69] |
| **v-PDT | 12 | Significantly lower v-PDT frequency and greater improvement in the visual acuity than AMD. | [70] |
| *v-PDT | 12 | The visual acuity improved in 56.3% of patients, remained the same in 31.3%, and decreased in 12.5% and. No patient had any long lasting complication after the treatment. | [68] |
| *v-PDT | 36 | 75% of the treated eyes had no significant loss of vision, and 14.8% showed significant improvement in visual acuity. | [65] |
| *v-PDT | 60 | Mean VA letter score loss is similar between patients with AMD (-7.25) and with PCV (-5.36) at the month 60examination. Significantly more frequent retreatments in the years 3 and 4 than patients with AMD. | [155] |
| *v-PDT+ranibizumab 0.5 mg | 12 | Complete regression of polypoidal lesions in combination therapy group. | [119] |
| *v-PDT+ranibizumab 0.5 mg | 12 | The mean BCVA change from baseline was +12.3 letters. 58.3% of patients had a BCVA gain of 15 letters or more. All patients underwent regression of polyps without recurrence. | [156] |
| *v-PDT+bevacizumab 1.25 mg | 12 | Lower rate of post v-PDT hemorrhage. Recurrence rate was unchanged. | [157] |
| ranibizumab 0.5 mg+*v-PDT | 12 | Improved VA and reduced exudation. | [158] |
| **v-PDT+ bevacizumab 1.25 mg | 12 | Improved VA in 56% treated eyes. | [73] |
*standard fluence: 50 J/cm2, 600 mw/cm2 at 689 nm;
**reduced fluence: 25 J/cm2 for 70 sec, 300 mW/cm2 at 689 nm;
BCVA: best corrected visual acuity;
v-PDT: Visudyne®-photodynamic therapy.
Table 2: Summary of clinical trials with v-PDT alone or in combination with other treatment modalities in PCV patients.
Even though all polypoidal lesions regressed with PDT, its effect on the branching vascular network does not seem to be permanent, and thus recurrence of polypoidal lesions may occur in the long run. Another problem is the heterogeneous localization of the polyps, especially those in the peripapillary area, which are not always easily accessible by laser light. Finally, repeated v-PDT may induce persistent choroidal atrophy. Hirami et al. [67] retrospectively reviewed the data from 91 patients who underwent PDT for the treatment of PCV. In this study, during the follow-up period after PDT, postoperative subretinal hemorrhages were seen in 30.8% of patients. In 78.6% of these, the subretinal hemorrhage was absorbed without treatment. Although visual acuity was the same or increased in 81.8% of eyes with subretinal hemorrhage alone, it decreased significantly in 50.0% of the eyes with postoperative vitreous hemorrhage. Akaza et al. [72] with a follow-up of 24 months showed regression of the polypoidal lesions in 29 out of 31 eyes (94%). However, recurrence of polypoidal lesions appeared in 10 of 29 eyes (34%) and additional PDT was suggested. V-PDT could reduce the size of polypoidal lesions, but did not destroy them completely. Thus, the persistent branching vessels in the network are supposed to be at the origin of the new polypoidal lesions.
Summarizing, v-PDT demonstrated very positive short- to medium-term results in PCV. Retreatment is necessary in some cases, but at a relatively low frequency. It may well be that vaso-occlusive PDT, possibly applied at reduced fluence in combination with giving anti-angiogenic compound, may lead to a quite good long-term clinical outcome for PCV [73,59].
Inflammation: CNV can also occur as a complication of punctate inner choroidopathy (PIC), uveitis, multifocal choroiditis (MC) and panuveitis. PIC is a relatively uncommon inflammatory multifocal chorioretinopathy that affects predominantly young myopic women. It is characterized by the presence of multiple, small and well-defined yellow-white fundus lesions restricted to the posterior pole of the eye, where there is an absence of flare and inflammatory cells in the anterior chamber or vitreous cavity [74]. MC is a chronic inflammatory disease also affecting myopic women. It is characterized by multiple punchedout chorioretinal lesions at the posterior pole and midperiphery, as well as abnormalities associated with vitreitis. Visual deterioration can be a result of a number of complications, including cystoid macular edema, CNV and epiretinal membrane scar. The prognosis in PIC and MC patients is generally good. However, in about 30% of cases, patients develop subfoveal CNV leading to vision loss. A pool of evidence has indicated the potential success of v-PDT or its combination with anti-inflammatory or anti-angiogenic compounds in the treatment of these disorders. Prospective and retrospective studies on patients with diagnosed CNV due to PIC treated with v-PDT have shown improved VA in most cases, as elegantly reviewed by Chan et al. [75]. Combining PDT with intravitreal triamcinolone (IVTA, 4 mg) seems to be a promising treatment modality for both idiopathic CNV and CNV secondary to PIC, as it requires fewer treatment sessions and results in superior visual improvement than other treatment options [46].
Angioid streaks: Angioid streaks (AS) are small breaks in a weakened Bruch's membrane. They may be seen in patients with various systemic diseases, such as pseudoxanthoma elasticum or sickle hemoglobinopathy. There are relatively few case reports of patients with CNV due to AS that were treated with v-PDT and the results vary between different reports. In one case study, eight patients with subfoveal CNV secondary to AS were treated with v-PDT and their vision was monitored for an average of 8.75 months [76]. Treatment was well tolerated and no deterioration of vision was observed. Two patients showed no improvement in VA and the increase in median best-corrected VA was 1.37 lines (SD 1.59 lines). On the other hand, several retrospective studies have indicated that v-PDT does not appear to help significantly in the stabilization of vision loss or lesion size in patients with CNV secondary to AS [77,78]. This is even the case when re-treatment was performed earlier than the standard 3 months [79]. One report indicated that treatment efficacy seem to be dependent on baseline VA [80], while others have reported that even though v-PDT did not prevent the progression of vision loss, it helped to stabilize macular function and slow disease progression [81,82]. It was suggested that further advances in treatment efficacy could be achieved by improvements to treatment protocols and parameters or through combination therapies. The recent review by Gliem et al. [83] summarized fifty four relevant studies, which evaluated different therapies for CNV due to AS. The primary outcome measure was a change in best-corrected visual acuity (BCVA). Treatment with anti- VEGF compounds improved or stabilized BCVA in all case series. V-PDT slowed down disease progression with the stabilization or a decrease in BCVA. Individual BCVA and follow-up data for each treated eye was reported in >160 cases for both the treatment groups, VEGF inhibitors and v-PDT. In a pooled analysis of those studies, the difference in the mean change of BCVA between both treatment groups was estimated at approximately 6 letters. Laser photocoagulation gave comparable results as v-PDT with treatment performed in extrafoveal lesions, but resulted in frequent recurrences and led to more retinal damage. Additionally, combination therapies were not seen to be superior to monotherapy. Although v-PDT was generally well-tolerated and may have limited and/or slowed the progression of vision loss as compared to the natural expected progression of CNV due to AS, the overall treatment benefits and improvements in vision loss are not clear, indicating that better treatment options or combination therapies are still needed.
Central serous chorioretinopathy (CSCR): Central serous chorioretinopathy (CSCR) is a disorder characterized by the serous detachment of the neurosensory retina due to increased permeability and leakage from the choriocapillaries through RPE lesions [84]. Visual loss or permanent symptoms may appear in cases with persistent focal leakage or chronic diffuse leakage. CSCR most commonly occurs in populations of Asian or Caucasian descent and in middle-aged men. CSCR can develop as a complication of CNV, in which case, PDT can provide an effective treatment. The effects of standard fluence v-PDT on CSCR have been shown to not only promote the resolution of acute CSCR, but also to prevent recurrences [85,86]. More recently, reducedfluence v-PDT (25 J/cm2) has been used in CSCR with anatomical and functional success [87]. Although focal laser and PDT are the standard procedures for persistent sub-retinal fluid (SRF) in CSCR, they are not appropriate for all patients and the optimal timing of intervention remains to be defined [88].
As previous studies have indicated, v-PDT has been explored as a form of treatment for a variety of non-malignant eye disorders associated with or occurring as a result of CNV. In some cases, it has been shown to improve VA and reduce disease related symptoms. For many of these indications, however, there is still only a limited amount of data on the investigation at the effects of v-PDT therapy and more long-term studies are needed to make definite conclusions.
