Research Article Open Access
The Relationship between Choline Acetyltransferase and Nitric Oxide Synthase Isoform Expression in Alzheimer's Disease
B.E. Kalisch1*, J.C. Baskey2 and R.J. Rylett2
1Department of Biomedical Sciences, University of Guelph, Guelph, Ontario, Canada
2Department of Physiology and Pharmacology, University of Western Ontario and Cell Biology Research Group, Robarts Research Institute, London, Ontario, Canada
- Corresponding Author:
- B.E. Kalisch
Department of Biomedical Sciences
University of Guelph, Guelph, Ontario
Canada, N1G 2W1
Tel: 519-824-4120
Fax: 519-787-1640
E-mail: bkalisch@uoguelph.ca
Received date: November 14, 2011; Accepted date: December 20, 2011; Published December 22, 2011
Citation: Kalisch BE, Baskey JC, Rylett RJ (2012) The Relationship between Choline Acetyltransferase and Nitric Oxide Synthase Isoform Expression in Alzheimer’s Disease. J Alzheimers Dis 2:105. doi:10.4172/2161-0460.1000105
Copyright: © 2012 Kalisch BE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Nitric oxide (NO) plays an important role in cholinergic neurotransmission and has been implicated in the progression of Alzheimer’s disease (AD). Cholinergic neurotransmission has been associated with regulation of NO synthase (NOS) expression, and we determined previously that NO modulates choline acetyltransferase (ChAT) expression. In spite of the important links identified between NOS and ChAT, little is known about the relationship between these two enzymes in AD. Therefore in the present study, we compared the expression levels of ChAT and NOS in necropsy brain of individuals with AD and non-demented controls. ChAT and NOS levels were assessed in control and AD caudate, nucleus basalis of Meynert (nbM), cortex and hippocampus by radioenzymatic assay, immunoblot analysis and RT-PCR. We detected a significant decrease in ChAT activity in the cortex of AD cases, but no alterations in NOS activity were observed in any of the brain regions examined. At the mRNA level, no significant decrease in total ChAT mRNA was detected but the decrease in M-ChAT mRNA levels approached significance in the nbM. No difference in nNOS and iNOS mRNA and protein levels was observed between control and AD tissue in any of the four brain regions sampled, but a statistically significant decrease in eNOS mRNA levels was detected in both the cortex and hippocampus of AD brain. Finally, the levels of ChAT and NOS activity, protein or mRNA isoforms did not correlate in most of the brain regions examined, but a reduction in ChAT activity and eNOS mRNA in basal forebrain projection regions was observed. These data suggest that in general, there is no correlation between the levels of NOS and ChAT in control or AD subjects, but a reduction in eNOS levels in the hippocampus and cortex indicates there could be an interaction between eNOS containing cells and basal forebrain projections neurons in AD.
Keywords
Cholinergic; Nitric oxide; Human brain; Enzyme activity; mRNA levels
Abbreviations
ACh: Acetylcholine; AD: Alzheimer’s Disease; ChAT: Choline Acetyl Transferase; nbM: Nucleus Basalis of Meynert; NGF: Nerve Growth Factor; NO: Nitric Oxide; NOS: Nitric Oxide Synthase; PMI: Post-Mortem Interval; SEM: Standard Error of the Mean; TrkA: Neurotrophic Tyrosine Kinase Receptor, type I.
Introduction
Alzheimer disease’s (AD) is characterized by memory loss and a decline in cognitive and functional abilities. Neuropathological features include β-amyloid (Aβ)-containing neuritic plaques and neurofibrillary tangles (reviewed in [1]), as well as synaptic loss and dysfunction [2]. While abnormalities occur in several neurotransmitter systems, alteration in cholinergic neuronal markers is found early in AD [3,4] with dementia severity correlating best with decreased activity of the cholinergic marker choline acetyltransferase (ChAT) in basal forebrain [5-7], hippocampus [8] and cortex [9,10]. Cholinergic neurons are dependent on the neurotrophin nerve growth factor (NGF) for survival and phenotypic maintenance [11-16]. In AD brains, decreased levels of NGF [17] and mRNA for the high-affinity NGF receptor (TrkA) [18-20] have been observed in surviving basal forebrain neurons, implying that reduced NGF-TrkA signalling could contribute to cholinergic degeneration in AD.
Information regarding NGF-mediated signalling events and enhancement of cholinergic function has come from animal model and cell culture studies. Nitric oxide (NO) has been identified as an important modulator of NGF-mediated neurotrophic responses. NGF increases expression of all three isoforms of nitric oxide synthase (NOS) in PC12 cells [21,22] and the neuronal NOS isoform (nNOS) in cultured rat basal forebrain cholinergic neurons [23,24]. We found that NOS inhibitors attenuate NGF-mediated increases in ChAT mRNA levels and activity in PC12 cells [21]. These studies show that NGF can regulate NOS expression and that NO can modulate ChAT expression, thus linking TrkA signalling and NO production with the maintenance of cholinergic phenotype. It is not known if these signalling events are altered in AD brain, but recent studies have focused on the role of NO in AD and data are emerging regarding the expression levels of NOS isozymes in AD [25-37].
Some studies support the neurotoxic role of NO and depict elevations in NOS isoform expression during AD progression [25-32], whereas others describe reductions in NOS expression and the data are consistent with NO-mediated neuroprotection [33-37]. Hyman et al. [25] found that NOS-containing hippocampal neurons were relatively spared in AD, suggesting that NO produced by these cells was toxic to neighbouring neurons. In support of this, the number of cortical neurons immunoreactive for nNOS and the inducible NOS isoform (iNOS) are increased as AD progresses with the increase linked to neurofibrillary tangle formation [26-28]. As well, increased serum NO levels correlate inversely with Mini-Mental State Examination scores in AD patients [29], and it is suggested that Aβ-induced NO release from neurons [28] and iNOS expression in astrocytes [31,32] contribute to neuron damage in AD. In contrast, Norris et al. [33] found reduced nNOS-containing neurons in the frontal cortex and hippocampus of AD brains and in the end-stage of the disease, the expression of constitutive endothelial NOS (eNOS; [34] and nNOS [35]) in cortical neurons was decreased. In addition, cortical plaque and tangle burden has been correlated with a reduction in capillary eNOS levels [36]. It has also been suggested that elevations in NOS expression occurring early in the disease process are a protective response to early neurodegenerative events [37]. While NO likely contributes to both neuroprotective and neurotoxic events in AD, the changes described in NOS expression could play a role in the phenotypic maintenance of cholinergic neurons. In addition, nNOS-containing cortical neurons and micro vessels receive input from basal forebrain cholinergic neurons [38-40], and loss of this input may contribute to the reduction in cortical blood flow and decreased cognitive abilities in AD [39]. These data suggest that cholinergic neuron degeneration could lead to alterations in NOS expression in AD. Taken together, these studies support the interaction between cholinergic neurotransmission and NOS expression.
Therefore, we set out to investigate whether there was a correlation between cholinergic deficit and NOS expression in post-mortem tissue from AD subjects and age-matched controls. Since the predominant form of AD is late onset, we only examined tissues from individuals over the age of 65 years. In this more common form of the disease, inheritance of the ε4 allele of apolipoprotein (apo) E is a major risk factor [41,42]. In fact, greater cholinergic deficits have been detected in tissues obtained from AD subjects that express apoE4 [43-45] as compared with individuals that express ApoE2 and/or 3. In addition, ApoE4 has been reported to increase NO release, enhance arginine (NO precursor) transport and increase oxidative stress [46-51], which could make ApoE4 carriers more vulnerable to neuronal damage. We therefore also investigated whether the levels of ChAT and NOS in necropsy brain tissue differed between ApoE4 carriers and non-carriers.
