Review Article Open Access
The Molecular Pathophysiology, Differential Diagnosis, and Treatment of MPO Deficiency
Rongqin Ren1, Yuri Fedoriw2 and Monte S. Willis2,3*1Department of Pathology, East Carolina University, Greenville, NC, USA
2Department of Pathology & Laboratory Medicine, University of North Carolina, Chapel Hill, NC, USA
3McAllister Heart Institute, University of North Carolina, Chapel Hill, NC, USA
- *Corresponding Author:
- Monte S. Willis
McAllister Heart Institute
University of North Carolina at Chapel Hill
2340B Medical Biomolecular Research Building
103 Mason Farm Road, Chapel Hill, NC 27599-7525, USA
Tel: (919) 843-1938
Fax: (919) 843-4585
E-mail: monte_willis@med.unc.edu
Received date: December 13, 2011; Accepted date: March 28, 2012; Published date: March 29, 2012
Citation: Ren R, Fedoriw Y, Willis MS (2012) The Molecular Pathophysiology, Differential Diagnosis, and Treatment of MPO Deficiency. J Clinic Experiment Pathol 2:109. doi:10.4172/2161-0681.1000109
Copyright: © 2012 Ren R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Keywords
Myeloperoxidase; Pathophysiology; Recurrent infections; Phagocytes; Neutrophils; Laboratory testing; Molecular testing
Introduction
Myeloperoxidase (MPO) is an enzyme found in the azurophilic granules of neutrophils and in monocyte lysosomes, and is one of many enzymes involved in defense against common infections, such as fungi and bacteria. Myeloperoxidase was originally discovered in neutrophils in 1868 [1]; it was used in the differential diagnosis of myeloid and lymphoid leukemias beginning in 1916 [2] and first isolated in 1941 [3]. Beyond its use diagnostically, it was not until 1954, 1963, and 1966 that the first cases of human MPO deficiency were reported that could be localized to neutrophils and monocytes [4,5]. Initially this disease was believed to be quite rare; it wasn’t until large scale analysis of white cells using hemocytometers dependent upon MPO staining, was it realized that MPO deficiency is quite common. Complete MPO deficiency is found in 1 in 4000 people; partial MPO defects are found in 1 in 2000 individuals in the United States [6-9]. Despite this high prevalence, greater than 95% of people with this disorder are asymptomatic throughout their lifetime.
Until recently, MPO deficiency has been classified as a primary immunodeficiency. However, in 2005, the International Union of Immunological Societies Primary Immunodefiency Diseases Classification Committee omitted MPO deficiency because of its “marginal clinical relevance” [10]. However, in a small percentage of patients (<5%) with MPO deficiency, serious infections have been reported from Candida species, which can present as mucocutaneous, meningeal, bone, and even disseminated infections. Other studies have reported severe Staphylococcus aureus infections after injuries or surgical procedures and inflammatory diseases. Patients with diabetes mellitus or cancer have been linked to increased susceptibility to Candida infections in the context of MPO deficiency. So while MPO deficiency is rarely a problem, it potentially can be serious. In this review, we outline the detection of MPO deficiency in the laboratory, present an overview of the anti-microbial systems in phagocytic cells, and discuss the biosynthesis and process of MPO in the context of hereditary mutations. We then discuss the clinical manifestations and treatment of MPO deficiency, focusing on the differential diagnosis of similarly presenting diseases.
Detection of MPO Deficiency in the Laboratory
Until the 1970’s, only 15 cases of MPO deficiency had been reported. The use of automated flow cytometry and other modern laboratory techniques have allowed the screening of large study populations to determine a more accurate prevalence of MPO deficiency [11]. Patients with MPO deficiency are most commonly detected incidentally through routine screening tests in the clinical lab and present without any symptoms. The use of MPO staining was initially utilized in the determination of the cell lineages of immature cells in acute leukemias more than 30 years ago. Since MPO is found exclusively in the neutrophil lineage, the presence of MPO allows the differentiation of acute myeloid leukemia (AML) of granulocytic lineage from acute lymphoblastic leukemia and other AML’s of monocytic, erythroid, and megakaryocytic lineages. This staining characteristic has been adapted to many of the currently used automated hemocytometers, which use MPO staining to determine the WBC differential [12]. These automated systems stain white blood cells with 4-chloro-1-naphthol to label MPO, which are then analyzed by flow cytometry for size, complexity, and the presence of peroxidase activity [13]. Many hematology analyzers using this technique identify MPO deficiency by the identification of increased large unstained cells (LUCs), which are large cells with a low intensity of MPO staining. These large unstained cells can represent blasts, variant and atypical lymphocytes, or more commonly as patients with MPO deficiency, where their large neutrophils have decreased MPO activity. The presence of LUCs allows clinicians to differentiate the possibility of a hematological malignancy, activated lymphocytes, or the presence of MPO deficient patients in the larger clinical context.
Overview of Anti-microbial Systems in Phagocytic Cells
MPO is found in both neutrophils and monocytes. The original description of MPO was made by Agner [3], who identified this protein from the pus of a dog. His initially called it verdoperoxidase, since it gave both the pus and neutrophils their green color [3]. Neutrophil lysosomes predominantly contain MPO, in addition to other antimicrobial proteins. As such, MPO accounts for 2-5% of the total cellular protein in neutrophils (2-4 g per million cells) [14].