Ophthalmic tumors
Benign tumors: There are many malignant eye diseases for which v-PDT treatment has shown clinical efficacy. Eye tumors can be of benign or malignant origin. Vascular tumors of the choroid or retina, while being considered benign, may be associated with complications and significant visual impairment. Treatment options for eye tumors generally include photodynamic therapy, argon laser photocoagulation, trans-scleral diathermy, cryotherapy, anti-angiogenic agents, plaque radiotherapy, proton beam radiotherapy, local resection or adjuvant chemotherapy. PDT provides two major clinical advantages over the other therapeutic options. First of all, v-PDT has been shown to be relatively selective, resulting in limited damage to surrounding structures and minimal visual impairment, allowing for a substantial increase in the tolerance of the eye to treatment. Secondly, due to its selectivity, v-PDT can provide the ability to treat posterior neoformations on the macula, which are close to critical structures, such as the optic disk, while preserving the adjacent anatomic structures. This may allow v-PDT to provide efficient tumor control with desirable cosmetic results. Moreover, this treatment modality might be an option in patients who require ocular treatment only. The most well-known vascular tumors in the eye include retinal capillary hemangioma, choroidal hemangioma, retinal vasoproliferative tumor or Wyburn-Mason syndrome, some of which will be discussed in more detail below.
Choroidal hemangioma: Vascular tumors of the choroidal layer of the retina include circumscribed choroidal hemangioma and diffuse choroidal hemangioma. The treatment of these tumors can be relatively challenging, as symptoms appear secondary to exudative retinal detachment. Circumscribed choroidal hemangiomas (CCH) are benign hamartomas, which can be identified as an orange choroidal body with indistinct margins. The treatment decision is made on the basis of tumor location, the presence of SRF, as well as the extent of symptoms. Current treatment options, based on VA potential and the extent of detachment, include argon laser photocoagulation, plaque brachytherapy, proton beam radiotherapy [89] and PDT [90]. All of these techniques have limited efficacy and lesion recurrences are not uncommon, additionally scaring can affect the fovea in centrally located lesions. PDT seems to provide a viable treatment strategy in subfoveal or juxtafoveal CCHs, since other treatments may frequently be associated with significant morbidity. Tumor regression is most effective after the first PDT session, and is usually significant after 1 [91] to 3 months [92] post treatment. Additionally, some case studies have reported on the use of v-PDT in the treatment of diffuse choroidal hemangioma in patients with Sturge-Weber syndrome (SWS) [90]. In patients diagnosed with SWS, hemangiomas mainly appear as spread lesions in the choroid or leptomeninges and skin. These diffuse choroidal hemangiomas contain areas of enhanced thickening that may simulate CCH. In these patients, there is an increased probability of secondary retinal detachment, with shifting of the SRF. Next to vision loss caused by exudative retinal detachment, congenital glaucoma is diagnosed in the majority of patients with SWS. Case studies have indicated that PDT can be an effective treatment option for visual deterioration from exudative retinal detachment in patients with diffuse choroidal hemangiomas.
Diffuse choroidal hemangiomas appear during ophthalmic examination as orange, diffuse choroidal thickenings. Similarly to CCH, they may be associated with exudative retinal detachment, which is frequently quiescent. Short-term results obtained with PDT are promising as compared to radiotherapy. Moreover, PDT is associated with fewer side effects [93]. To date, large studies with long-term follow-up to fully assess the efficacy of PDT in the treatment of diffuse choroidal hemangioma have not been performed.
Retinal vasoproliferative tumors: Retinal vasoproliferative tumors of the retina are very rare. Their pathogenesis is not fully known, but can be caused by inflammation. In a fundoscopy, they present as heavily vascularized yellowish nodular masses localized in the pre-equatorial retina or inferior to the retina, and are often associated with intraretinal hemorrhages. Secondary tumors may occur in the section of abnormal retina or at the edge of a chorioretinal scar. The relative rarity of retinal vasoproliferative tumors has resulted in the lack of an evidence-based consensus on how to best treat these lesions. The treatment options include cryotherapy or plaque brachytherapy, photocoagulation and PDT [94]. A number of case reports have shown the successful use of a PDT to treat both primary [95] and secondary [96] vasoproliferative tumors.
Malignant tumors: Malignant ophthalmic tumors may arise from various eye and orbital structures. The most common primary tumors include melanomas and squamous cell carcinomas. Metastases that appear in and around the eye are usually from breast (in women) or lung carcinoma (in men). Other less common sites of origin include the prostate, kidney, thyroid and gastrointestinal tract. Photodynamic therapy does not seem to play a crucial role in treatment of these malignancies; however, some successful treatments have been reported in Table 3.
| Indication | Treatment regimen | Results | Ref. |
|---|---|---|---|
| Choroidal melanoma | |||
| Amelanotic choroidal melanoma | *v-PDT with 3 overlapping spots | Tumor regression to a flat scar; stable for 50 months follow-up. | [97] |
| Choroidal melanoma | v-PDT | Complete regression of tumor in 2 cases; 2 cases required enucleation | [101] |
| Amelanotic choroidal melanoma | v-PDT | Complete regression of lesion. | [99] |
| Amelanotic choroidal melanoma | *v-PDT | V-PDT was highly effective in causing regression of posteriorly located amelanotic choroidal melanomas, without a detrimental effect on vision | [98] |
| Amelanotic choroidal melanoma | *v-PDT | *v-PDT caused transient increased exudation of retinal/subretinal fluid. Complete absorption of this fluid with improvement of visual acuity to 20/20 was noted 3-4 weeks post *v-PDT. | [102] |
| Pigmented choroidal melanoma | *v-PDT vs. *v-PDT+ bevacizumab |
Tumors receiving v-PDT alone had continued tumor growth resulting in enucleation years later; combination treatment showed reduced tumor vascularity, but biopsy stilled showed viable tumor cells. | [100] |
| Squamous cell carcinoma | |||
| SCC of the conjunctiva | *v-PDT | Tumor regression in all patients at 1 month, 2 patients had complete regression after 1-2 treatments for whole follow-up period; 3rd patient had larger tumor and only treated areas showed regression. | [159] |
| SCC of the conjunctiva | *v-PDT | Regression of half the lesion after first PDT session and near-complete cure of lesion 2 weeks after second treatment; stable disease for 13 months. | [160] |
| Choroidal metastasis | |||
| Choroidal metastasis | *v-PDT | Complete control and resolution of SRF in 7 tumors (78%). 2 tumors failed to respond and were treated with plaque radiotherapy. | [104] |
| Choroidal metastasis (chemo- and radiation therapy resistant) | *v-PDT | Resolution of exudative detachment and 50% decrease in tumor volume | [161] |
| Choroidal metastasis | *v-PDT | Visual acuity following decrease in the tumor vascular permeability and absorption of subretinal fluid was observed. | [162] |
*v-PDT: Standard fluence, 50 J/cm2, 600 mW/cm2 at 689 nm.
Table 3: Selected reports on v-PDT in treatment of ophthalmic malignant tumors.
Choroidal melanoma: In the case of choroidal melanoma, treatment results based mostly on case studies have indicated that small and preferably low or non-pigmented melanomas responded favorably to PDT [97-99], where as pigmented melanomas were less or not at all [100,101] responsive due to light absorption by melanin and hemoglobin. For example, Canal-Fontcuberta et al. [100] reported on a case study where pigmented choroidal melanomas receiving PDT as a primary treatment showed progressive tumor growth that led to enucleation years later, suggesting that a single session of v-PDT was not effective as a primary treatment for pigmented small choroidal melanomas. In a similar case study, one patient was treated with v-PDT as primary therapy for peripapillary amelanotic choroidal melanomas and another patient received v-PDT in combination with an intravitreal injection of bevacizumab. In both cases, the single v-PDT session was not sufficient to stop tumor growth and both patients suffered from enucleating of the eye 16 months and 4 years after the initial treatment [100]. PDT seemed to also give promising results in low pigmented or amelanotic tumors in patients unresponsive to brachytherapy [97]. Transient increased leakage causing increased retinal edema or subretinal fluid has also been reported following PDT for different types of intraocular tumors, including amelanotic choroidal melanoma [102]. Further studies are needed to identify effective methods for the prevention of this potentially vision-threatening complication. Since melanoma is a highly immunogenic malignancy, it is likely that the combination of PDT with immunostimulatory therapies will increase anti-tumor efficacy.