Materials and Methods
Tissue samples
Necropsy brain samples used in this study were obtained from the University of California, Irvine, Institute for Brain Aging and Dementia. Brains were collected at autopsy of control and AD subjects with written consent of next-of-kin and in compliance with Research Ethics Review Boards. The present studies were carried out in compliance with Canadian Institutes for Health Research (CIHR) guidelines for handling of human tissues, and received local institutional Biosafety Committee approval. Neuropathological evaluation of tissues was carried out at UC Irvine and used to confirm the diagnosis of AD through the presence of Aβ-containing neuritic plaques and neurofibrillary tangles, or to document the absence of these AD markers in control subjects. Brain samples from 6 age-matched controls (81.8 ± 3.6 years) and 13 AD patients (78.8 ±1.9 years) were used in this study. For the experiments described, when available, the frontal cortex, hippocampus, caudate and nucleus basalis of Meynert (nbM) were dissected and the tissue was frozen at –80°C until preparation of samples for enzyme assays, immunoblots or RNA isolation.
Materials
Trizol and reverse transcriptase (RT)-polymerase chain reaction (PCR) reagents were from Invitrogen Life Technologies (Burlington, ON, Canada). NOS isoforms were detected by immunoblot analysis using a polyclonal rabbit anti-rat nNOS (Santa Cruz Biotechnology Inc.), rabbit polyclonal iNOS antibody (a gift from Dr. David W. Mercer, University of Texas-Houston Medical School), and mouse monoclonal anti-eNOS (Biomol International). Acetylcoenzyme A (acetylCoA) trilithium salt was obtained from Boehringer Mannheim (Mannheim, Germany), acetylCoA [acetyl-3H], specific activity 7.2 Ci/mmol, was purchased from ICN (East Hill, NY, USA) and [14C] arginine, specific activity 0.319 Ci/mmol, was purchased from New England Nuclear Research Products (Mississauga, ON, Canada) Liquid scintillation cocktail and anti-rabbit IgG-horse radish peroxidase secondary antibody was from Amersham Pharmacia Biotech (Piscataway, NJ, USA). All other chemicals were obtained from Sigma Chemical Company or VWR (Mississauga, ON, Canada).
RNA Isolation and RT-PCR amplification
Total RNA was isolated from frozen human tissue using Trizol. Extracted RNA was reverse transcribed with Thermoscript, using oligodt as the primer for 60 min at 55°C and the cDNA generated was used for PCR with Platinum Taq DNA polymerase. All PCR reactions were performed in a final volume of 50 μL with an initial strand separation at 94°C for 2 min and a final elongation for 2 min at 72°C after the last cycle. Primer pairs and cycling conditions are presented in Table 1.
PCR products were separated on 1.5% agarose gels and visualized with ethidium bromide under UV light. Semi-quantitative evaluation of relative amounts of PCR product formed was carried out by densitometric analysis [BioRad Multi-Analyst/PC version 1.1] and the value obtained for each sample was compared with the corresponding β-actin sample. The specificity of PCR products was verified by Southern blot analysis or DNA sequencing.
Determination of nitric oxide synthase activity
NOS activity in tissue samples was measured by a radiolabelled arginine to citrulline conversion assay using modifications of the method of Bredt and Snyder [52]. Frozen brain tissue was homogenized in 5 vols of ice-cold 50 mM HEPES buffer (pH 7.4) containing 0.1 mM EDTA and 10 μg/mL each of leupeptin, AEBSF and pepstatin. Duplicate aliquots of homogenates (25 μL) were incubated for 30 min at 37°C with 100 μL of reaction buffer consisting of 25 μM arginine, 5 nCi [14C] arginine, 5 μM each of FAD and FMN, 10 μM tetrahydrobiopterin, 1 μM NADPH, 1.2 mM MgCl2, 1 mM CaCl2, 1 mM valine and 10 μg/ mL calmodulin in 50 mM HEPES buffer (pH 7.4). Reactions were stopped by the addition of 500 μL ice-cold 20 mM HEPES buffer containing 2 mM EDTA (pH 5.5). Each sample was passed through a 1 mL Dowex 50WX8-400 (Na+) column to separate [14C] arginine from [14C] citrulline. The eluate and two 0.5 mL washes were collected, 10 mL of scintillation cocktail added and the resulting [14C] citrulline counted. Protein content of tissue homogenates was determined by the method of Bradford [53] and NOS activity was expressed as nmol [14C] citrulline formed/mg protein/h.
Determination of choline acetyltransferase activity
Aliquots of tissue homogenates prepared for the NOS assay were further solubilized by addition of an equal volume of ice-cold 0.1M sodium phosphate buffer (pH 7.4) containing 0.87 mM EDTA, 0.1% Triton X-100 and 0.15 mM eserine and incubated for 30 min at 4°C. ChAT activity was measured by monitoring the conversion of [3H] acetylCoA to [3H] acetylcholine (ACh) using a modification of the method of Fonnum [54]. Each supernatant sample (10 μL) was incubated with 10 μL of reaction buffer for 30 min at 37°C. The reaction buffer consisted of 0.285 M NaCl, 0.095% BSA, 0.19 mM eserine, 3.8 mM choline, 0.2 mM acetylCoA and 37 μM [3H] acetylCoA (7.2 Ci/mmole) in 0.1 M sodium phosphate buffer, pH 7.4. Each reaction was stopped by the addition of 300 μL of 3-heptanone containing 20 mg/mL sodium tetraphenylboron. Samples were vortex mixed for 30 s and centrifuged for 5 min at 5700 g. Subsequently, 200 μL of the upper organic layer was added to 3.5 mL scintillation cocktail and the radioactivity measured. The amount of protein present in tissue homogenates was determined [53] and ChAT activity was expressed as nmol [3H] ACh formed /mg protein/h.
Immunoblots
Aliquots of tissue homogenate prepared for the NOS assay were mixed with an equal volume of ice-cold RIPA buffer containing (final concentration): 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM each of EDTA, sodium fluoride, AEBSF and sodium orthovanadate and 1 μg/mL each of leupeptin, aprotinin and pepstatin and shaken on ice for 30 min. Samples were centrifuged at 17 500 g for 15 min and protein content of the supernatant measured [53]. SDS sample buffer was added to aliquots containing 100 μg of protein, boiled for 5 min and loaded onto 7.5% SDS-PAGE gels for electrophoresis. Separated proteins were transferred to nitrocellulose membranes that were then blocked in 8% non-fat milk in PBS containing 0.5% Triton X-100 for 1 h at room temperature. Primary antibody (1:2000 rabbit anti-human anti-ChAT antibody [55]) was added into fresh blocking solution and the blots incubated for 2 h at room temperature. Antibody detection was achieved using 1:2500 donkey anti-rabbit IgG-horse radish peroxidase conjugated secondary antibody followed by chemiluminescence detection (ECL, Amersham). Blots were stripped and probed for iNOS (1:2000, rabbit polyclonal anti-iNOS), nNOS (1.25 μg/mL rabbit polyclonal anti-rat nNOS) and eNOS (1:2000, mouse monoclonal anti-eNOS).
Data analysis
Enzyme activity for ChAT and NOS is presented as mean ±standard error of the mean (SEM) and parametric statistical analysis was performed using Student’s t-test to compare samples obtained from AD brain to those from age-matched controls. Semi-quantitative analysis of steady-state mRNA levels was obtained using MultiAnalyst image analysis software (BioRad). Data were calculated as the ratio of arbitrary densitometric units of ChAT, NOS or TrkA transcripts normalized to values obtained for β-actin from the same sample. Data are presented as the mean ± SEM and parametric statistical analysis was performed using Student’s t-test. Mean values were considered different if p<0.05. Correlations were assessed using Pearsons Linear correlation and were considered significant if p<0.05.