Neutrophils
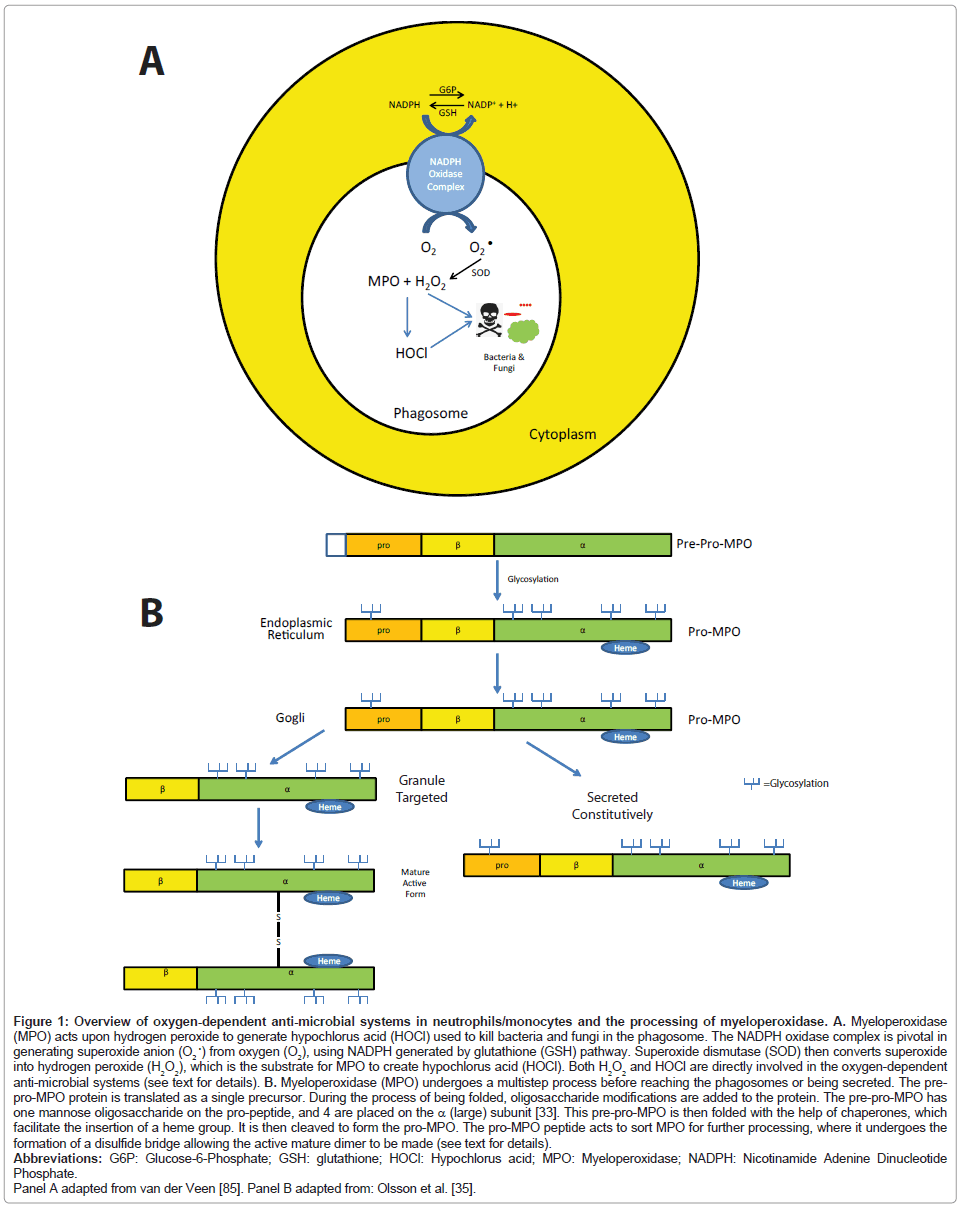
Polymorphonuclear neutrophils (PMNs) are an important component of the innate immune system essential for optimal antimicrobial defense [15]. Mature PMNs contain two types of granules based on cytochemical staining: 1) peroxidase negative; and 2) peroxidase positive (azurophilic) granules [16]. The peroxidasenegative granules are composed of the formyl peptide receptor, fusogenic proteins (secretory carrier membrane protein and vesicleassociated membrane protein-2), β2 integrins, the NADPH oxidase component flavocytochrome b558, and urokinase-type plasminogenactivating receptor [17-22]. Peroxidase-positive (azurophilic) granules contain MPO, elastase and cathepsins (proteases), and other direct mediators of anti-microbial activity including azurocidin, defensins, and bactericidal-permeability-increasing protein [16]. When pathogens are taken up by granulocytes and placed into phagosomes, granules fuse and release their contents into the phagosome. NADPH produces reactive oxygen species which are converted to H2O2 by superoxide dismutase, which kill ingested microbes in an oxygen-dependent manner (summarized in Figure 1A). MPO, a unique member of the peroxidase family, is capable of converting H2O2 to hypochlorous acid by oxidizing Cl- to Cl+ (hypochlorous acid). The MPO-Hydrogen peroxide-Cl system is thought to be a prominent response in the terminating the respiratory burst in neutrophils as individuals with MPO deficiency have prolonged respiratory bursts. The importance of the MPO reaction forming hypochlorous acid in pathogen killing is also highlighted in neutrophils from patients with MPO deficiency. MPO deficient neutrophils have a pathogen killing time that is twice as long compared to neutrophils from control patients in vitro [23]. MPO also functions in the production of tyrosyl radical production, chlorination, generation of tyrosine peroxide, mediates myeloid cell adhesion through a β2 integrin mechanism [24-27], and is involved in the oxidation of lipoproteins found in the serum, as discussed in more detail below.