Squamous cell carcinoma (SCC): Currently available therapeutic options to treat squamous cell carcinoma (SCC) include surgical excision, cryotherapy and radiation, as well as investigational approaches with topical chemotherapy, interferon and antiviral drugs. There are only a few case studies showing a successful v-PDT treatment in SCC [103]. Kevany and Palczewski [104] noted SCC tumor regression in all patients 1 month after standard dose v-PDT. Two patients experienced complete regression (clinical and angiographic observation) after one or two treatments for the entire follow-up time. One particular tumor encompassed large areas of the conjunctiva and cornea. In this case, only the treated areas showed tumor regression. PDT induced minimal, temporary local irritation in two patients and small conjunctival hemorrhages and mild transient chemosis in the eyes, directly after treatment. There are also case reports on successful v-PDT treatment of conjunctival ocular surface squamous neoplasia extending into the cornea [103]. In this report, half of the mass regressed after the first PDT session. A second treatment of PDT resulted in a near-complete cure of the remaining lesion within 2 weeks, after which the patient remained stable for 13 months. Future study is needed to investigate the role of PDT in the management of squamous cell carcinoma.
Choroidal metastasis: The most common form of intraocular cancer, however, is still the appearance of metastases from cancers in distant organs. These metastases can lead to significant vision loss. The uvea is the most common site for ocular metastasis. Within the uvea, 88% of metastases occur in the choroid, followed by metastases to the iris (9%) and ciliary body (2%). Metastases that appear in and around the eye are usually from breast (in women) or lung carcinoma (in men). Treatment options of choroidal metastasis include laser photocoagulation, cryotherapy, chemotherapy, radiotherapy or PDT. The main advantage of PDT for this indication lies in intraluminal photothrombosis in endothelial structures. Kaliki et al. [105] reported a case study on choroidal metastases that were treated with v-PDT. After v-PDT, complete control with resolution of subretinal fluid was achieved in 7 of 9 tumors (78%). These tumors had a mean tumor thickness reduction of 39%. Two tumors failed to respond to PDT and were further treated with plaque radiotherapy. Improvement or stabilization of vision was achieved in 7 of the 8 eyes.
This study confirmed that PDT could be used to effectively destroy malignant tissue and to induce antitumor activity. However, there is a very limited experience of the PDT in melanoma, and these sporadic reports are to be followed by extensive clinical studies, in order for PDT to be accepted as an effective adjuvant procedure in treatment of choroidal metastasis.
Limitations of PDT
The actual biodistribution and selectivity of verteporfin after intravenous infusion is still unknown. It seems that the verteporfin distribution within the fundus was determined by angiographic studies in the past. The selectivity of v-PDT in ophthalmology was often presumed and based essentially on fluorescein angiographic studies [106]. The first published article about a CNV histological study after PDT was from Schnurrbusch et al. [107]. Two patients with recurrences after v-PDT underwent surgical extraction of the CNV. Electron photomicrographs showed occluded vessels within the CNV containing thrombotic masses, as well as morphological damage of the neovascular endothelium. Most of the vessels presented regressive changes with vacuolisation and fragmentation of the neovascular endothelium accompanied by disintegration of the endothelial cell layer. Moreover, the extravasation of erythrocytes was observed. The authors concluded that their findings might be non-specific and would not definitely be all attributed to v-PDT. The findings could be related to the original pathology or could indicate that PDT treatment might result in RPE atrophy. Later major choriocapillaris photothrombosis after standard v-PDT in the normal fundus areas was reported by Schimdt-Erfurth et al. [108], thus terminating the presumed high neovascular tissue selective targeting of v-PDT in humans. It was also very difficult for Ghazi et al. [109] to find intraluminal CNV thrombus formation in their specimens. Only a few areas at the lesion periphery revealed to be occluded, whereas the CNV center was practically intact. Two additional studies [26,110] confirmed a lack of intraluminal and selective CNV thrombus formation after v-PDT. In Moshfeghi et al. [110] histopathologic evaluation of CNV 3 days after v-PDT showed partial vascular occlusion that was not present in later specimens, indicating that v-PDT did not appear to lead to permanent and complete occlusion of the CNV. Since these studies, no real improvement in the selectivity of PDT of CNV has been developed.
The treatment of various ocular disorders with v-PDT is also frequently associated with the gradual recurrence of disease symptoms and reductions in VA, requiring regular re-treatments to maintain treatment benefits. This regression is generally attributed to angiogenesis and the recurrence of neovascularization, partially in response to tissue hypoxia and inflammation caused by v-PDT, but in many cases, angiogenesis is also innately associated with the pathology of neovascular based eye disorders and limited efficacy can be attributed to disease-related reperfusion of the targeted vasculature.
Limited treatment efficacy has also been seen in many of the case studies examining the PDT treatment of ocular tumors. Hypoxia and inflammation, as a result of PDT, may play an important role in tumor recurrence; however, PDT efficacy may also be limited by how deep the light can penetrate into the tumor tissue in order to activate the photosensitizer. This hypothesis is supported by studies showing that the successful treatment of ocular melanomas be highly dependent on their degree of tumor pigmentation [111]. It is believed that many of these limitations may be partially overcome by combination of v-PDT with other treatment modalities, as discussed in depth in the subsequent chapter.
Improvement of PDT Efficacy by Combination Therapy
Previous research and clinical studies have demonstrated that v-PDT treatment of eye disorders involving CNV can provide beneficial clinical effects in patients, despite some limitations. The most relevant of these limitations, with respect to treatment efficacy, include the effects of v-PDT on the RPE cells and the requirement for retreatment in order to maintain treatment benefits. These limitations have prompted researchers in this field to explore strategies to improve the efficiency and selectivity of v-PDT, and to reduce treatment burdens. This includes the combination of PDT with anti-angiogenic strategies or angiostatic steroids, most frequently reported for the treatment of CNV due to AMD or PCV in clinical applications, as shown in Table 1.
Unique characteristics in the pathogenesis of AMD, such as oxidative stress and inflammation, lead to angiogenesis and the increased expression of various cytokines, which provide unique targets for therapeutic intervention. Additionally, it seems reasonable to presume that more effective therapy can be achieved with long-term improvement in VA by targeting more than one of these processes at the same time.
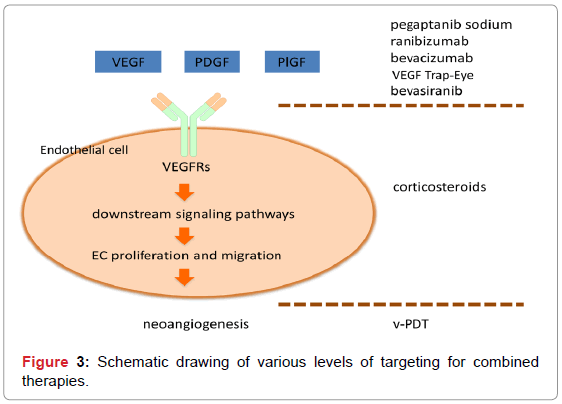
For example, pharmacological intervention in the activity of VEGF-A decreases the proliferation of ECs and the recruitment of other cell types, such as leukocytes, which can express the cytokines and proteases necessary for the development and maintenance of neovessels. However, once this neovascularization is mature, it may no longer be responsive to anti-VEGF treatment. PDT, on the other hand, destroys existing neovessels effectively acting as a “CNV eraser”, and could be combined with an anti-VEGF strategy aimed at inhibiting further neovessel outgrowth and leakage. Combination with corticosteroids will further improve the therapy by its anti-inflammatory and antifibrosis effects (Figure 3).
The closure of unwanted neovasculature by the combination of PDT with angiostatic compounds to prevent neovascularization, as well as corticosteroids to prevent inflammation, may provide an effective multilevel treatment for AMD. This combination therapy is also expected to be more effective due to the fact that PDT tends to result in the increased production VEGF-A [10,112], and thus the induction of angiogenesis [113,114], as well as some degree of inflammation. By affecting separate pathways, one may accomplish synergies that might lead to a better treatment outcome.
Anti-angiogenic strategies have already been implemented as monotherapies in the treatment of eye diseases involving CNV with relative success. For example, the VISION (VEGF Inhibition Study in Ocular Neovascularization) study demonstrated that in the treatment group receiving pegaptanib sodium, a pegylated anti-VEGF aptamer, at 0.3 mg, 70% of patients lost less than 15 letters of VA, as compared to 55% in the control group. However, pegaptanib sodium therapy did not lead to any improvement in mean VA [41,115]. Additionally, the ANCHOR (anti-VEGF antibody for the treatment of predominantly classic choroidal neovascularization in AMD) phase III study compared patients treated with either two doses of monthly ranibizumab or with v-PDT. Patients were enrolled into either v-PDT or monthly intravitreal ranibizumab (0.3 mg or 0.5 mg) injections. Ranibizumab treatments lead to a greater clinical benefit after 2 years than v-PDT [41]. The search for a long-lasting treatment for AMD is still underway, and the current status of combination trials is summarized below.