Results
Analysis of ChAT and NOS expression was performed on samples of frontal cortex, hippocampus, caudate and nbM from postmortem brain of confirmed AD subjects and age-matched controls. Neuropathological identification of AD along with age, sex, postmortem interval (PMI) and ApoE genotype was determined by the University of California, Irvine, Institute for Brain Aging and Dementia and these data are presented in Table 2.
Expression of ChAT in Alzheimer’s and control brains
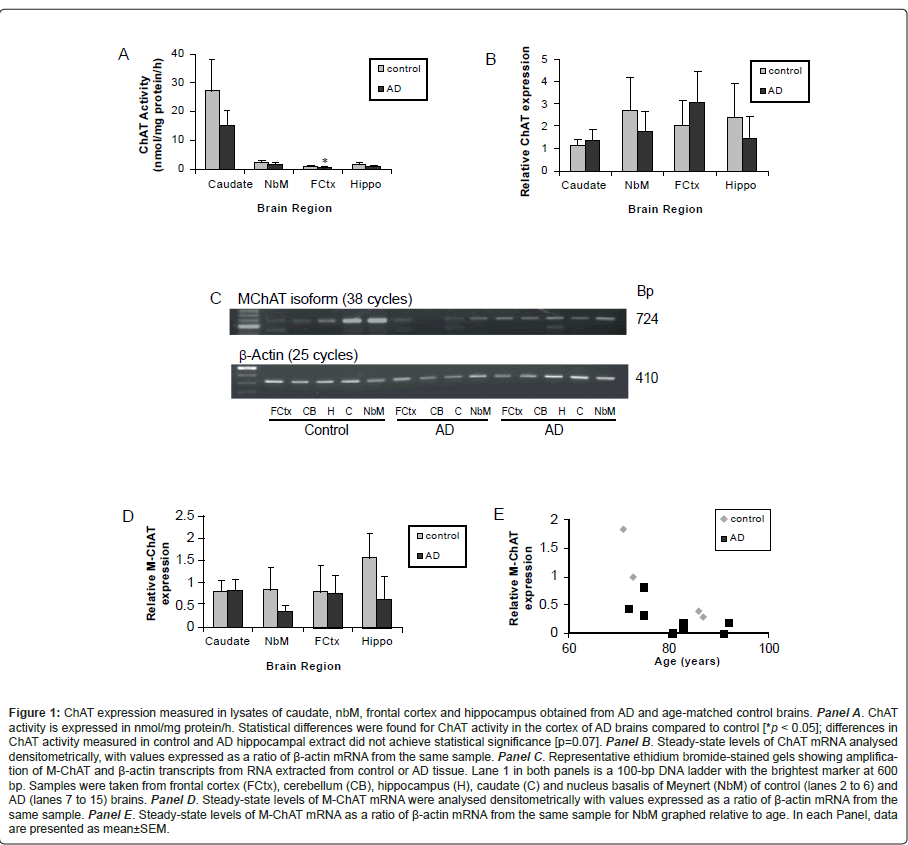
ChAT levels were assessed in brain samples by radioenzymatic assay, immunoblot analysis and RT-PCR. Figure 1A illustrates a significant decrease in ChAT activity in frontal cortex samples from individuals with confirmed AD as compared to age-matched controls. A trend towards decreased ChAT activity was found in the other brain regions, but as there was substantial variability in the activity measured from caudate samples and the sample size for hippocampal tissues from AD cases was smaller (n=6), ChAT activity values for these tissues from control and AD cases did not achieve statistical significance (p=0.07). We also evaluated if age or PMI correlated with ChAT activity, but did not find significant correlations in any of the brain regions examined for either control or AD cases (data not shown). Similar findings were observed when ChAT protein levels were assessed by immunoblot analysis. Densitometric analysis revealed similar amounts of ChAT in caudate samples obtained from control and AD brain and, while levels were variable in the nbM and hippocampus, no difference was observed between tissues obtained from control or AD brain. Of note, in contrast to what is observed in samples from control subjects, ChAT protein was generally below detectable levels using immunoblot in the frontal cortex in tissue obtained from AD brains.
We also compared steady-state levels of total ChAT mRNA (Figure 1B), as well as the M-ChAT transcript that encodes a form of the enzyme that is unique to humans [56-58]. Surprisingly, there were no significant differences between control and AD subjects when comparing total ChAT mRNA levels, but comparison of the mean densitometric values of M-ChAT mRNA levels revealed a trend towards a decrease in the nbM of AD subjects (p=0.06) (Figure 1C and D). Moreover, there was a correlation between increased age and decreased levels of M-ChAT mRNA in this brain region in tissues from both age-matched controls and AD cases (Figure 1E). No other correlations were found between total ChAT or M-ChAT mRNA and PMI or age in any of the other brain regions examined for either control or AD cases (data not shown). We compared mRNA levels and activity for ChAT and found that while total ChAT mRNA did not correlate with ChAT activity in any of the brain regions, relative M-ChAT mRNA levels correlated with ChAT activity in the caudate. We also compared ChAT activity in hippocampus and cortex to ChAT mRNA levels in nbM and again, there was no correlation between total ChAT mRNA and ChAT activity. However importantly, in AD samples, ChAT activity in frontal cortex correlated with the levels of M-ChAT transcript in nbM with the same correlation almost achieving statistical significance for hippocampus. A summary of the changes in ChAT expression in AD is presented in Table 3.
| Case | Age (yrs) | Sex | PMI (h) | ApoE genotype |
|---|---|---|---|---|
| C1 | 87 | F | 4.5 | 3 / 3 |
| C2 | 86 | F | 9.5 | 3 / 3 |
| C3 | 87 | F | 6.5 | 3 / 3 |
| C4 | 71 | F | 4.0 | 3 / 3 |
| C5 | 73 | M | 10.5 | 2 / 2 |
| AD1 | 92 | M | 2.7 | 3 / 3 |
| AD2 | 72 | F | 12.0 | N/A |
| AD3 | 75 | M | 3.0 | 3 / 4 |
| AD4 | 91 | M | 5.0 | 3 / 3 |
| AD5 | 76 | M | 16.0 | N/A |
| AD6 | 75 | F | 8.5 | N/A |
| AD7 | 72 | M | 4.25 | 3 / 3 |
| AD8 | 79 | M | 7.0 | 3 / 3 |
| AD9 | 83 | F | 6.0 | N/A |
| AD10 | 83 | M | 7.0 | N/A |
| AD11 | 75 | F | 4.25 | 3 / 4 |
| AD12 | 70 | M | 5.0 | 3 / 3 |
| AD13 | 81 | M | 5.0 | 3 / 4 |
Table 2: Patient information integrated in the present study.
Figure 1: ChAT expression measured in lysates of caudate, nbM, frontal cortex and hippocampus obtained from AD and age-matched control brains. Panel A. ChAT activity is expressed in nmol/mg protein/h. Statistical differences were found for ChAT activity in the cortex of AD brains compared to control [*p < 0.05]; differences in ChAT activity measured in control and AD hippocampal extract did not achieve statistical significance [p=0.07]. Panel B. Steady-state levels of ChAT mRNA analysed densitometrically, with values expressed as a ratio of β-actin mRNA from the same sample. Panel C. Representative ethidium bromide-stained gels showing amplification of M-ChAT and β-actin transcripts from RNA extracted from control or AD tissue. Lane 1 in both panels is a 100-bp DNA ladder with the brightest marker at 600 bp. Samples were taken from frontal cortex (FCtx), cerebellum (CB), hippocampus (H), caudate (C) and nucleus basalis of Meynert (NbM) of control (lanes 2 to 6) and AD (lanes 7 to 15) brains. Panel D. Steady-state levels of M-ChAT mRNA were analysed densitometrically with values expressed as a ratio of β-actin mRNA from the same sample. Panel E. Steady-state levels of M-ChAT mRNA as a ratio of β-actin mRNA from the same sample for NbM graphed relative to age. In each Panel, data are presented as mean±SEM.