Figure 1: Overview of oxygen-dependent anti-microbial systems in neutrophils/monocytes and the processing of myeloperoxidase. A. Myeloperoxidase (MPO) acts upon hydrogen peroxide to generate hypochlorus acid (HOCl) used to kill bacteria and fungi in the phagosome. The NADPH oxidase complex is pivotal in generating superoxide anion (O2 •) from oxygen (O2), using NADPH generated by glutathione (GSH) pathway. Superoxide dismutase (SOD) then converts superoxide into hydrogen peroxide (H2O2), which is the substrate for MPO to create hypochlorus acid (HOCl). Both H2O2 and HOCl are directly involved in the oxygen-dependent anti-microbial systems (see text for details). B. Myeloperoxidase (MPO) undergoes a multistep process before reaching the phagosomes or being secreted. The prepro- MPO protein is translated as a single precursor. During the process of being folded, oligosaccharide modifications are added to the protein. The pre-pro-MPO has one mannose oligosaccharide on the pro-peptide, and 4 are placed on the a (large) subunit [33]. This pre-pro-MPO is then folded with the help of chaperones, which facilitate the insertion of a heme group. It is then cleaved to form the pro-MPO. The pro-MPO peptide acts to sort MPO for further processing, where it undergoes the formation of a disulfide bridge allowing the active mature dimer to be made (see text for details).
Monocytes
Like neutrophils, monocytes contain MPO-positive granules in lysosomes. MPO-granules form during the promonocytic stage in the bone marrow, and are easily detectable in circulating monocytes, although there are generally only 1/3 the amount found in neutrophils [28]. As monocytes differentiate into macrophages in tissue, their MPO expression has been reported to be lost, which might be regained under certain conditions [29]. Macrophages in liver, microglia, and granulecontaining neurons have also been reported to express MPO [30,31].
MPO Biosynthesis and Processing
Our understanding of the biosynthesis and processing of MPO has been characterized in some detail and offers a glimpse into the diversity of the underlying mechanisms, which lead to MPO deficiency [32-35]. Myeloperoxidase is encoded by a single gene (MPO) found at the 17q22-23 chromosome locus, which consists of 12 exons and 11 introns [36-38]. When translated, the MPO gene forms a peptide precursor, which consists of an amino-terminal signal peptide, a propeptide following, which is trailed by a small, then a large subunit [39]. The synthesized MPO protein is next glycosylated to form an enzymatically inactive pre-pro-MPO. The pre-pro-MPO is then folded, modified with oligosaccharides, and sorted into granule storage (Figure 1B). Pre-pro-MPO is glycosylated with oligosaccharides once on the propeptide region and four times on the heavy subunit [33]. Pre-pro-MPO binds to the chaperone calnexin and calreticulin to facilitate its folding in the endoplasmic reticulum; calreticulin facilitates the insertion of a heme group [40]. When heme is added, an enzymatically active precursor is formed, called the pro-MPO (Figure 1B) [41]. Pro-MPO spends a long time in the ER before export, a process independent of the chaperones calreticulin and calnexin, which generally are involved in ER quality control [42]. This may have to do with the time necessary to integrate heme into the protein. The pro-peptide acts to sort MPO into the granules (Figure 1B) [35]. Pro-MPO undergoes additional conversations to eventually become mature MPO; a process involving the formation of a disulfide bridge between 2 MPO proteins, forming the active mature dimer of MPO (Figure 1B) [35]. The mechanisms of the process of pro-MPO are not fully understood.
Hereditary “Primary” MPO Deficiency
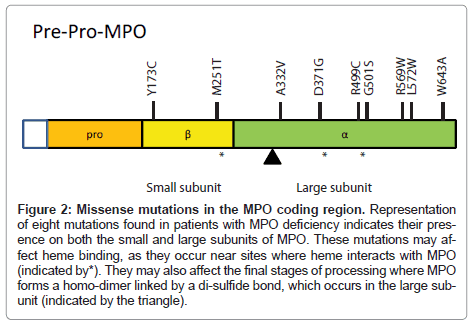
In the research setting, multiple missense mutations in the MPO gene have been identified in patients with MPO deficiency (summarized in Figure 2). However, the identification of mutations is not part of the diagnosis of MPO deficiency. These studies have helped in elucidating how MPO is processed and may further inform the underlying mechanisms of MPO deficiency. These mutations also demonstrate the diversity of disease phenotypes, outlined below.
Figure 2: Missense mutations in the MPO coding region. Representation of eight mutations found in patients with MPO deficiency indicates their presence on both the small and large subunits of MPO. These mutations may affect heme binding, as they occur near sites where heme interacts with MPO (indicated by*). They may also affect the final stages of processing where MPO forms a homo-dimer linked by a di-sulfide bond, which occurs in the large subunit (indicated by the triangle).
R569W
The substitution of tryptophan for arginine at codon 569 of the MPO gene has been one mutation described in patients with MPO deficiency. Compound heterozygotes, or the presence of 2 single mutations in R569W, have been most commonly identified in MPO deficiency patients the United States [43]. One person with homozygous (i.e. two) R569W mutations has been described. This missense mutation prevents the maturation of the pre-pro-MPO, resulting in enzymatically inactive protein that is not delivered to the azurophilic granules [43].
Y173C
The substitution of tyrosine for cysteine at codon 173 leads to misfolding of the protein, which is due to the additional intramolecular sulfide bonds that are formed. The Y173C protein is converted to pro- MPO, but misfolded at this step and sequestered by the chaperone calnexin and retained in the ER [44]. This prevents it from entering the secretory pathway, targeting it to the azurophilic granules. Protein quality controls systems, including the ubiquitin proteasome system, then degrade the Y173C protein at an accelerated rate [45].