Ranibizumab in combination with PDT has been studied in the FOCUS (rhufab V2 Ocular Treatment Combining the Use of Visudyne® to Evaluate Safety) phase I/II trial, and the PROTECT phase II trial (Ranibizumab administrated in conjugation with v-PDT in patients with occult or predominantly classic subfoveal CNV due to AMD). Both studies showed that the combination groups exhibited less lesion growth and greater reductions in CNV leakage and SRF accumulation [116,117].
The SUMMIT trial was a multicenter, 12-month double-blind non-inferiority randomized phase III clinical program, including a European (MONT BLANC), Northern American (DENALI) and Asian (EVEREST) study. This trial was aimed at determining if the combination of ranibizumab and standard dose v-PDT is better than ranibizumab monotherapy in patients with subfoveal CNV secondary to AMD. At the 12-month time point of the MONT BLANC trial, an average VA improvement of 2.5 letters was seen in the combination therapy treatment group, as compared to 4.4 letters in the monotherapy arm [118]. This confirmed the non-inferiority of combination therapy over ranibizumab monotherapy at 12 months. The randomized prospective study (EVEREST) showed the efficacy of v-PDT in combination with ranibizumab for PCV [119]. Complete regression of polypoidal lesions was achieved at 6 months in 77.8% of patients after combination therapy, as compared to 71.4% after PDT alone and 28.6% after intravitreal injection of ranibizumab. Patients gained an average of 10.9, 7.5, and 9.2 letters in VA, respectively. The VA after PDT was maintained for 2 years after the initial treatment. Patients with AMD and PCV had stable visual outcome within 1 year, but not afterwards [119].
To date, there is limited information on combination therapy for PCV that is refractory to anti-VEGF therapy, but due to the limited effectiveness of anti-VEGF treatment itself in PCV, combined therapy might be an option when persistent or recurrent exudative change is seen post anti-VEGF treatments. Reduced fluence v-PDT in combination with intravitreal bevacizumab was reported by Sagong et al. [73]. At 12 months, VA improved in 56% of eyes by 3 lines or more, was the same in 37% of eyes and decreased in 1 eye due to the recurrence of polyps.
In the DENALI study, patients received standard fluence v-PDT and intravitreal ranibizumab (0.5 mg) combination therapy, reduced fluence v-PDT and intravitreal ranibizumab (0.5 mg) combination therapy, or monthly ranibizumab monotherapy (0.5 mg). Patients receiving v-PDT combination therapy were administered ranibizumab treatment on day 1 and months 1 and 2. At the 12 month time point, patients in the standard fluence combination group gained more letters as compared to baseline (5.3 letters) than patients in the reduced fluence combination group (4.4 letters), but which was less than patients receiving the monthly monotherapy of ranibizumab (8.1 letters). This study did not show that v-PDT combination therapy was more effective in terms of gain in VA, as compared to ranibizumab monthly monotherapy [120].
The impact of inflammatory processes in the progression of AMD has also been investigated by many groups [121,122]. For example, the formation of drusen, which is a common feature of dry AMD, has been associated with localized inflammation and the activation of the complement cascade [123]. Additionally, it has been shown that patients with AMD express elevated systemic biomarkers of inflammation, including CRP, IL-6 and homocysteine. The role of inflammation in the pathogenesis of AMD has led to the investigation of the use of corticosteroids, a class of anti-inflammatory agents, in the treatment of AMD. PDT has also been shown to induce inflammatory processes and the potential advantage of combining PDT with corticosteroids for other indications has already been investigated. Therefore, the combination of PDT with corticosteroids could provide additional benefit in the treatment of neovascular eye diseases. For example, one study showed that in patients with AMD, treatment with v-PDT in combination with the anti-inflammatory triamcinolone acetonide (TA) resulted in a reduction in the need for retreatment, as compared to v-PDT treatment alone. In this study, however, many of the patients did not show an improvement in VA, as compared to the v-PDT only treatment group [123]. Additionally, the combination of v-PDT with corticosteroids has been associated with some adverse side effects, including cataract progression and ocular hypertension [124], which are likely to be a direct result of the corticosteroids. Another retrospective analysis reported on patients with PCV who underwent v-PDT, with or without IVTA with a follow-up of 2 years or more [125]. In this study, twentyseven eyes of 27 patients were analyzed, out of which 12 were treated with v-PDT monotherapy and 15 were treated with the combination of PDT and IVTA. The results showed that v-PDT reduced the short-term risk of vision loss in these patients. The combined therapy, however, did not appear to result in any additional benefit for the treated patients.
The use of triple therapies, including v-PDT, anti-VEGF therapies and anti-inflammatory therapies, has also been investigated. For example, a phase II, multicenter, randomized study (the RADICAL trial) was designed to compare the effectiveness of reduced fluence v-PDT, in combination with ranibizumab combination therapies, with or without the anti-inflammatory agent dexamethasone, to the effects of ranibizumab monotherapy [126]. At the 24-month followup, significantly fewer retreatment visits were required in the groups of patients receiving combination therapies, as compared to ranibizumab monotherapy. However, no statistically significant difference was seen in the average improvement in VA between the different treatment groups, as sample sizes were insufficient to draw definitive conclusions.
In a study involving 104 patients, Augustin [127], used v-PDT applied at a reduced fluence (42 J/cm2, 300 mw/cm2 at 689 nm), followed by a vitrectomy 16 hours later and the administration of dexamethasone (800 μg) and bevacizumab (1.5 mg) into the center of the eye. Significant improvements in VA (an average of 1.8 lines) and a decreases in retinal thickness (182 μm) were seen at the 40-week followup. Additionally, no serious adverse effects were observed and less than 25% of patients’ required additional treatment, which if administered was either a repeat of the triple therapy or anti-VEGF monotherapy.
Triple therapy was also investigated in another study, including 36 eyes of patients with CNV secondary to AMD. Patients were treated with standard fluence v-PDT followed immediately by the administration bevacizumab (1.25 mg) through intravitreal injection and the corticosteroid, triamcinolone acetonide (4 mg) [128]. This was followed by bevacizumab (1.25 mg) treatment every 3 months. The six-month results of this study showed a reduction in the number of re-treatments required, sustained vasculature closure and improvements in VA [128]. A final study investigated the use of sameday triple therapy with reduced fluence v-PDT (25 J/cm2, 300 mw/ cm2), intravitreal dexamethasone (4 mg), and bevacizumab (1.25 mg) in patients with AMD [129]. In this study, VA was maintained, and decreased macular thickness was observed in patients with and without previous anti-VEGF therapy. The triple therapy appeared to reduce the number of anti-VEGF injections and stabilized vision in some patients not responding to anti-VEGF therapy [129].
Future Directions in Ophthalmic PDT
Photodynamic therapy is no longer generally recommended as a monotherapy regimen, but its success has been confirmed in combination with pharmacological treatments [122]. Currently, the standard of care for wet AMD or PCV is either a monthly dose of ranibizumab or bevacizumab (the latter has shown non-inferior results when compared to ranibizumab (the CATT trial; [130])). However, not all patients respond to anti-VEGF monotherapy, and long-term effects may not be reached in those who do respond [6]. Combinatorial approaches may, therefore, turn out beneficial as the disease is targeted through multiple mechanisms, likely resulting in reduced treatment frequency and improved visual outcome.