Expression of NOS in Alzheimer’s and control brains
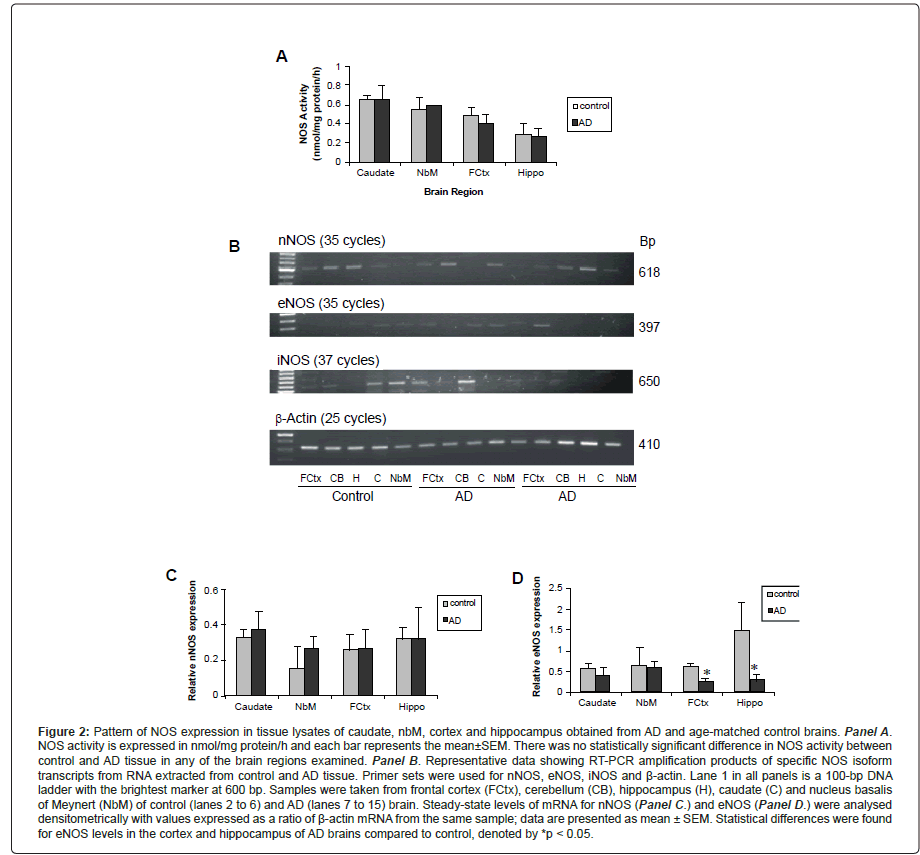
Both elevations and reductions in NOS expression in AD brain have been reported in the literature. In our study, we measured NOS activity and steady-state levels of NOS mRNA from necropsy samples obtained from the cortex, caudate, nbM and hippocampus of individuals with confirmed AD and age-matched controls, first to evaluate NOS expression and secondly to compare the levels of NOS with ChAT. As seen in Figure 2A, there was no difference in total NOS activity between control and AD tissue in any of the brain regions examined. We also assessed levels of iNOS activity (in the absence of calcium), but did not find detectable levels in any of the brain regions in samples from either control or AD brain (data not shown).
| Brain Region | ChAT activity | ChAT mRNA | ChAT protein | NOS activity | NOS protein | NOS mRNA |
|---|---|---|---|---|---|---|
| Frontal Cortex | ↓(*) | Total:↔ M-ChAT:↔ |
ND | ↔ | nNOS: ↔ eNOS:ND iNOS:↔ |
nNOS:↔ eNOS:↓(*) iNOS:ND |
| Hippocampus | ↓(+) | Total:↔ M-ChAT:↔ |
variable | ↔ | nNOS: ↔ eNOS:ND iNOS:↔ |
nNOS:↔ eNOS:↓(*) iNOS:ND |
| Nucleus basalis of Meynert | ↔ | Total:↔ M-ChAT: ↓ |
variable | ↔ | nNOS: ↔ eNOS:ND iNOS:↔ |
nNOS:↔ eNOS:↓(*) iNOS:ND |
| Caudate | ↔ | Total:↔ M-ChAT:↔ |
↔ | ↔ | nNOS: ↔ eNOS:ND iNOS:↔ |
nNOS:↔ eNOS:↓(*) iNOS:ND |
ChAT, choline acetyltransferase; NOS, nitric oxide synthase; n, neuronal; e, endothelial; I, inducible; ↓, decrease; (*), statistically significant difference; (+), difference approached statistical significance; ↔, no change compared to control tissue; ND, not detected in most/all samples.
Table 3: Summary of changes in ChAT and NOS expression in AD.
Figure 2: Pattern of NOS expression in tissue lysates of caudate, nbM, cortex and hippocampus obtained from AD and age-matched control brains. Panel A. NOS activity is expressed in nmol/mg protein/h and each bar represents the mean±SEM. There was no statistically significant difference in NOS activity between control and AD tissue in any of the brain regions examined. Panel B. Representative data showing RT-PCR amplification products of specific NOS isoform transcripts from RNA extracted from control and AD tissue. Primer sets were used for nNOS, eNOS, iNOS and Ã�-actin. Lane 1 in all panels is a 100-bp DNA ladder with the brightest marker at 600 bp. Samples were taken from frontal cortex (FCtx), cerebellum (CB), hippocampus (H), caudate (C) and nucleus basalis of Meynert (NbM) of control (lanes 2 to 6) and AD (lanes 7 to 15) brain. Steady-state levels of mRNA for nNOS (Panel C.) and eNOS (Panel D.) were analysed densitometrically with values expressed as a ratio of Ã�-actin mRNA from the same sample; data are presented as mean ± SEM. Statistical differences were found for eNOS levels in the cortex and hippocampus of AD brains compared to control, denoted by *p < 0.05.
To examine if age or PMI affected NOS activity, we evaluated the relationship between these factors in all four brain regions from both control and AD tissue samples. There was no correlation between age and NOS activity levels, but increasing PMI correlated with a decrease in NOS activity in the caudate and approached statistical significance in the frontal cortex (p=0.06). Expression of NOS protein isoforms was investigated by immunoblot analysis. In both control and AD, nNOS was present in tissue from all four brain regions, with more variable expression observed in the AD samples. Similarly, iNOS was present in all tissue samples but levels did not appear to differ between control and AD subjects. A comparison of eNOS protein levels in control and AD samples was difficult as the bands observed were either weak or not present in several of the tissue lysates. Interestingly, eNOS appeared to be absent from the frontal cortex and hippocampus from most of the AD cases studied.