M251T
The non-conservative substitution of methionine with threonine at codon 251 leads to a protein with significantly decreased activity. This mutation is located in the carboxy-terminal end of the light chain region of the protein near the heme binding region [46]. Stable transfectants of the M251T proteins are inefficiently processed into mature MPO, but they do enter the secretary pathway [41,47]. Mature MPO is formed, but the overall enzymatic activity is very low [46].
R499C
The substitution of an arginine for cysteine at codon 499 in exon 9 has been identified in a single patient with complete MPO deficiency. While the MPO mRNA was transcribed properly, no MPO protein was made, indicating that it was degraded by some protein quality control mechanisms [48].
G501S
This missense mutation occurs in the pocket where heme binds; it has been reported in a patient with complete MPO deficiency [49]. In Japanese populations, both R499C and G501S mutations lead to complete MPO deficiency, and may be due to their localization near H502, which is important region for heme association [48,49]. These mutations may affect the structural stability of the MPO protein as their proximity to three heme sites appears to be important in maintaining structural integrity [50].
Other MPO mutations
Since hereditary MPO deficiency is the most common biochemical defect of neutrophils, recent larger population-based studies have begun to elucidate how common each of these mutations are. Approximately 40,000 Italian individuals were studied; 7 partial and 8 total MPO deficient patients were identified [51,52]. They found 3 previously identified mutations (T752C, C1705T, 1566_1579del14), 6 novel mutations, four missense mutations (C995T, A1112G, T1715G, and T1927C), a deletion of an adenine within exon 3 and mutation within the 3’ splice site of intron 11 [51,52]. The relative frequency of novel mutations discovered in these larger studies highlights our limited understanding of the genetic basis of MPO deficiency.
The identification of MPO mutations is still performed only on a research basis and is not available for clinical diagnosis at this time. There are a number of reasons for this limitation. First, the full scope of mutations that cause MPO deficiency has not been determined, and in some cases like the R499C mutations, only one patient has been identified. Second, the currently available MPO activity tests are readily available and can give results within a short period time, whereas sequencing techniques take much longer. Lastly, the cost effectiveness of the determining MPO activity cannot match the expense of reagents and technician time required for sequencing MPO. As the scope of specific mutations that cause MPO become clearer, the use of directed molecular tests to identify specific mutations may become available. Currently, these tests will take more time and cost more than MPO activity tests.
Secondary “Acquired” MPO Deficiency
Secondary or “acquired” MPO deficiency can be differentiated from primary MPO deficiency by the variability of MPO activity in neutrophils and medical history (Table 1). Multiple medical conditions have been associated with secondary MPO deficiency, in which a partial deficiency is seen, generally in a subset of neutrophils [53]. These conditions include lead intoxication [54], iron deficiency, severe infection [55], thrombotic diseases [56], renal transplantation, diabetes mellitus [6,57,58], drugs, disseminated cancers, as well as hematological disorders and neoplasms of the developing granulocyte lineage [53]. These include myeloid leukemias (acute and chronic AML, CML), polycythemia vera [59], Hodgkin disease, refractory anemia, aplastic anemia, primary myelofibrosis, and myelodysplastic syndromes [53,60]. While the underlying mechanisms of many of these causes are not completely delineated, a few of them have been more clearly defined. For example, lead intoxication inhibits heme synthesis, which is a necessary component in the maturation of MPO [53]. Additionally, severe infections can cause PMNs to be activated, leading to the consumption of MPO, which then appears as an MPO deficiency [53]. Deficiency in MPO in the secondary “acquired” forms is transient and improves with resolution of the primary illness. Most patients with an acquired form of MPO deficiency only have a fraction of their cells affected; that is, they have cells present with full MPO activity. However, in patients with myeloproliferative disorders, the deficiency may affect several granulocyte lines [61,62]. In the setting of iron deficiency, iron replacement of is necessary for MPO biosynthesis to continue [63].
| Primary “Hereditary” MPO Deficiency | Secondary “Acquired” MPO Deficiency | |
|---|---|---|
| MPO Activity in neutrophils | Absent (or low) in all cells | Variable activity cell to cell (absentànormal) |
| MPO Activity in Bone Marrow | Absent | Variable |
| Concomitant diseases | Sometimes | Always |
| Inherited | Yes | No |
Table 1: Differentiation of primary and secondary MPO deficiency. Adapted from Lanza et al. [53].
Clinical Manifestations, Differential Diagnosis, and Treatment of MPO Deficiency
The most common infections in MPO deficient patients are from different Candida species, which can present as mucocutaneous, meningeal, bone, and disseminated fungal infection [9,64-68]. Some patients with complete MPO deficiency can also develop severe Staphylococcus aureus infections after injuries or surgical procedures, and inflammatory diseases such as spondylarthritis [11]. Patients with diabetes mellitus or cancer are more susceptible to Candida infections in the context of MPO deficiency [6,69-72]. Many of these reports associating infection risk in MPO deficiency were published in the 1970’s and 1980’s, although they continue to be published. More recent data suggests that there may be less of an association of MPO deficiency with increased infections than previously thought.