In our view, the development of better strategies against neovascular eye disorders should be conducted in two directions: (i) the development of improved and more selective or targeted photosensitizers, and (ii) the combination of PDT with other treatment approaches targeting distinct pathways involved in the disease progression.
i. To date, the development of photosensitizers for application in cancer seems to have been driven chemically, rather than biologically or clinically, with a focus on improving optical properties. The difficulties to overcome are the limited penetration depth of excitation light into the target tissue and the problem of the selectivity of PDT exclusively towards the target cells. Modification of the photosensitizing moiety through its physicochemical properties, making compounds with longer excitation wavelengths, is a necessary step. The selectivity of PDT can be enhanced through the design of targeted photosensitizers, homing in to the target cells of choice. This is currently attempted by targeting to growth factor receptors (as shown, e.g. in a rat laser-injury model for CNV for VEGFR2 [131]), or by using targeting moieties such as antibodies, polymers [132] and peptide scaffolds [133]. These strategies, which may include new photosensitizers with increased clearance rates and better selectivity, might overcome the present limitations of ophthalmic PDT. Furthermore, some attempts to modify the drug-light time interval in the treatment of PCV have already been reported (e.g. the VALIO trial, Table 2). Another improvement could be the strategy of oscillatory photodynamic therapy (OPDT). Although performed only in a few patients [134], it was demonstrated to be an effective treatment for CNV lesions and central serous chorioretinopathy and caused reduced side effects, as compared to standard-dose PDT. Thus, more representative groups of patients should be included to give conclusive results.
ii. Advances mainly in the field of oncology have led to the development of new, more selective anti-angiogenic compounds. Next to ranibizumab, several other anti-VEGF strategies have been developed for eye disorders. The FDA approved Aflibercept (VEGF Trap-eye/Eylea; Regeneron, Tarrytown, NY, USA/Bayer Plc, UK), which differs from ranibizumab and bevacizumab in the mode of action by trapping VEGF via acting as a dummy receptor for VEGF, thus effectively inhibiting the angiogenic response. Based on a phase III clinical trial [135], it was approved for exudative AMD in 2011. Pivotal trials with ranibizumab, bevacizumab and Aflibercept show that patients receiving protocol-driven treatment have a 30-40% chance of achieving a 15-letter improvement in visual acuity [39]. It is expected that the combination of v-PDT with Aflibercept (and possibly a steroid drug) would give superior results over conventional combination strategies.
VEGF can also be targeted by the use of small interfering RNAs, e.g. bevasiranib [136,137]. Bevasiranib targets the production of VEGF protein, but does not affect existing VEGF protein, suggesting that it may give a synergistic effect when applied in combination with other treatment regimens such as v-PDT. Distinct therapeutic approaches are promising in this regard, such as inhibition of oxidative stress, interference with immunomodulation and the retinoid visual cycle occurring between the rods and cones and the retinal pigmented epithelium [138]. The maintenance of this cycle is essential to sustain physiological vision. Accumulation of cytotoxic side products of this cycle might be correlated with several eye disorders. Moreover, due to the success of first generation tyrosine kinase inhibitors such as sunitinib, erlotinib and sorafenib, a large number of angiogenesis inhibitors have been developed and tested in combination with PDT [114], including agents that target more upstream pathways, such as mTOR and HIF-1α. Agents that target integrins [139], PDGF [140], or receptor tyrosine kinases (e.g. vatalanib, pazopanib, TG 100801, TG 101095, AG 013958, AL 39324, [137]) are also enhancing the growing list of anti-angiogenic drug family members. Another class of anti-vascular drugs is the family of vascular disrupting agents, such as tubulin-binding combretastatin A-4 phosphate. These were tested recently in a clinical phase I/II dose-escalation safety and tolerability trial, and the results are pending [141]. Recently, we have suggested the use of ruthenium-based drugs as anti-angiogenic anti-tumor agents for combination with PDT [142,143]. There is discussion on the use of immune potentiating strategies, such as targeting of CTLA4 by ipilimumab [144], or targeting of programmed cell death gene (PD- 1) [145], and its ligand as immune checkpoint blockers. These drugs activate the immune system and may not exert wanted activity. It might be beneficial for ocular tumors, but most likely should be avoided for non-malignant neovascular eye disorders such as AMD.
A very promising approach for the treatment of AMD is the replacement of the damaged RPE cells with healthy progenitor cells. In a clinical study, which included four patients with AMD, and with vision of 20/200 or worse, treatment was performed with transplants of human neural retinal progenitor cell layers and RPE. In this study, all four AMD patients showed improvement in VA as a result of the therapy. Moreover, graft rejection was not observed over a period of 6-years of follow-up [146].
Last but not least, the use of photodynamic treatment is being developed of other ocular disorder, retinoblastoma, which is the most common malignant intraocular tumor in children. Positive treatment outcomes have been reported in vitro or in vivo models of human retinoblastoma xerographs using various photosensitizers, e.g. verteporfin [147], photofrin II [148], glycol-linked glycoconjugated porphyrins [149], LDL-conjugated chlorin e6 [150], or metatetra( hydroxyphenyl)chlorin (m-THPC) [151]. PDT may represent an alternative conservative treatment for these tumors and needs further investigation and development.
Conclusions
PDT has been a useful treatment modality in the clinical management of a variety of intraocular neovascular disorders in the “pre anti-VEGF era”. Now-a-days, PDT is used as a second-line therapy in wet AMD patients not responding to monotherapy with anti-VEGF agents, or in whom the treatment burden of monthly injections is too heavy. Next to this, it is still virtually the only effective treatment for PCV-diagnosed patients. PDT seems to give promising results when applied for benign, and particularly vascular tumors, such as circumscribed choroidal hemangioma. For primary malignant tumors, such as choroidal melanoma, PDT does not offer local tumor control rates that are equivalent or better than that achieved with plaque or proton radiation therapy. On the other hand, for choroidal metastases PDT can be applied as a palliative treatment. Increased knowledge concerning the pathogenesis of neovascular-based diseases would likely result in the gradual development of more selective photosensitizers and of combination strategies with improved therapeutic benefits for patients and increased potential for long-term vision improvement.
Acknowledgement
The authors are grateful for financial support from Dr. J. Jacobi and the Dutch Science Foundation NWO.
References
- Mennel S, Barbazetto I, Meyer CH, Peter S, Stur M (2007) Ocular photodynamic therapy--Standard applications and new indications (Part 1). Review of the literature and personal experience. Ophthalmologica 221: 216-226.
- Pachydaki S, Sobrin L, Miller JW (2007) Photodynamic therapy and combination treatments. Int Ophthalmol Clin 47: 95-115.
- Kaiser PK; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group (2006) Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol 244: 1132-1142.
- Kaiser PK (2007) Verteporfin photodynamic therapy and anti-angiogenic drugs: potential for combination therapy in exudative age-related macular degeneration. Curr Med Res Opin 23: 477-487.
- Nowak-Sliwinska P (2012) Anti-angiogenic treatment for exudative age-related macular degeneration: new strategies are underway. Current Angiogenesis 1: 318-334.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, et al. (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology.
- Van den Bergh H, Ballini JP (2003) Photodynamic therapy: Basic principles and mechanisms. Lasers in Opthalmology 183-195.
- Nelson JS, Liaw LH, Berns MW (1987) Tumor destruction in photodynamic therapy. Photochem Photobiol 46: 829-835.
- Schmidt-Erfurth U, Hasan T, Gragoudas E, Michaud N, Flotte TJ, et al. (1994) Vascular targeting in photodynamic occlusion of subretinal vessels. Ophthalmology 101: 1953-1961.
- Fingar VH (1996) Vascular effects of photodynamic therapy. J Clin Laser Med Surg 14: 323-328.
- Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, et al. (1989) Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol 256: F794-802.
- Doukas J, Hechtman HB, Shepro D (1989) Vasoactive amines and eicosanoids interactively regulate both polymorphonuclear leukocyte diapedesis and albumin permeability in vitro. Microvasc Res 37: 125-137.
- Chang WH, Petry JJ (1980) Platelets, prostaglandins, and patency in microvascular surgery. J Microsurg 2: 27-35.
- Ogletree ML (1987) Overview of physiological and pathophysiological effects of thromboxane A2. Fed Proc 46: 133-138.
- Mennel S, Barbazetto I, Meyer CH, Peter S, Stur M (2007) Ocular photodynamic therapy--Standard applications and new indications. Part 2. Review of the literature and personal experience. Ophthalmologica 221: 282-291.
- Koh A, Lim TH, Au Eong KG, Chee C, Ong SG, et al. (2011) Optimising the management of choroidal neovascularisation in Asian patients: Consensus on treatment recommendations for anti-VEGF therapy. Singapore Med J 52: 232-240.
- Qazi Y, Maddula S, Ambati BK (2009) Mediators of ocular angiogenesis. J Genet 88: 495-515.
- Klein R, Klein BE, Linton KL (1992) Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 99: 933-943.
- Biarnés M, Monés J, Alonso J, Arias L (2011) Update on geographic atrophy in age-related macular degeneration. Optom Vis Sci 88: 881-889.
- Thomas EL, SnET2 SG (2004) SnET2 photodynamic therapy for age-related macular degeneration: Visual acuity efficacy outcomes from two parallel phase III trials. ARVO 45: 2214.
- Mori K, Yoneya S, Anzail K, Kabasawa S, Sodeyama T, et al. (2001) Photodynamic therapy of experimental choroidal neovascularization with a hydrophilic photosensitizer: mono-L-aspartyl chlorin e6. Retina 21: 499-508.