Finally, NOS isoform mRNA levels were assessed with representative data depicting steady-state levels of nNOS (top panel), eNOS (upper middle panel), iNOS (lower middle panel) and β-actin (bottom panel) mRNA in frontal cortex, hippocampus, caudate and nbM shown in Figure 2B. The level of nNOS mRNA from AD tissues was similar to that of controls (Figure 2C), but the levels of eNOS were decreased in both the hippocampus and frontal cortex. There was no correlation between age or PMI and the level of nNOS or eNOS, with the exception of the caudate where increased PMI correlated with increased eNOS expression. In some samples, iNOS mRNA was not detected and the number of samples in which iNOS was observed in each of the brain regions, for control or AD tissue samples, was not large enough to make valid statistical comparisons. Interestingly, when we compared NOS mRNA isoform levels within the various brain regions, we observed an inverse correlation between the expression of nNOS and iNOS; when levels of nNOS appeared lower, iNOS bands of greater density were observed (see Figure 2B). The only significant correlation between NOS activity and steady-state mRNA levels was between eNOS expression and NOS activity in the frontal cortex. A summary of changes in NOS expression in AD is presented in Table 3.
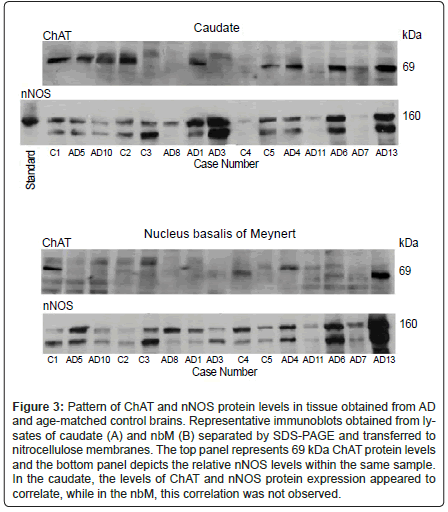
Figure 3: Pattern of ChAT and nNOS protein levels in tissue obtained from AD and age-matched control brains. Representative immunoblots obtained from lysates of caudate (A) and nbM (B) separated by SDS-PAGE and transferred to nitrocellulose membranes. The top panel represents 69 kDa ChAT protein levels and the bottom panel depicts the relative nNOS levels within the same sample. In the caudate, the levels of ChAT and nNOS protein expression appeared to correlate, while in the nbM, this correlation was not observed.
Relationship between ChAT and NOS expression in Alzheimer’s and control brains
To investigate the potential link between ChAT and NOS in the frontal cortex, hippocampus, caudate and nbM of AD and age-matched control brain, we compared the levels of ChAT and NOS activity and mRNA in these regions. A comparison of the ratios of ChAT to NOS activity between control and AD tissues revealed no difference in these values in any of the brain regions examined, but a trend towards a decrease in this ratio was observed in the hippocampus of individuals with AD (p= 0.06).
As well, there was no correlation between the level of ChAT and NOS activity in the caudate, nbM or hippocampus, but in samples of frontal cortex from both control and AD cases, there was a significant correlation between the measured enzyme activity levels. To determine if the NOS isoforms expressed in the various brain regions correlated with ChAT expression, protein levels were compared following immunoblot analysis. As shown in Figure 3 (upper panel), levels of the 160 kDa splice variant of nNOS appeared to correlate with ChAT protein levels in the caudate from both control and AD samples. Levels of 160 kDa nNOS protein did not correlate with ChAT protein expression in the nbM (Figure 3, lower panel) cortex or hippocampus. As seen in Figure 3, a second band was consistently observed on blots probed for nNOS. There are four splice variants of human nNOS (reviewed in [59]). The most abundant is nNOSα which corresponds with the 160 kDa band observed in the nNOS standard and with the upper band depicted on the nNOS blots in Figure 3. Although the identity of the lower band was not determined, it is possible that it could be made up of one or both of the two smaller molecular mass splice variants, nNOSβ and nNOSγ, which are 136 and 125 kDa respectively. Levels of this smaller band did not correlate with nNOSα or with ChAT expression in either the caudate or nbM, and the levels of this protein were variable in both control and AD samples. For all other NOS isoforms in all other brain regions, there was no correlation between the levels of NOS and ChAT protein. At the level of mRNA expression, the only correlations detected were in the caudate, where increasing levels of steady-state ChAT mRNA corresponded to increasing levels of eNOS transcript.
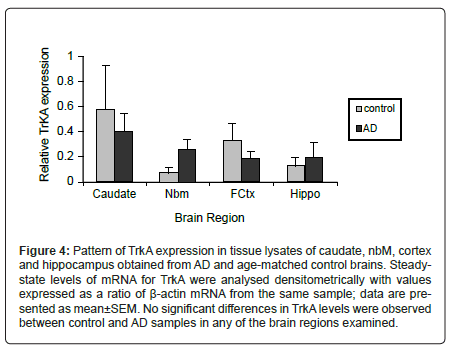
Figure 4: Pattern of TrkA expression in tissue lysates of caudate, nbM, cortex and hippocampus obtained from AD and age-matched control brains. Steadystate levels of mRNA for TrkA were analysed densitometrically with values expressed as a ratio of Ã�-actin mRNA from the same sample; data are presented as mean±SEM. No significant differences in TrkA levels were observed between control and AD samples in any of the brain regions examined.
Relationship between TrkA mRNA levels and ChAT and NOS expression
Since TrkA levels were reported to be decreased in basal forebrain neurons of AD subjects [18-20] and NGF-TrkA signalling regulates expression of NOS [21-24] and ChAT [21,60-65], we also compared steady-state mRNA levels of TrkA, ChAT and NOS isoforms. Figure 4 depicts ratios of the densitometric analysis of TrkA mRNA normalized to β-actin. No significant difference in relative TrkA transcript levels was observed between control and AD cases in any of the brain regions examined. There was no correlation between age or PMI and the level of TrkA in the four brain regions examined or between the levels of ChAT or NOS mRNA isoforms and TrkA. The only significant correlation observed was between TrkA and eNOS levels in the frontal cortex.
Expression of ChAT and NOS in AD patients with and without an ApoE ε4 allele
To evaluate the impact of ApoE genotype on the levels of ChAT and NOS in the frontal cortex, hippocampus, caudate and nbM, enzyme activities and steady-state mRNA levels measured from AD brains were compared from individuals with and without an ApoE4 allele. There was no statistically significant difference in ChAT mRNA levels or activity in any of the brain regions examined, but a trend towards an increase in ChAT activity was observed in the hippocampus of AD subjects having one E4 allele (3/3 = 0.43±0.11, 3/4 = 1.87±0.84, p=0.07). As well, there was no statistically significant difference between the levels of NOS mRNA isoforms or activity in the caudate, nbM or hippocampus, but in samples of the frontal cortex a trend towards decreased NOS activity was observed in individuals with one E4 allele (3/3 = 0.68±0.05, 3/4 = 0.37±0.20, p=0.07).
Discussion
The present study examined the relationship between ChAT and NOS expression in necropsy samples of nbM, hippocampus, frontal cortex and caudate from confirmed cases of AD and age-matched controls. Our results support previous findings of a decrease in ChAT activity in the cortex of AD patients, with a similar trend observed in the hippocampus. In contrast to previous reports [66-67], we did not detect a decrease in total ChAT mRNA levels in samples obtained from the nbM of AD patients, but a trend towards a reduction in the M-ChAT transcript that encodes both 69- and 82-kDa forms of ChAT was observed. Interestingly, this decrease in M-ChAT mRNA levels also correlated with increasing age. We also did not detect differences in NOS activity between control and AD tissue in any of the brain regions examined. However, PMI may play a critical role in the evaluation of NOS activity, as we found an inverse correlation between increasing PMI and NOS activity in the caudate; a similar trend was observed with cortical NOS activity. While we did not observe differences in nNOS and iNOS mRNA and protein levels between control and AD tissue in any of the brain regions examined, eNOS appeared to decrease in the frontal cortex and hippocampus, with statistically significant decreases in eNOS mRNA levels detected in AD tissue in both of these brain regions. In general, there was no correlation between the levels of NOS and ChAT in control or AD patients in any of the brain regions examined, but a reduction in eNOS levels in the projection areas of basal forebrain cholinergic neurons suggests there is an association between eNOS containing cells and cholinergic neurons in AD.