In patients with these symptoms, a fairly broad differential diagnosis should be considered in the context of the patient’s presentation. The differential diagnosis should include Chronic granulomatous disease (CGD), Glycogen-Storage Disease Type 1 (GSD1), Hyperimmunoglobulinemia E Syndrome, Kostmann Disease, Leukocyte Adhesion Deficiency, and Shwachman-Diamond Syndrome. All of these disorders have the recurrent life-threatening bacterial and fungal infections in common, however, they differ in their broader spectrum of disease (Table 2). For example, chronic granulomatous disease can only be differentiated by laboratory tests as it affects the same killing pathways as MPO deficiency. This contrasts to Shwachman-Diamond syndrome in which pancreatic insufficiency and skeletal abnormalities often overshadow the neutrophil deficiency that can be associated with recurrent infections. The often simple clinical and laboratory distinction from MPO is summarized in Table 2. The definitive diagnosis of MPO deficiency is made by histochemical staining of neutrophils for myeloperoxidase [73]. No specific therapy is indicated in patients with MPO deficiency beyond microbe-specific therapy for their infections, along with strict control of blood glucose in diabetic patients.
| Summary | Distinction from MPO Deficiency | |
|---|---|---|
| Chronic Granulomatous Disease (CGD) | An inherited disorder of phagocytic cells. Inability of phagocytes to produce superoxide anions (O2-). Recurrent life-threatening bacterial and fungal infections. Caused by defect(s) in the NADPH oxidase complex | In periods of health, CGD patients have normal MPO levels. Tests of superoxide generation (e.g. The nitro blue-tetrazoium (NBT) test, the dihydrorhodamine (DHR), or the cytochrome C reduction assay) are all normal in MPO patients (and diagnostic of CGD). |
| Glycogen-Storage Disease Type 1 (GSD1) (=Glucose-6-phosphate dehydrogenase deficiency) |
Patients have large livers with excessive glycogen. Patients present with hypoglycemia, lactic acidosis, hypertriglyceridemia, and hyperuricemia. A common manifestation of this disease is neutropenia, putting the patient at risk of increased infection. |
The only similarity with MPO is the increased infection. MPO is not associated with a decrease in neutrophil or the other constellation of symptoms found in GSD1. |
| Hyperimmunoglobulinemia E (Job) Syndrome | Characterized by recurrent skin abscesses, pneumonia, eczematous dermatitis, and elevated IgE levels (>10 times normal values). Both autosomal dominant and recessive forms have been described. Caused by mutations in the STAT3 and DOCK8 genes. Caused by abnormal neutrophil chemotaxis due to decreased IFNγ by T lymphocytes. | MPO patients have normal IgE levels (in the absence of allergies), whereas Job Syndrome patients have 10-100 fold increases and none of the dermatologic manifestations of disease. |
| Kostmann Disease | Rare disease characterized by decreased neutrophil counts (ANC<200/ml). Severe persistent neutropenia results in susceptibility to bacterial infections. The autosomal recessive form is caused by mutations in the HAX1 genes; the autosomal dominant form is caused by mutations in the neutrophil elastase gene (ELS2). | Bone marrow normal in MPO patients; Kostmann disease patients have early granulocytes (promyelocyte/myelocyte arrest), but few maturing forms. |
| Leukocyte Adhesion Deficiency (LAD) | Rare primary immunodeficiency characterized by marked leukocytosis and localized bacterial infections. Caused by a failure to express CD18, which composes the CD11a/CD18 (LFA-1) expressed on lymphocytes and antigen presenting cells. | LAD patients have extremely elevated neutrophils (6-10 x normal) as they cannot leave the vessels. Flow cytometric analysis demonstrates a lack of CD18 on peripheral blood. |
| Shwachman-Diamond Syndrome | Rare autosomal recessive disorder characterized by pancreatic insufficiency, bone marrow dysfunction, and skeletal abnormalities. Symptoms of malnutrition secondary to pancreatic insufficiency predominate. Caused by mutations in the SBDS gene believed to be involved in RNA metabolism or ribosome assembly. SDS patients have a low neutrophil count the leaves patients at risk of developing severe recurrent infections, along with anemia and thrombocytopenia. Bone marrow typically is hypocellular with maturation arrest of all myeloid lineages. |
MPO patients lack evidence of pancreatic insufficiency, bone defects, and abnormal peripheral blood cell counts. |
Table 2: Differential diagnosis in patients presenting with recurrent infections. Adapted from: Rosenzweig and Holland [73] and Petersen and Sheikh [86].
How Can Most MPO Patients be Asymptomatic?
Greater than 95% of patients lacking MPO are asymptomatic, despite studies of human MPO deficient neutrophils indicating they have abnormalities in killing Candida albicans and hyphal forms a Aspergillus fumigatus [74,75]. Despite the lack of MPO, MPO deficient neutrophils retain much of their ability to kill a variety of microbes. Initially, S. aureus, Serratia spp., and E. coli killing is impaired, but reaches normal levels after time [76]. This suggests alternative but overlapping mechanisms may compensate in this context.
Much of what is known about the differential killing ability in MPO deficiency comes from MPO-knockout mice. Like neutrophil depleted mice, MPO knockout mice have a high susceptibility to infection with C. albicans [77-79]. There is considerable more susceptibility to pulmonary infections after intranasal challenge with C. tropicalis, Trichosporon asahii, and Pseudomonas aeruginosa, and to a lesser degree Cryptococcus neoformans, Klebsiella pneumoneiae, and Aspergillus fumigates [78,80,81]. However, there is no increased susceptibility in MPO-deficient mice to C. glabrata, S. aureus, and S. pneumonia [81]. The finding that there is no susceptibility to S. aureus is consistent with observations make with MPO deficient human neutrophils, where early, but not late killing of S. aureus is impaired, but long term killing is not affected [76].