- Blumenkranz MS, Woodburn KW, Qing F, Verdooner S, Kessel D, et al. (2000) Lutetium texaphyrin (Lu-Tex): a potential new agent for ocular fundus angiography and photodynamic therapy. Am J Ophthalmol 129: 353-362.
- Sickenberg M, Ballini JP, Zographos L, Piguet B, van den Bergh H, et al. (1999) Preliminary clinical results of Lu-Tex fluorescence pharmacokinetic and photodynamic therapy to treat choroidal neovascularization. In: Proceedings of the Twelfth Congress of the European Society of Ophthalmology, Stockholm, Sweden.
- Bressler NM, (2001) Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol 119: 198-207.
- Costa RA, Farah ME, Cardillo JA, Calucci D, Williams GA (2003) Immediate indocyanine green angiography and optical coherence tomography evaluation after photodynamic therapy for subfoveal choroidal neovascularization. Retina 23: 159-165.
- Gelisken F, Lafaut BA, Inhoffen W, Voelker M, Grisanti S, et al. (2004) Clinicopathological findings of choroidal neovascularisation following verteporfin photodynamic therapy. Br J Ophthalmol 88: 207-211.
- Rudolf M, Michels S, Schlötzer-Schrehardt U, Schmidt-Erfurth U (2004) Expression of angiogenic factors by photodynamic therapy. Klin Monbl Augenheilkd 221: 1026-1032.
- Weiss A, den Bergh Hv, Griffioen AW, Nowak-Sliwinska P (2012) Angiogenesis inhibition for the improvement of photodynamic therapy: the revival of a promising idea. Biochim Biophys Acta 1826: 53-70.
- Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, et al. (2003) Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer 88: 1772-1779.
- Schmidt-Erfurth U, Niemeyer M, Geitzenauer W, Michels S (2005) Time course and morphology of vascular effects associated with photodynamic therapy. Ophthalmology 112: 2061-2069.
- Zhang X, Jiang F, Zhang ZG, Kalkanis SN, Hong X, et al. (2005) Low-dose photodynamic therapy increases endothelial cell proliferation and VEGF expression in nude mice brain. Lasers Med Sci 20: 74-79.
- Schmidt-Erfurth U, Schlotzer-Schrehard U, Cursiefen C, Michels S, Beckendorf A, et al. (2003) Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sc 44: 4473-4480.
- Grossniklaus HE, Green WR (2004) Choroidal neovascularization. Am J Ophthalmol 137: 496-503.
- Hera R, Keramidas M, Peoc'h M, Mouillon M, Romanet JP, et al. (2005) Expression of VEGF and angiopoietins in subfoveal membranes from patients with age-related macular degeneration. Am J Ophthalmol 139: 589-596.
- Das A, McGuire PG (2003) Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res 22: 721-748.
- Sivaprasad S, Hykin P (2013) What is new in the management of wet age-related macular degeneration? Br Med Bull 105: 201-211.
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 119: 1388-1398.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology 119: 1399-1411.
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, et al. (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119: 2537-2548.
- Kishan AU, Modjtahedi BS, Morse LS, Lee P (2013) Radiation therapy for neovascular age-related macular degeneration. Int J Radiat Oncol Biol Phys 85: 583-597.
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, et al. (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 116: 57-65.
- Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, et al. (2003) Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial--VIP report no. 3. Ophthalmology 110: 667-673.
- Lam DS, Chan WM, Liu DT, Fan DS, Lai WW, et al. (2004) Photodynamic therapy with verteporfin for subfoveal choroidal neovascularisation of pathologic myopia in Chinese eyes: a prospective series of 1 and 2 year follow up. Br J Ophthalmol 88: 1315-1319.
- Cohen SY (2009) Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina 29: 1062-1066.
- Sickenberg M, Schmidt-Erfurth U, Miller JW, Pournaras CJ, Zografos L, et al. (2000) A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Arch Ophthalmol 118: 327-336.
- Chan WM, Lai TY, Lau TT, Lee VY, Liu DT, et al. (2008) Combined photodynamic therapy and intravitreal triamcinolone for choroidal neovascularization secondary to punctate inner choroidopathy or of idiopathic origin: one-year results of a prospective series. Retina 28: 71-80.
- Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA (2004) Polypoidal choroidal vasculopathy. Surv Ophthalmol 49: 25-37.
- Cackett P, Wong D, Yeo I (2009) A classification system for polypoidal choroidal vasculopathy. Retina 29: 187-191.
- Hayashi H, Yamashiro K, Gotoh N, Nakanishi H, Nakata I, et al. (2010) CFH and ARMS2 variations in age-related macular degeneration, polypoidal choroidal vasculopathy, and retinal angiomatous proliferation. Invest Ophthalmol Vis Sci 51: 5914-5919.
- Gotoh N, Nakanishi H, Hayashi H, Yamada R, Otani A, et al. (2009) ARMS2 (LOC387715) variants in Japanese patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Am J Ophthalmol 147: 1037-1041, 1041.
- Chen H, Liu K, Chen LJ, Hou P, Chen W, et al. (2012) Genetic associations in polypoidal choroidal vasculopathy: A systematic review and meta-analysis. Mol Vis 18: 816-829.
- Honda S, Bessho H, Kondo N, Kusuhara S, Tsukahara Y, et al. (2012) Positive association of CD36 gene variants with the visual outcome of photodynamic therapy in polypoidal choroidal vasculopathy. Mol Vis 18: 2796-2804.
- Cheng Y, Huang L, Li X, Zhou P, Zeng W, et al. (2013) Genetic and functional dissection of ARMS2 in age-related macular degeneration and polypoidal choroidal vasculopathy. PLoS One 8: e53665.
- Kanda A, Chen W, Othman M, Branham KE, Brooks M, et al. (2007) A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A 104: 16227-16232.
- Goto A, Akahori M, Okamoto H, Minami M, Terauchi N, et al. (2009) Genetic analysis of typical wet-type age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese population. J Ocul Biol Dis Infor 2: 164-175.
- Lee KY, Vithana EN, Mathur R, Yong VH, Yeo IY, et al. (2008) Association analysis of CFH, C2, BF and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 49: 2613-2619.
- Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A (2008) Elastin gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 49: 1101-1105.
- Kociok N, Joussen AM (2007) Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefes Arch Clin Exp Ophthalmol 245: 101-113.
- Nowak-Sliwinska P, Sickenberg M, van den Bergh H, Koh AHC (2013) Photodynamic therapy for polypoidal choroidal vasculopathy. Progress in Eye and Retinal Research.
- Tsujikawa A, Ooto S, Yamashiro K, Tamura H, Otani A, et al. (2010) Treatment of polypoidal choroidal vasculopathy by intravitreal injection of bevacizumab. Jpn J Ophthalmol 54: 310-319.
- Hikichi T, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, et al. (2010) Improvement of angiographic findings of polypoidal choroidal vasculopathy after intravitreal injection of ranibizumab monthly for 3 months. Am J Ophthalmol 150: 674-682.
- Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, et al. (2008) Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 92: 70-73.
- Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA (2010) Polypoidal choroidal vasculopathy: A review. Surv Ophthalmol 55: 501-515.
- Chan WM, Lam DS, Wong TH, Lai TY, Kwok AK, et al. (2003) Photodynamic therapy with verteporfin for subfoveal idiopathic choroidal neovascularization: one-year results from a prospective case series. Ophthalmology 110: 2395-2402.
- Leal S, Silva R, Figueira J, Cachulo ML, Pires I, et al. (2010) Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy: Results after 3 years of follow-up. Retina 30: 1197-1205.
- Tsuchiya D, Yamamoto T, Kawasaki R, Yamashita H (2009) Two-year visual outcomes after photodynamic therapy in age-related macular degeneration patients with or without polypoidal choroidal vasculopathy lesions. Retina 29: 960-965.
- Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, et al. (2007) Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina 27: 335-341.
- Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, et al. (2002) Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina 22: 529-535.
- Honda S, Imai H, Yamashiro K, Kurimoto Y, Kanamori-Matsui N, et al. (2009) Comparative assessment of photodynamic therapy for typical age-related macular degeneration and polypoidal choroidal vasculopathy: A multicenter study in Hyogo prefecture, Japan. Ophthalmologica 223: 333-338.
- Yamashita A, Shiraga F, Shiragami C, Ono A, Tenkumo K (2010) One-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 149: 465-471.
- Yamashita A, Shiraga F, Shiragami C, Shirakata Y, Fujiwara A (2013) Two-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 155: 96-102.
- Akaza E, Mori R, Yuzawa M (2008) Long-term results of photodynamic therapy of polypoidal choroidal vasculopathy. Retina 28: 717-722.