Reductions in ChAT expression in AD have been described previously [5-8] and in general, our findings are consistent with these reports. In frontal cortex, ChAT activity was significantly lower in AD patients when compared to controls. A similar trend was observed in hippocampus, but this was not statistically significant (p=0.07) likely due to the smaller number of hippocampal samples available relative to the other brain regions examined. ChAT expression was also evaluated at the mRNA level. A critical finding is that, while there was no change detected in total ChAT mRNA levels in nbM, lower levels of M-ChAT transcript were observed (p=0.06) and the level of M-ChAT mRNA correlated with the loss of enzyme activity in frontal cortex. We showed recently that primate-specific 82-kDa ChAT is found in the nuclei of cholinergic neurons in human brain and spinal cord, and compared the subcellular localization patterns and temporal expression of this cholinergic protein in necropsy brain of control subjects of varying ages and AD patients [68]. Importantly, the decline in M-ChAT transcript found in the present study extends our previous findings as we observed an age-related decline in 82-kDa ChAT protein encoded by the M-ChAT mRNA in brain cholinergic neurons between birth and the eighth decade of life and in mild cognitive impairment and AD; this age-related change was associated with reduced nuclear localization of 82-kDa ChAT [68]. A larger sample size would be needed to determine whether M-ChAT transcript levels in the nbM correlate with the expression of 69 and 82-kDa ChAT protein in the hippocampus and cortex of control individuals and AD patients.
In contrast to ChAT, NOS activity was not altered in AD patients in any of the brain regions examined. However, increasing PMI correlated with decreasing NOS activity in tissue obtained from both AD patients and age-matched controls indicating that caution must be taken when interpreting NOS activity data from cases with very different PMIs. When examining the specific isoforms of NOS, we did not detect differences between control and AD tissue for expression of nNOS or iNOS at either the mRNA or protein level in the brain regions examined. These data support the findings of Hyman et al. [25] who reported that in AD, hippocampal NOS-containing neurons were relatively spared, but appear to contrast several others that found alterations in NOS isoform expression. A number of studies observed an increase in the number of nNOS and iNOS positive neurons [25-27], while Norris et al. [33] and Thorns et al. [69] detected a reduction in nNOS-containing neurons in cortex and hippocampus of AD brains. A similar decrease in nNOS immunoreactive neuron number was also reported by Yew et al. [35] but an increase in staining intensity was observed, suggesting that in AD, nNOS levels in surviving neurons may be increased. While our results indicate there was no overall change in the tissue levels of nNOS or iNOS expression, it is possible that the changes in the level of these isoforms occur within individual neurons, astrocytes and glia and that these alterations are a consequence of or contribute to AD progression. In contrast to nNOS and iNOS, we observed a significant decrease in eNOS expression in hippocampus and cortex of tissue from AD patients. These data support the findings of de la Monte and Bloch [34] who detected a decrease in cortical neuronal eNOS levels in endstage AD, but these authors also detected elevations in eNOS in glial cells. While we do not know whether the reduction in eNOS detected in our samples results from changes in neuronal, endothelial or glial cells, these results indicate that eNOS levels are altered in late-onset AD. Since the reduction was only detected in the projection areas of basal forebrain cholinergic neurons, this suggests an interaction between basal forebrain cholinergic neurons and eNOS-containing cells of the hippocampus and cortex.
We examined the relationship between NOS and ChAT in AD further by comparing the mRNA and protein levels of these enzymes in frontal cortex, hippocampus, caudate and nbM in AD and age-matched control brain. With respect to NOS and ChAT activity, we found no difference in the ratio of these enzymes between control and AD tissue in the brain regions examined and the only significant correlation between the activity levels of the two enzymes was observed in frontal cortex samples of both control and AD brain. As well, few correlations were observed at the RNA and protein level for specific NOS isoforms and ChAT. Finally, since NGF-mediated TrkA activation increases NOS and ChAT expression, we compared the steady-state mRNA levels of TrkA, ChAT and NOS isoforms. We found no difference in the level of TrkA mRNA in tissue from AD and control brains, and the only correlation observed was between TrkA and eNOS mRNA levels in cortex. Taken together, these data suggest that ChAT and NOS are not regulated together in cholinergic neurons of the nbM, but we did not examine the location of the NOS isoforms and levels of expression within the cholinergic neurons themselves.
In the basal forebrain of rodents, colocalization of ChAT, NOS and TrkA has been reported, with the proportion of colocalization varying between regions. The majority of NOS-containing neurons in rat basal forebrain are also immunopositive for TrkA, but many of the TrkApositive neurons do not stain for NOS [70]. The proportion within the nbM is lower, with approximately one-third of NOS-positive cells exhibiting TrkA immunoreactivity [70]. Similarly, a proportion of basal forebrain cholinergic neurons also express NOS, with higher levels of colocalization occurring in the substantia innominata and medial septum as compared to the nbM [71-73]. Since the proportion of TrkAresponsive cholinergic neurons in the nbM that also express NOS is small, co-regulation of these enzymes by NGF will only occur in a limited subpopulation of cells. This could explain the lack of correlation between ChAT and NOS levels in the nbM in our study. While the majority of cholinergic neurons in the nbM do not express NOS, the coordinated regulation of ChAT and NOS in a small population of neurons in this brain region may be important for the modulation of cortical neuron function and blood flow [38,39].
This subpopulation of cholinergic neurons may also exhibit differences with respect to neurodegeneration and alterations ChAT isoform levels. We did not detect a difference in overall expression of ChAT mRNA but, compared to control, the decrease in steady-state levels of M-ChAT in the nbM of AD samples approached significance (p=0.06). We reported previously that in PC12 cells, NO modulates NGF-signalling [74] and is necessary for NGF-mediated increases in ChAT expression [21]. In the human brain, the presence of NOS in a subpopulation of cholinergic cells could lead to important differences in cell signalling and/or the transcription of 69- and 82-kDa ChAT. While no correlations between ChAT and NOS activity were observed in the projection regions of basal forebrain cholinergic neurons, we detected significant decreases in eNOS mRNA levels in both hippocampus and frontal cortex. In the cortex, cholinergic neurons project onto NOS immunoreactive neurons and regulate local cerebral blood flow [38-40,75]. Similarly, cholinergic neurons project from the medial septum and diagonal band to the hippocampus and stimulation of these pathways increases hippocampal blood flow [76]. As well, lesioning of the basal forebrain cholinergic neurons reduces NOS catalytic activity in hippocampal and cortical regions [38]. Although nNOS protein levels were not affected in these animals, other isoforms of NOS were not investigated. Since eNOS also plays a role in basal forebrain-mediated regulation of cerebral blood flow [77], it is possible that in end-stage AD, prolonged loss of cholinergic input affects eNOS levels in cortex and hippocampus. It has been suggested that changes in cholinergic input not only alters local blood flow but may affect the activity of neurons in these brain regions as well [38]. Therefore, loss of eNOS may be occurring in neurons as well as endothelial cells.
Since NO plays a role in cholinergic neuron function and is implicated in AD pathogenesis, it is important to understand the relationship between ChAT and NOS in the brain. The results from this study suggest that the levels of NOS and ChAT isoforms are regulated independently in cortex, hippocampus and nbM in AD. However, a reduction in eNOS levels in the projection regions of basal forebrain cholinergic neurons suggests that loss of cholinergic input could affect eNOS levels, ultimately altering local cerebral blood flow. Also, this study evaluated NOS and ChAT levels in specific brain regions. Modest changes in NO levels in neurons and surrounding cells may have important influences on cholinergic neuron function. Examining the levels of NOS and ChAT isoforms within cells could yield critical information regarding the role of NO in the neurodegeneration observed in AD.