MPO-deficient mice are variably susceptible to E. coli depending on the exposure. Challenging MPO-deficient mice with sepsis induced by cecal ligation and puncture results in enhanced infection (103). However, challenging MPO-deficient mice with E. coli intraperitoneally resulted in a reduction of lung infections [82]. This study identified that MPO-deficient mice had an increased expression of vessel inducible nitric oxide synthase (iNOS) in their lungs and neutrophils, resulting in a 2-6 fold increased in NO production compared to mice with MPO [82]. The enhanced NO is able to react with O2 • to form ONOO•, which can kill microbes (Figure 1A) and may compensate for the decreased HOCl-mediated killing of bacteria in MPO deficient mice [82]. The number of neutrophils in mice is only 10-20% of that found in humans [83,84], so mice are normally less reliant on neutrophil killing and may not reflect what happens in humans. However, mice have been useful in delineating the non-MPO derived killing mechanisms that may be clinically relevant in understanding our defenses against different types of microbes [85].
Summary
Both complete and partial MPO deficiency is common, being present in 1:4000 and 1:2000 people in the United States, respectively. Despite this high prevalence, most patients are asymptomatic throughout their lifetime. It is this minimal clinical significance which led to the International Union of Immunological Society Primary Immunodeficiency Diseases Classification Committee to recently remove MPO deficiency from the classification as a primary immunodeficiency. However, a small percentage of people present with severe, often recurrent, microbial infections necessitating a understanding of its diagnosis and underlying pathophysiology. MPO is present in both neutrophils and monocytes and is a critical component in creating hypochlorous acid in phagosomes to secure the killing of microbes (summarized in Figure 1A). MPO can be defective due to mutations in MPO (Figure 2), which can result in improper processing (Figure 1B). Secondary causes are also common due to a variety of exposures and concomitant diseases, including lead intoxication, iron deficiency, severe infection, thrombotic diseases, renal transplantation, diabetes mellitus, drugs, disseminated cancers, as well as hematological disorders. The differentiation of primary and secondary causes can generally be made by assessing the clinical history and identifying different patterns of MPO expression (summarized in Table 1). Patients presenting with recurrent infections should be considered to have other, less common diseases, in addition to MPO (outlined in Table 2). Generally simple laboratory tests and/or clinical presentation and history can differentiate the underlying cause (Table 2). Currently, molecular diagnosis of MPO mutations is performed on a research basis only and not used clinically. No specific therapy is indicated in patients with MPO deficiency beyond microbe-specific therapy for their infections, along with strict control of blood glucose in diabetic patients.
References
- Czylarz Ev, Fürth O (1907) Über tierische Peroxidase. Chem Physiol Path 10: 358-362.
- Graham GS (1916) The oxidizing Ferment of the Myelocyte Series of Cells and its Demonstration by an Alphanaphthol-Pyronin Method. J Med Res 35: 231-242.
- Agner K (1941) Verdoperoxidase: a ferment isolated from leucocytes. Acta Chem Scand 2: 1-62.
- Undritz E (1966) [The Alius-Grignaschi anomaly: the hereditary constitutional peroxidase defect of the neutrophils and monocytes]. Blut 14: 129-136.
- Grignaschi VJ, Sperperato AM, Etcheverry MJ, Macario AJL (1963) Rev Assoc Med Argentina 77: 218–221.
- Parry MF, Root RK, Metcalf JA, Delaney KK, Kaplow LS, et al. (1981) Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med 95: 293-301.
- Kitahara M, Eyre HJ, Simonian Y, Atkin CL, Hasstedt SJ (1981) Hereditary myeloperoxidase deficiency. Blood 57: 888-893.
- Nauseef WM, Root RK, Malech HL (1983) Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest 71: 1297-1307.
- Nauseef WM (1988) Myeloperoxidase deficiency. Hematol Oncol Clin North Am 2: 135-158.
- Notarangelo L, Casanova JL, Conley ME, Chapel H, Fischer A, et al. (2005) Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee Meeting in Budapest, 2005. J Allergy Clin Immun 117: 883-896.
- Kutter D (1998) Prevalence of myeloperoxidase deficiency: population studies using Bayer-Technicon automated hematology. J Mol Med (Berl) 76: 669-675.
- Larrocha C, Fernández de Castro M, Fontan G, Viloria A, Fernández-Chacón JL, et al. (1982) Hereditary myeloperoxidase deficiency: study of 12 cases. Scand J Haematol 29: 389-397.
- Ross DW, Kaplow LS (1985) Myeloperoxidase deficiency. Increased sensitivity for immunocytochemical compared to cytochemical detection of enzyme. Arch Pathol Lab Med 109: 1005-1006.
- Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77: 598-625.
- Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64: 328-340.
- Borregaard N, Cowland JB (1997) Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89: 3503-3521.
- Sengeløv H, Boulay F, Kjeldsen L, Borregaard N (1994) Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J 299 : 473-479.
- Sengelov H, Kjeldsen L, Diamond MS, Springer TA, Borregaard N (1993) Subcellular localization and dynamics of Mac-1 (alpha m beta 2) in human neutrophils. J Clin Invest 92: 1467-1476.
- Borregaard N, Heiple JM, Simons ER, Clark RA (1983) Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol 97: 52-61.
- Kjeldsen L, Sengeløv H, Lollike K, Nielsen MH, Borregaard N (1994) Isolation and characterization of gelatinase granules from human neutrophils. Blood 83: 1640-1649.
- Brumell JH, Volchuk A, Sengelov H, Borregaard N, Cieutat AM, et al. (1995) Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J Immunol 155: 5750-5759.
- Plesner T, Ploug M, Ellis V, Rønne E, Høyer-Hansen G, et al. (1994) The receptor for urokinase-type plasminogen activator and urokinase is translocated from two distinct intracellular compartments to the plasma membrane on stimulation of human neutrophils. Blood 83: 808-815.
- Hampton MB, Kettle AJ, Winterbourn CC (1998) Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92: 3007-3017.
- Winterbourn CC, Pichorner H, Kettle AJ (1997) Myeloperoxidase-dependent generation of a tyrosine peroxide by neutrophils. Arch Biochem Biophys 338: 15-21.