- Sagong M, Lim S, Chang W (2012) Reduced-fluence photodynamic therapy combined with intravitreal bevacizumab for polypoidal choroidal vasculopathy. Am J Ophthalmol 153: 873-882.
- Amer R, Lois N (2011) Punctate inner choroidopathy. Surv Ophthalmol 56: 36-53.
- Chan WM, Lim TH, Pece A, Silva R, Yoshimura N (2010) Verteporfin PDT for non-standard indications--A review of current literature. Graefes Arch Clin Exp Ophthalmol 248: 613-626.
- Karacorlu M, Karacorlu S, Ozdemir H, Mat C (2002) Photodynamic therapy with verteporfin for choroidal neovascularization in patients with angioid streaks. Am J Ophthalmol 134: 360-366.
- Arias L, Pujol O, Rubio M, Caminal J (2006) Long-term results of photodynamic therapy for the treatment of choroidal neovascularization secondary to angioid streaks. Graefes Arch Clin Exp Ophthalmol 244: 753-757.
- Shaikh S, Ruby AJ, Williams GA (2003) Photodynamic therapy using verteporfin for choroidal neovascularization in angioid streaks. Am J Ophthalmol 135: 1-6.
- Ladas ID, Georgalas I, Rouvas AA, Gotsis S, Karagiannis DA, et al. (2005) Photodynamic therapy with verteporfin of choroidal neovascularization in angioid streaks: Conventional versus early retreatment. Eur J Ophthalmol 15: 69-73.
- Menchini U, Virgili G, Introini U, Bandello F, Ambesi-Impiombato M, et al. (2004) Outcome of choroidal neovascularization in angioid streaks after photodynamic therapy. Retina 24: 763-771.
- Heimann H, Gelisken F, Wachtlin J, Wehner A, Völker M, et al. (2005) Photodynamic therapy with verteporfin for choroidal neovascularization associated with angioid streaks. Graefes Arch Clin Exp Ophthalmol 243: 1115-1123.
- Jurklies B, Bornfeld N, Schilling H (2006) Photodynamic therapy using verteporfin for choroidal neovascularization associated with angioid streaks--long-term effects. Ophthalmic Res 38: 209-217.
- Gliem M, Finger RP, Fimmers R, Brinkmann CK, Holz FG, et al. (2013) Treatment of choroidal neovascularization due to angioid streaks: a comprehensive review. Retina 33: 1300-1314.
- Yannuzzi LA (2012) Type A behavior and central serous chorioretinopathy. Retina 32: 709.
- Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM (2003) Photodynamic therapy for chronic central serous chorioretinopathy. Retina 23: 752-763.
- Battaglia Parodi M, Da Pozzo S, Ravalico G (2003) Photodynamic therapy in chronic central serous chorioretinopathy. Retina 23: 235-237.
- Smretschnig E, Ansari-Shahrezaei S, Moussa S, Glittenberg C, Krebs I, et al. (2012) Half-fluence photodynamic therapy in acute central serous chorioretinopathy. Retina 32: 2014-2019.
- Nicholson B, Noble J, Forooghian F, Meyerle C (2013) Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv Ophthalmol 58: 103-126.
- Zografos L, Egger E, Bercher L, Chamot L, Munkel G (1998) Proton beam irradiation of choroidal hemangiomas. Am J Ophthalmol 126: 261-268.
- Tsipursky MS, Golchet PR, Jampol LM (2011) Photodynamic therapy of choroidal hemangioma in sturge-Weber syndrome, with a review of treatments for diffuse and circumscribed choroidal hemangiomas. Surv Ophthalmol 56: 68-85.
- Boixadera A, Garcia-Arumi J, Martinez-Castillo V, Encinas JL, Elizalde J, et al. (2009) Prospective clinical trial evaluating the efficacy of photodynamic therapy for symptomatic circumscribed choroidal hemangioma. Ophthalmology 116: 100-105.
- Madreperla SA (2001) Choroidal hemangioma treated with photodynamic therapy using verteporfin. Arch Ophthalmol 119: 1606-1610.
- Anand R (2003) Photodynamic therapy for diffuse choroidal hemangioma associated with Sturge Weber syndrome. Am J Ophthalmol 136: 758-760.
- Rennie IG (2010) Retinal vasoproliferative tumours. Eye (Lond) 24: 468-471.
- Saldanha MJ, Edrich C (2008) Treatment of vasoproliferative tumors with photodynamic therapy. Ophthalmic Surg Lasers Imaging 39: 143-145.
- Osman SA, Aylin Y, Arikan G, Celikel H (2007) Photodynamic treatment of a secondary vasoproliferative tumour associated with sector retinitis pigmentosa and Usher syndrome type I. Clin Experiment Ophthalmol 35: 191-193.
- Tuncer S, Kir N, Shields CL (2012) Dramatic regression of amelanotic choroidal melanoma with PDT following poor response to brachytherapy. Ophthalmic Surg Lasers Imaging 43: e38-40.
- Campbell WG, Pejnovic TM (2012) Treatment of amelanotic choroidal melanoma with photodynamic therapy. Retina 32: 1356-1362.
- Donaldson MJ, Lim L, Harper CA, Mackenzie J, G Campbell W (2005) Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Experiment Ophthalmol 33: 548-549.
- Canal-Fontcuberta I, Salomão DR, Robertson D, Cantrill HL, Koozekanani D, et al. (2012) Clinical and histopathologic findings after photodynamic therapy of choroidal melanoma. Retina 32: 942-948.
- Barbazetto IA, Lee TC, Rollins IS, Chang S, Abramson DH (2003) Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol 135: 898-899.
- Mashayekhi A, Shields CL, Shields JA (2013) Transient increased exudation after photodynamic therapy of intraocular tumors. Middle East Afr J Ophthalmol 20: 83-86.
- Cekic O, Bardak Y, Kapucuoglu N (2011) Photodynamic therapy for conjunctival ocular surface squamous neoplasia. J Ocul Pharmacol Ther 27: 205-207.
- Kevany BM, Palczewski K (2010) Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 25: 8-15.
- Kaliki S, Shields CL, Al-Dahmash SA, Mashayekhi A, Shields JA (2012) Photodynamic therapy for choroidal metastasis in 8 cases. Ophthalmology 119: 1218-1222.
- Sickenberg M, Ballini JP, van den Bergh H (1999) A computer-based method to quantify the classic pattern of choroidal neovascularization in order to monitor photodynamic therapy. Graefes Arch Clin Exp Ophthalmol 237: 353-360.
- Schnurrbusch UE, Welt K, Horn LC, Wiedemann P, Wolf S (2001) Histological findings of surgically excised choroidal neovascular membranes after photodynamic therapy. Br J Ophthalmol 85: 1086-1091.
- Schmidt-Erfurth U, Laqua H, Schlötzer-Schrehard U, Viestenz A, Naumann GO (2002) Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol 120: 835-844.
- Ghazi NG, Jabbour NM, De La Cruz ZC, Green WR (2001) Clinicopathologic studies of age-related macular degeneration with classic subfoveal choroidal neovascularization treated with photodynamic therapy. Retina 21: 478-486.
- Moshfeghi DM, Kaiser PK, Grossniklaus HE, Sternberg P Jr, Sears JE, et al. (2003) Clinicopathologic study after submacular removal of choroidal neovascular membranes treated with verteporfin ocular photodynamic therapy. Am J Ophthalmol 135: 343-350.
- Favilla I, Favilla ML, Gosbell AD, Barry WR, Ellims P, et al. (1995) Photodynamic therapy: A 5-year study of its effectiveness in the treatment of posterior uveal melanoma, and evaluation of haematoporphyrin uptake and photocytotoxicity of melanoma cells in tissue culture. Melanoma Res 5: 355-364.
- Nowak-Sliwinska P, van Beijnum JR, van Berkel M, van den Bergh H, Griffioen AW (2010) Vascular regrowth following photodynamic therapy in the chicken embryo chorioallantoic membrane. Angiogenesis 13: 281-292.
- van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, et al. (2013) Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene 32: 363-374.
- Nowak-Sliwinska P, Weiss A, van Beijnum JR, Wong TJ, Ballini JP, et al. (2012) Angiostatic kinase inhibitors to sustain photodynamic angio-occlusion. J Cell Mol Med 16: 1553-1562.
- Sivaprasad S, Hykin P, Saeed A, Beatty S, Grisanti S, et al. (2010) Intravitreal pegaptanib sodium for choroidal neovascularisation secondary to age-related macular degeneration: Pan-European experience. Eye (Lond) 24: 793-798.