Acknowledgements
This research was funded by operating grants to RJR from the CIHR and Alzheimer Society of Canada. BEK was the recipient of a Fellowship from CIHRAlzheimer Society of Canada-Astra Zeneca. Special thanks to Dr. David W. Mercer from the University of Texas-Huston Medical School who provided the iNOS antibody used in this study.
References
- Selkoe DJ (2004) Alzheimer Disease: Mechanistic understanding predicts novel therapies. Ann Inter Med 140: 627-638.
- Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298: 789-791.
- Sims NR, Bowen DM, Smith CC, Flack RH, Davison AN, et al. (1980) Glucose metabolism and acetylcholine synthesis in relation to neuronal activity in Alzheimer's disease. Lancet 1: 333-336.
- Ikonomovic MD, Abrahamson EE, Isanski BA, Mufson EJ, Dekosky ST (2007) Superior frontal cortex cholinergic axon density in mild cognitive impairment and early Alzheimer disease. Arch Neurol 64: 1312-1317.
- Collerton D (1986) Cholinergic function and intellectual decline in Alzheimerââ�¬â�¢s disease. Neurosci 19: 1-28.
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, et al. (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Mol Brain Res 1: 53-62.
- Wilcock GK, Esiri MM, Bowen DM, Smith CC (1982) Alzheimer's disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci 57: 407-417.
- Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ (2000) Choline acetyltransferase activity and cognitive domain scores of Alzheimer's patients. Neurobiol Aging 21: 11-17.
- Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, et al. (1995) Neurochemical correlates of dementia severity in Alzheimerââ�¬â�¢s disease: relative importance of the cholinergic deficits. J Neurochem 64: 749-760.
- Samuel W, Alford M, Hofstetter CR, Hansen L (1997) Dementia with Lewy bodies versus pure Alzheimer disease: differences in cognition, neuropathology, cholinergic dysfunction, and synapse density. J Neuropathol Exp Neurol 56: 499-508.
- Chen S, Bentivoglio M (1993) Nerve growth factor receptor-containing cholinergic neurons of the basal forebrain project to the thalamic reticular nucleus in the rat. Brain Res 606: 207-212.
- Cuello AC, Maysinger D, Garafalo L (1993) Trophic factor effects on cholinergic innervation in the cerebral cortex of the adult rat brain. Mol Neurobiol 6: 451-461.
- Hefti W, Weiner WJ (1986) Nerve growth factor and Alzheimerââ�¬â�¢s disease. Ann Neurol 20: 275-281.
- Lapchak PA (1993) Nerve growth factor pharmacology: application to the treatment of cholinergic neurodegeneration in Alzheimer's disease. Exp Neurol 124: 16-20.
- Mufson EJ, Conner JM, Kordower JH (1995) Nerve growth factor in Alzheimer's disease: defective retrograde transport to nucleus basalis. Neuroreport 6: 1063-1066.
- Whittemore SR, Seiger A (1987) The expression, localization and functional significance of beta-nerve growth factor in the central nervous system. Brain Res 434: 439-464.
- Scott SA, Mufson EJ, Weingartner JA, Skau KA, Crutcher KA (1995) Nerve growth factor in Alzheimerââ�¬â�¢s disease: increased levels throughout the brain coupled with declines in nucleus basalis. J Neurosci 15: 6213-6221.
- Boissiere F, Faucheux B, Ruberg M, Agid Y, Hirsch EC (1997) Decreased TrkA gene expression in cholinergic neurons of the striatum and basal forebrain of patients with Alzheimer's disease. Exp Neurol 145: 245-252.
- Ginsberg SD, Che S, Wuu J, Counts SE, Mufson E (2006) Down regulation of trk but no p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimerââ�¬â�¢s disease. J Neurochem 97: 475-487.
- Mufson EJ, Li JM, Sobreviela T, Kordower JH (1996) Decreased trkA gene expression within basal forebrain neurons in Alzheimer's disease. Neuroreport 8: 25-29.
- Kalisch BE, Bock NA, Davis W, Rylett RJ (2002) Inhibitors of nitric oxide synthase attenuate nerve growth factor-mediated increases in choline acetyltransferase gene expression in PC12 cells. J Neurochem 81: 624-635.
- Peunova N, Enikolopov G (1995) Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature 375: 68-73.
- Holtzman DM, Le S, Li Y, Chua-Couzens J, Xia H, et al. (1996) Expression of neuronal-NOS in developing basal forebrain cholinergic neurons: regulation by NGF. Neurochem Res 21: 861-868.
- Holtzman DM, Kilbridge J, Bredt DS, Black SM, Li Y, et al. (1994) NOS induction by NGF in basal forebrain cholinergic neurones: evidence for regulation of brain NOS by a neurotrophin. Neurobiol Dis 1: 51-60.
- Hyman BT, Marzloff K, Wenniger JJ, Dawson TM, Bredt DS, et al. (1992) Relative sparing of nitric oxide synthase-containing neurons in the hippocampal formation in Alzheimer's disease. Ann Neurol 32: 818-820.
- Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Encinas JM, Serrano J, et al. (2004) Expression of nitric oxide system in clinically evaluated cases of Alzheimer's disease. Neurobiol Dis 15: 287-305.
- Luth HJ, Holzer M, Gertz HJ, Arendt T (2000) Aberrant expression of nNOS in pyramidal neurons in Alzheimer's disease is highly co-localized with p21ras and p16INK4a. Brain Res 852: 45-55.
- Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie QW, et al. (1996) Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J Exp Med 184: 1425-1433.
- Dildar K, Sinem F, Gokhan E, Orhan Y, Filiz M (2010) Serum nitrosative stress levels are increased in Alzheimer disease but not in vascular dementia. Alzheimer Dis Assoc Disord 24: 194-197.
- Hu J, El-Fakahany EE (1993) Beta-amyloid activates nitric oxide synthase in neuronal clone. Neuroreport 4: 760-764.
- Rossi F, Bianchini E (1996) Synergistic induction of nitric oxide by beta-amyloid and cytokines in astrocytes. Biochem Biophys Res Commun 225: 474-478.
- Wallace MN, Geddes JG, Farquhar DA, Masson MR (1997) Nitric oxide synthase in reactive astrocytes adjacent to beta-amyloid plaques. Exp Neurol 144: 266-272.
- Norris PJ, Faull RL, Emson PC (1996) Neuronal nitric oxide synthase (nNOS) mRNA expression and NADPH-diaphorase staining in the frontal cortex, visual cortex and hippocampus of control and Alzheimer's disease brains. Brain Res Mol Brain Res 41: 36-49.
- de la Monte SM, Bloch KD (1997) Aberrant expression of the constitutive endothelial nitric oxide synthase gene in Alzheimer disease. Mol Chem Neuropathol 30: 139-159.
- Yew DT, Wong HW, Li WP, Lai HW, Yu WH (1999) Nitric oxide synthase neurons in different areas of normal aged and Alzheimer's brains. Neuroscience 89: 675-686.
- Jeynes B, Provias J (2009) Significant negative correlations between capillary expressed eNOS and Alzheimer lesion burden. Neurosci Lett 463: 244-248.
- Hartlage-Rubsamen M, Apelt J, Schliebs R (2001) Fibrillary beta-amyloid deposits are closely associated with atrophic nitric oxide synthase (NOS)-expressing neurons but do not upregulate the inducible NOS in transgenic Tg2576 mouse brain with Alzheimer pathology. Neurosci Lett 302: 73-76.