- Pichorner H, Metodiewa D, Winterbourn CC (1995) Generation of superoxide and tyrosine peroxide as a result of tyrosyl radical scavenging by glutathione. Arch Biochem Biophys 323: 429-437.
- Stanbro WD (1998) A kinetic model of the myeloperoxidase-hydrogen peroxide-chloride ion system in phagolysosomes. J Theor Biol 193: 59-68.
- Queiroz RF, Vaz SM, Augusto O (2011) Inhibition of the chlorinating activity of myeloperoxidase by tempol: revisiting the kinetics and mechanisms. Biochem J 439: 423-431.
- Nichols BA, Bainton DF (1973) Differentiation of human monocytes in bone marrow and blood. Sequential formation of two granule populations. Lab Invest 29: 27-40.
- Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, et al. (2001) Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol 158: 879-891.
- Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, et al. (2004) Neuronal expression of myeloperoxidase is increased in Alzheimer's disease. J Neurochem 90: 724-733.
- Nagra RM, Becher B, Tourtellotte WW, Antel JP, Gold D, et al. (1997) Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J Neuroimmunol 78: 97-107.
- Arnljots K, Olsson I (1987) Myeloperoxidase precursors incorporate heme. J Biol Chem 262: 10430-10433.
- Nauseef WM (1987) Posttranslational processing of a human myeloid lysosomal protein, myeloperoxidase. Blood 70: 1143-1150.
- Olsson I, Persson AM, Strömberg K (1984) Biosynthesis, transport and processing of myeloperoxidase in the human leukaemic promyelocytic cell line HL-60 and normal marrow cells. Biochem J 223: 911-920.
- Olsson I, Bulow E, Hansson M (2004) Biosynthesis and sorting of myeloperoxidase in hematopoietic cells. Jpn J Infect Dis 57: S13-14.
- Inazawa J, Inoue K, Nishigaki H, Tsuda S, Taniwaki M, et al. (1989) Assignment of the human myeloperoxidase gene (MPO) to bands q21.3----q23 of chromosome 17. Cytogenet Cell Genet 50: 135-136.
- Latos-Bielenska A, Klett C, Just W, Hameister H (1991) Refinement of localization of the human genes for myeloperoxidase (MPO), protein kinase C, alpha polypeptide, PRKCA, and the DNA fragment D17S21 on chromosome 17q. Hereditas 115: 69-72.
- van Tuinen P, Johnson KR, Ledbetter SA, Nussbaum RL, Rovera G, et al. (1987) Localization of myeloperoxidase to the long arm of human chromosome 17: relationship to the 15; 17 translocation of acute promyelocytic leukemia. Oncogene 1: 319-322.
- Yamada M, Hur SJ, Hashinaka K, Tsuneoka K, Saeki T, et al. (1987) Isolation and characterization of a cDNA coding for human myeloperoxidase. Arch Biochem Biophys 255: 147-155.
- Nauseef WM (1998) Insights into myeloperoxidase biosynthesis from its inherited deficiency. J Mol Med (Berl) 76: 661-668.
- Nauseef WM (2004) Lessons from MPO deficiency about functionally important structural features. Jpn J Infect Dis 57: S4-5.
- Bülow E, Nauseef WM, Goedken M, McCormick S, Calafat J, et al. (2002) Sorting for storage in myeloid cells of nonmyeloid proteins and chimeras with the propeptide of myeloperoxidase precursor. J Leukoc Biol 71: 279-288.
- Nauseef WM, Cogley M, McCormick S (1996) Effect of the R569W missense mutation on the biosynthesis of myeloperoxidase. J Biol Chem 271: 9546-9549.
- Hansson M, Olsson I, Nauseef WM (2006) Biosynthesis, processing, and sorting of human myeloperoxidase. Arch Biochem Biophys 445: 214-224.
- DeLeo FR, Goedken M, McCormick SJ, Nauseef WM (1998) A novel form of hereditary myeloperoxidase deficiency linked to endoplasmic reticulum/proteasome degradation. J Clin Invest 101: 2900-2909.
- Romano M, Dri P, Dadalt L, Patriarca P, Baralle FE (1997) Biochemical and molecular characterization of hereditary myeloperoxidase deficiency. Blood 90: 4126-4134.
- Suzuki K, Muso E, Nauseef WM (2004) Contribution of peroxidases in host-defense, diseases and cellular functions. Jpn J Infect Dis 57: S1-2.
- Persad AS, Kameoka Y, Kanda S, Niho Y, Suzuki K (2006) Arginine to cysteine mutation (R499C) found in a Japanese patient with complete myeloperoxidase deficiency. Gene Expr 13: 67-71.
- Ohashi YY, Kameoka Y, Persad AS, Koi F, Yamagoe S, et al. (2004) Novel missense mutation found in a Japanese patient with myeloperoxidase deficiency. Gene 327: 195-200.
- Banerjee S, Stampler J, Furtmüller PG, Obinger C (2011) Conformational and thermal stability of mature dimeric human myeloperoxidase and a recombinant monomeric form from CHO cells. Biochim Biophys Acta 1814: 375-387.
- Marchetti C, Patriarca P, Solero GP, Baralle FE, Romano M (2004) Genetic studies on myeloperoxidase deficiency in Italy. Jpn J Infect Dis 57: S10-12.
- Marchetti C, Patriarca P, Solero GP, Baralle FE, Romano M (2004) Genetic characterization of myeloperoxidase deficiency in Italy. Hum Mutat 23: 496-505.
- Lanza F (1998) Clinical manifestation of myeloperoxidase deficiency. J Mol Med (Berl) 76: 676-681.