- Antoszyk AN, Tuomi L, Chung CY, Singh A, et al. (2008) Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): Year 2 results. Am J Ophthalmol 145: 862-874.
- Schmidt-Erfurth U, Wolf S, PROTECT Study Group (2008) Same-day administration of verteporfin and ranibizumab 0.5 mg in patients with choroidal neovascularisation due to age-related macular degeneration. Br J Ophthalmol 92: 1628-1635.
- Larsen M, Schmidt-Erfurth U, Lanzetta P, Wolf S, Simader C, et al. (2012) Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: Twelve-month MONT BLANC study results. Ophthalmology 119: 992-1000.
- Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, et al. (2012) EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 32: 1453-1464.
- Kaiser PK, Boyer DS, Cruess AF, Slakter JS, Pilz S, et al. (2012) Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: Twelve-month results of the DENALI study. Ophthalmology 119: 1001-1010.
- Cook KM, Figg WD (2010) Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J Clin 60: 222-243.
- Couch SM, Bakri SJ (2011) Review of combination therapies for neovascular age-related macular degeneration. Semin Ophthalmol 26: 114-120.
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, et al. (2005) Complement factor H variant increases the risk of age-related macular degeneration. Science 308: 419-421.
- Frimpong-Boateng A, Bunse A, Rüfer F, Roider J (2009) Photodynamic therapy with intravitreal application of triamcinolone acetonide in age-related macular degeneration: functional results in 54 patients. Acta Ophthalmol 87: 183-187.
- Lai TY, Lam CP, Luk FO, Chan RP, Chan WM, et al. (2010) Photodynamic therapy with or without intravitreal triamcinolone acetonide for symptomatic polypoidal choroidal vasculopathy. J Ocul Pharmacol Ther 26: 91-95.
- Hudson HL (2008) The RADICAL trial: Exploring combination therapies. Retina Today 1: 59-62.
- Augustin A (2009) Triple therapy for age-related macular degeneration. Retina 29: S8-S11.
- Yip PP, Woo CF, Tang HH, Ho CK (2009) Triple therapy for neovascular age-related macular degeneration using single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone. Br J Ophthalmol 93: 754-758.
- Bakri SJ, Couch SM, McCannel CA, Edwards AO (2009) Same-day triple therapy with photodynamic therapy, intravitreal dexamethasone, and bevacizumab in wet age-related macular degeneration. Retina 29: 573-578.
- CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, et al. (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364: 1897-1908.
- Renno RZ, Terada Y, Haddadin MJ, Michaud NA, Gragoudas ES, et al. (2004) Selective photodynamic therapy by targeted verteporfin delivery to experimental choroidal neovascularization mediated by a homing peptide to vascular endothelial growth factor receptor-2. Arch Ophthalmol 122: 1002-1011.
- Konan YN, Gurny R, Allémann E (2002) State of the art in the delivery of photosensitizers for photodynamic therapy. J Photochem Photobiol B 66: 89-106.
- Verwilst P, David CC, Leen V, Hofkens J, de Witte PA, et al. (2013) Synthesis and in vitro evaluation of a PDT active BODIPY-NLS conjugate. Bioorg Med Chem Lett 23: 3204-3207.
- Peyman GA, Tsipursky M, Nassiri N, Conway M (2011) Oscillatory photodynamic therapy for choroidal neovascularization and central serous retinopathy; a pilot study. J Ophthalmic Vis Res 6: 166-176.
- Thomas M, Mousa SS, Mousa SA (2013) Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration. Clin Ophthalmol 7: 495-501.
- Zampros I, Praidou A, Brazitikos P, Ekonomidis P, Androudi S (2012) Antivascular endothelial growth factor agents for neovascular age-related macular degeneration. J Ophthalmol 2012: 319728.
- Mousa SA, Mousa SS (2010) Current status of vascular endothelial growth factor inhibition in age-related macular degeneration. BioDrugs 24: 183-194.
- Travis GH, Golczak M, Moise AR, Palczewski K (2007) Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol 47: 469-512.
- Stupp R, Ruegg C (2007) Integrin inhibitors reaching the clinic. J Clin Oncol 25: 1637-1638.
- Dimitroff CJ, Klohs W, Sharma A, Pera P, Driscoll D, et al. (1999) Anti-angiogenic activity of selected receptor tyrosine kinase inhibitors, PD166285 and PD173074: Implications for combination treatment with photodynamic therapy. Invest New Drugs 17: 121-135.
- ClinicalTrials.gov (2012) Combretastatin A4 phosphate in patients with neovascular age-related macular degeneration.
- Nowak-Sliwinska P, van Beijnum JR, Casini A, Nazarov AA, Wagnieres G, et al. (2011) Organometallic ruthenium(II) arene compounds with antiangiogenic activity. J Med Chem 54: 3895-3902.
- Nazarov AA, Baquié M, Nowak-Sliwinska P, Zava O, van Beijnum JR, et al. (2013) Synthesis and characterization of a new class of anti-angiogenic agents based on ruthenium clusters. Sci Rep 3: 1485.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711-723.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455-2465.
- Yang Z, Stratton C, Francis PJ, Kleinman ME, Tan PL, et al. (2008) Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med 359: 1456-1463.
- Stephan H, Boeloeni R, Eggert A, Bornfeld N, Schueler A (2008) Photodynamic therapy in retinoblastoma: effects of verteporfin on retinoblastoma cell lines. Invest Ophthalmol Vis Sci 49: 3158-3163.
- Yamamoto N, Sery TW, Hoober JK, Willett NP, Lindsay DD (1994) Effectiveness of photofrin II in activation of macrophages and in vitro killing of retinoblastoma cells. Photochem Photobiol 60: 160-164.
- Laville I, Pigaglio S, Blais JC, Doz F, Loock B, et al. (2006) Photodynamic efficiency of diethylene glycol-linked glycoconjugated porphyrins in human retinoblastoma cells. J Med Chem 49: 2558-2567.
- Schmidt-Erfurth U, Diddens H, Birngruber R, Hasan T (1997) Photodynamic targeting of human retinoblastoma cells using covalent low-density lipoprotein conjugates. Br J Cancer 75: 54-61.
- Aerts I, Leuraud P, Blais J, Pouliquen AL, Maillard P, et al. (2010) In vivo efficacy of photodynamic therapy in three new xenograft models of human retinoblastoma. Photodiagnosis Photodyn Ther 7: 275-283.
- Rosenfeld PJ, Boyer DS, Bressler NM, Fish G, Grizzard WS, et al. (2007) Verteporfin therapy of subfoveal occult choroidal neovascularization in AMD using delayed light application: One-year results of the VALIO Study. Am J Ophthalmol 144: 970-972.
- Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, et al. (2008) One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 115: 141-146.
- Wakabayashi T, Gomi F, Sawa M, Tsujikawa M, Tano Y (2008) Marked vascular changes of polypoidal choroidal vasculopathy after photodynamic therapy. Br J Ophthalmol 92: 936-940.
- Tsuchihashi T, Mori K, Ueyama K, Yoneya S (2013) Five-year results of photodynamic therapy with verteporfin for Japanese patients with neovascular age-related macular degeneration. Clin Ophthalmol 7: 615-620.
- Ruamviboonsuk P, Tadarati M, Vanichvaranont S, Hanutsaha P, Pokawattana N (2010) Photodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: Results of a 1-year preliminary study. Br J Ophthalmol 94: 1045-1051.
- Gomi F, Sawa M, Wakabayashi T, Sasamoto Y, Suzuki M, et al. (2010) Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 150: 48-54.
- Saito M, Iida T, Kano M (2012) Combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Retina 32: 1272-1279.
- Barbazetto IA, Lee TC, Abramson DH (2004) Treatment of conjunctival squamous cell carcinoma with photodynamic therapy. Am J Ophthalmol 138: 183-189.
- Cekiç O, Bardak Y, Kapucuoglu N (2011) Photodynamic therapy for conjunctival ocular surface squamous neoplasia. J Ocul Pharmacol Ther 27: 205-207.
- Harbour JW (2004) Photodynamic therapy for choroidal metastasis from carcinoid tumor. Am J Ophthalmol 137: 1143-1145.
- Isola V, Pece A, Pierro L (2006) Photodynamic therapy with verteporfin of choroidal malignancy from breast cancer. Am J Ophthalmol 142: 885-887.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17673
- [From(publication date):
specialissue-2013 - Nov 18, 2025] - Breakdown by view type
- HTML page views : 12832
- PDF downloads : 4841