- Hartlage-Rubsamen M, Schliebs R (2001) Rat basal forebrain cholinergic lesion affects neuronal nitric oxide synthase activity in hippocampal and neocortical target regions. Brain Res 889: 155-164.
- Tong XK, Hamel E (1999) Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer's disease. Neuroscience 92: 163-175.
- Vaucher E, Linville D, Hamel E (1997) Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience79: 827-836.
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, et al. (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278: 1349-1356.
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, et al. (1993) Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43: 1467-1472.
- Allen SJ, Gowan SH, Tyler GK, Robertson AG, Holden PH, et al. (1997) Reduced cholinergic function in normal and Alzheimer's disease brain is associated with apolipoprotein E4 genotype. Neurosci Lett 239: 33-36.
- Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, et al. (1995) Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci USA 92: 12260-12264.
- Soininen H, Kosunen O, Helisalmi S, Mannermaa A, Paljarvi L, et al. (1995) A severe loss of choline acetyltransferase in the frontal cortex of Alzheimer patients carrying apolipoprotein epsilon 4 allele. Neurosci Lett 187: 79-82.
- Brown CM, Wright E, Colton CA, Sullivan PM, Laskowitz DT, et al. (2002) Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med 32: 1071-1075.
- Colton CA, Brown CM, Cook D, Needham LK, Xu Q, et al. (2002) APOE and the regulation of microglial nitric oxide production: a link between genetic risk and oxidative stress. Neurobiol Aging 23: 777-785.
- Colton CA, Brown CM, Czapiga M, Vitek MP (2002) Apolipoprotein-E allele-specific regulation of nitric oxide production. Ann N Y Acad Sci 962: 212-225.
- Colton CA, Czapiga M, Snell-Callanan J, Chernyshev ON, Vitek MP (2001) Apolipoprotein E acts to increase nitric oxide production in macrophages by stimulating arginine transport. Biochim Biophys Acta 1535: 134-44.
- Czapiga M, Colton CA (2003) Microglial function in human APOE3 and APOE4 transgenic mice: altered arginine transport. J Neuroimmunol 13: 44-51.
- Ramassamy C, Averill D, Beffert U, Theroux L, Lussier-Cacan S, et al. (2000) Oxidative insults are associated with apolipoprotein E genotype in Alzheimer's disease brain. Neurobiol Dis 7: 23-37.
- Bredt DS, Snyder SH (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87: 682-685.
- Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Fonnum F (1968) Choline acetyltransferase binding to and release from membranes. Biochem J 109: 389-398.
- Dobransky T, Davis WL, Xiao GH, Rylett RJ (2000) Expression, purification and characterization of recombinant human choline acetyltransferase: phosphorylation of the enzyme regulates catalytic activity. Biochem J 349: 141-151.
- Misawa H, Matsuura J, Oda Y, Takahashi R, Deguchi T (1997) Human choline acetyltransferase mRNAs with different 5'-region produce a 69-kDa major translation product. Brain Res Mol Brain Res 44: 323-333.
- Oda Y, Nakanishi I, Deguchi T (1992) A complementary DNA for human choline acetyltransferase induces two forms of enzyme with different molecular weights in cultured cells. Brain Res Mol Brain Res 16: 287-294.
- Toussaint JL, Geoffroy V, Schmitt M, Werner A, Garnier JM, et al. (1992) Human choline acetyltransferase (CHAT): partial gene sequence and potential control regions. Genomics 12: 412-416.
- Steinert JR, Chernova T, ForsytheI D (2010) Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16: 435-452.
- Baskey JC, Kalisch BE, Davis WL, Meakin SO, Rylett RJ (2002) PC12nnr5 cells expressing TrkA receptors undergo morphological but not cholinergic phenotypic differentiation in response to NGF. J Neurochem 80: 501-511.
- Cavicchioli L, Flanigan TP, Dickson JG, Vantini G, Dal Toso R, et al. (1991) Choline acetyltransferase messenger RNA expression in developing and adult rat brain: regulation by nerve growth factor. Brain Res Mol Brain Res 9: 319-325.
- Higgins GA, Koh S, Chen KS, Gage FH (1989) NGF induction of NGF receptor gene expression and cholinergic neuronal hypertrophy within the basal forebrain of the adult rat. Neuron 3: 247-256.
- Lorenzi MV, Knusel B, Hefti F, Strauss WL (1992) Nerve growth factor regulation of choline acetyltransferase gene expression in rat embryo basal forebrain cultures. Neurosci Lett 140: 185-188.
- Pongrac JL, Rylett RJ (1998) NGF-induction of the expression of ChAT mRNA in PC12 cells and primary cultures of embryonic rat basal forebrain. Mol Brain Res 62: 25-34.
- Tian X, Sun X, Suszkiw JB (1996) Developmental age-dependent upregulation of choline acetyltransferase and vesicular acetylcholine transporter mRNA expression in neonatal rat septum by nerve growth factor. Neurosci Lett 209: 134-136.
- Boissiere F, Faucheux B, Agid Y, Hirsch EC (1997) Choline acetyltransferase mRNA expression in the striatal neurons of patients with Alzheimerââ�¬â�¢s disease. Neurosci Lett 225: 169-172.
- Strada O, Vyas S, Hirsch EC, Ruberg M, Brice A, et al. (1992) Decreased choline acetyltransferase mRNA expression in the nucleus basalis of Meynert in Alzheimer disease: an in situ hybridization study. Proc Natl Acad Sci USA 89: 9549-9553.
- Gill S, Ishak M, Dobransky T, Hartoununian V, Davis K, et al. (2007) 82-kDa choline acetyltransferase is in nuclei of cholinergic neurons in human CNS and altered in aging and Alzheimer disease. Neurobiol Aging 28: 1028-1040.
- Thorns V, Hansen L, Masliah E (1998) nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer's disease. Exp Neurol 150: 14-20.
- Sobreviela T, Jaffar S, Mufson EJ (1998) Tyrosine kinase A, galanin and nitric oxide synthase within basal forebrain neurons in the rat. Neuroscience 87: 447-461.
- Sugaya K, McKinney M (1994) Nitric oxide synthase gene expression in cholinergic neurons in the rat brain examined by combined immunocytochemistry and in situ hybridization histochemistry. Brain Res Mol Brain Res 23: 111-125.
- Kitchener PD, Diamond J (1993) Distribution and colocalization of choline acetyltransferase immunoreactivity and NADPH diaphorase reactivity in neurons within the medial septum and diagonal band of Broca in the rat basal forebrain. J Comp Neurol 335: 1-15.
- Pasqualotto BA, Vincent SR (1991) Galanin and NADPH-diaphorase coexistence in cholinergic neurons of the rat basal forebrain. Brain Res 551: 78-86.
- Kalisch BE, Demeris CC, Isak M, Rylett RJ (2003) Modulation of nerve growth factor-induced activation of MAP kinase in PC12 cells by inhibitors of nitric oxide synthase. J Neurochem 87: 1321-1332.
- Sato A, Sato Y, Uchida S (2001) Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Int J Dev Neurosci 19: 327-337.
- Cao WH, Inanami O, Sato A, Sato Y (1989) Stimulation of the septal complex increases local cerebral blood flow in the hippocampus in anesthetized rats. Neurosci Lett 107: 135-140.
- Zhang F, Xu S, Iadecola C (1995) Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience 69: 1195-1204.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15661
- [From(publication date):
March-2012 - Nov 05, 2025] - Breakdown by view type
- HTML page views : 10925
- PDF downloads : 4736