- Caldwell KC, Taddeini L, Woodburn RL, Anderson GL, Lobell M (1979) Induction of myeloperoxidase deficiency in granulocytes in lead-intoxicated dogs. Blood 53: 588-593.
- Cocchi P, Mori S, Ravina A (1973) Myeloperoxidase-deficient leucocytes in streptococcal infections. Helv Paediatr Acta 28: 79-85.
- d'Onofrio G, Mancini R, Vallone R, Alfano G, Candido A, et al. (1983) Acquired neutrophil myeloperoxidase deficiency: an indicator of subclinical activation of blood coagulation? Blood Cells 9: 455-466.
- Cech P, Papathanassiou A, Boreux G, Roth P, Miescher PA (1979) Hereditary myeloperoxidase deficiency. Blood 53: 403-411.
- Pietrzyk JA, Palimaka W (1979) [The immunological system in diabetics]. Wiad Lek 32: 323-326.
- Bendix-Hansen K (1986) Myeloperoxidase-deficient polymorphonuclear leucocytes (VII): Incidence in untreated myeloproliferative disorders. Scand J Haemato 36: 8-10.
- Davey FR, Erber WN, Gatter KC, Mason DY (1988) Abnormal neutrophils in acute myeloid leukemia and myelodysplastic syndrome. Hum pathol 19: 454-459.
- Moretti S, Lanza F, Spisani S, Latorraca A, Rigolin GM, et al. (1994) Neutrophils from patients with myelodysplastic syndromes: relationship between impairment of granular contents, complement receptors, functional activities and disease status. Leuk Lymphoma 13: 471-477.
- Clark RA, Szot S (1981) The myeloperoxidase-hydrogen peroxide-halide system as effector of neutrophil-mediated tumor cell cytotoxicity. J Immunol 126: 1295-1301.
- Murakawa H, Bland CE, Willis WT, Dallman PR (1987) Iron deficiency and neutrophil function: different rates of correction of the depressions in oxidative burst and myeloperoxidase activity after iron treatment. Blood 69: 1464-1468.
- Weber ML, Abela A, de Repentigny L, Garel L, Lapointe N (1987) Myeloperoxidase deficiency with extensive candidal osteomyelitis of the base of the skull. Pediatrics 80: 876-879.
- Okuda T, Yasuoka T, Oka N (1991) Myeloperoxidase deficiency as a predisposing factor for deep mucocutaneous candidiasis: a case report. J Oral Maxillofac Surg 49: 183-186.
- Ludviksson BR, Thorarensen O, Gudnason T, Halldorsson S (1993) Candida albicans meningitis in a child with myeloperoxidase deficiency. Pediatr Infect Dis J 12: 162-164.
- Nguyen C, Katner HP (1997) Myeloperoxidase deficiency manifesting as pustular candidal dermatitis. Clin Infect Dis 24: 258-260.
- Chiang AK, Chan GC, Ma SK, Ng YK, Ha SY, et al. (2000) Disseminated fungal infection associated with myeloperoxidase deficiency in a premature neonate. Pediatr Infect Dis J 19: 1027-1029.
- Lehrer RI, Cline MJ (1969) Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest 48: 1478-1488.
- Cech P, Stalder H, Widmann JJ, Rohner A, Miescher PA (1979) Leukocyte myeloperoxidase deficiency and diabetes mellitus associated with Candida albicans liver abscess. Am J Med 66: 149-153.
- Koziol-Montewka M, Magrys A, Paluch-Oles J, Bogut A, Buczynski K, et al. (2006) MPO and cytokines in the serum of cancer patients in the context of Candida colonization and infection. Immunol Invest 35: 167-179.
- Magrys A, Koziol-Montewka M, Staroslawska E, Gabczynska B, Szczepanik A (2005) Immunological parameters of candidiasis in cancer patients. New Microbiol 28: 355-364.
- Rosenzweig SD, Holland SM (2011) Myeloperoxidase deficiency and other enzymatic WBC defects causing immunodeficiency. Waltham, MA.
- Rex JH, Bennett JE, Gallin JI, Malech HL, Melnick DA (1990) Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J Infect Dis 162: 523-528.
- Diamond RD, Clark RA, Haudenschild CC (1980) Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest 66: 908-917.
- Lehrer RI, Hanifin J, Cline MJ (1969) Defective bactericidal activity in myeloperoxidase-deficient human neutrophils. Nature 223: 78-79.
- Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, et al. (1999) Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun 67: 1828-1836.
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, et al. (2002) Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J Infect Dis 185: 1833-1837.
- Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, et al. (2001) Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest 107: 419-430.
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, et al. (2006) Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J Med Microbiol 55: 1291-1299.
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, et al. (2000) Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J Infect Dis 182: 1276-1279.
- Brovkovych V, Gao XP, Ong E, Brovkovych S, Brennan ML, et al. (2008) Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol 295: L96-103.
- Noguchi N, Nakano K, Aratani Y, Koyama H, Kodama T, et al. (2000) Role of myeloperoxidase in the neutrophil-induced oxidation of low density lipoprotein as studied by myeloperoxidase-knockout mouse. J Biochem 127: 971-976.
- Rausch PG, Moore TG (1975) Granule enzymes of polymorphonuclear neutrophils: A phylogenetic comparison. Blood 46: 913-919.
- van der Veen BS, de Winther MP, Heeringa P (2009) Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal 11: 2899-2937.
- Petersen MM, Mikita CP, Sheikh J (2010) Myeloperoxidase Deficiency. Medscape.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22600
- [From(publication date):
April-2012 - Nov 14, 2025] - Breakdown by view type
- HTML page views : 17653
- PDF downloads : 4947