Research Article Open Access
The Levels of Toxic Metals in Blue Crab Portunus segnis from Persian Gulf
Mehdi Hosseini1*, Seyed Mohammad Bagher Nabavi2 Jamileh Pazooki1 and Yaghoob Parsa21Department of Marine Biology, Faculty of Biological Science, Shahid Beheshti University, Tehran, Iran
2Department of Marine Biology, Faculty of Marine Science, Khoramshahr University of Marine Science and Technology, Iran
- *Corresponding Author:
- Mehdi Hosseini
Department of Marine Biology
Faculty of Biological Science
Shahid Beheshti University, Tehran, Iran
Tel: +98 21 29901
E-mail: smhbio@yahoo.com
Received date: December 01, 2013; Accepted date: January 21, 2014; Published date: January 28, 2014
Citation: Hosseini M, Nabavi SMB, Pazooki J, Parsa Y (2014) The Levels of Toxic Metals in Blue Crab Portunus segnis from Persian Gulf. J Marine Sci Res Dev 4:145. doi:10.4172/2155-9910.1000145
Copyright: © 2014 Hosseini M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Levels of heavy metals Ni, Co, Cd and Pb( in muscle, gill and hepatopancreas of blue swimming crab P. segnis and relationship with its food source from Persian Gulf, were investigated. In this study, there was a direct relationship between heavy metals levels in tissues crabs with organisms that feeding on them. Female crabs feed on plant and detritus and are close to bottom sediment and receive more sediment associated metals. Also, there was a positive correlation between metal concentrations in tissues with size of food items. Heavy metal concentrations were highest in female hepatopancreas whereas lowest in the muscle of all crab species. From the human consumption point of view, Heavy metals concentrations were below the admissible limits except for hepatopancreas of female crabs. Thus, precautions should be taken on account of higher content of metals as well as in other organs that could be affected by industrial pollution.
Keywords
Heavy metal; Monitoring; Portunus segnis; Persian Gulf
Introduction
Heavy metals can be classified as potentially toxic elements such as cadmium, lead and mercury and essential elements such as copper, zinc and iron. Toxic metals naturally occur in aquatic environments in very low concentrations, but their concentration levels have increased due to anthropogenic pollutants over time. Industrial activities as well as agriculture and mining create a potential source of metals pollution in aquatic environment.
Accumulation of metals in the organisms depends on various biological and environmental factors such as size, age, feeding habit, temperature and dissolved oxygen [1]. However, feeding habit plays a significant role in the accumulation of metals in organism’s tissues [2]. Because metals have tendency to be biomagnified through food chains [3]. Most aquatic food chains begin from invertebrates. However, ability of invertebrates to accumulate contaminants from water varies from species to species. Thus biomagnification of contaminants entered aquatic food chains is, significantly affected by invertebrate species.
It is known that certain forms of metals can readily accumulate within crustacean tissues at much higher levels than those in the water column and in sediment [1]. The blue swimming crab Portunus segnis belongs to the phylum; arthropoda which make up about three quarters of living animal species. For the fauna, the species most studied are the benthic macroinvertebrates, particularly those with low mobility, which accumulate larger concentrations of metals compared to animals that live in open water. Among the most-studied are the crab Portunidae which has important characteristics that allow the study of bioaccumulation: they feed mainly on a wide variety of fish, bivalves, plant, crustaceans and benthic animals [4], as well as the sediment itself [5] and they have a slow growth rate and long life cycle [4]. Therefore, this crab species is especially appropriate for use in studies of environmental impact by metals from an ecosystemic.
The Persian Gulf is a shallow and semi-enclosed sea that its environment is changing rapidly [6]. The discovery of oil in this sea led to a massive increase in anthropogenic activities in the area. In general, the agricultural use of fertilizers, herbicides, pesticides, petrochemical and oil industries are the major sources of pollution in this area [6]. Therefore, levels of Ni, Co, Cd and Pb in tissues (muscle, hepatopancreas and gills) of blue swimming crab Portunus segnis, from northwest of the Persian Gulf, were studied.
Materials and Methods
Sampling area was selected along the Persian Gulf (Figure 1). The Persian Gulf lies on the south Iran, between longitudes 48°25’ and 56°25” East, and latitudes 24°30” and 30°30’ North. It has an estimated area of 260 km2 and extends 600 km offshore to a depth average of about 30-40 m. This area is a sandy-muddy habitat that provides a suitable ecosystem for a lot of marine organisms such as crab, shrimp and fish [7]. Major sources of contamination in this area include the agricultural use of fertilizers, herbicides, pesticides, hazardous substance spills from various refineries, petrochemical and oil industries. Sampling was performed by trawl net. The samples placed on ice, immediately transported to the laboratory on the same day and stored at -20°C until analysis [8].
Each species was properly cleaned by rinsing with distilled water to remove debris, planktons and other external adherent, and the sex of each individual was determined according to morphological characteristics. Male crabs are bright blue and their abdomens (womb area) are narrow and in the form of a spear, while female crabs are green–brown and have round abdomens [9]. The samples were dissected with sterilized scissors and tweezers to remove samples of two tissues (muscle, gill and hepatopancreas), in standardized locations: (i) muscle of the chelar propodus, due to higher metal accumulation verified by Chen et al. [10]; (ii) hepatopancreas tissue, which has a particularly high metabolic rate [11] and (iii) gills, because of their osmoregulatory function [11]. It was then drained under folds of filter, weighed, wrapped in aluminum foil and then frozen at 10ºC prior to analysis. The tissues were placed in clean watch glasses and were oven dried at 105ºC for 1 hour and later cooled in the desiccators. Each sample of crab was homogenized in an acid-cleaned mortar and 2 g were digested in triplicate in a water bath at 60ºC for 6 h after adding 2.5 mL each of concentrated HNO3 and H2SO4 [12].
Each sample was analyzed for metals by the mineralization method with HNO3 at 65 percent, according to Basset et al. [13]. Analyses were optimized by hollow cathode lamps (LCO), according to the metallic element analyzed, and samples were read using a GBC-932 AA atomic absorption spectrophotometer [8]. The equipment was calibrated using metal stock solutions (1000 ppm). The recovery means for Ni, Co, Cd and Pb was 99%, 103%, 99% and 102% respectively.
All data were tested for normal distribution with Shapiro-wilk normality test. The comparisons of metals levels between muscle, gill and hepatopancreas of crab were carried out by t-test. All concentrations are reported in μg g-1 dry weight and a probability of p<0.01 was set to indicate statistical significance.
Results
Table 1 shows scientific name, sex, the mean body weight and food type for the blue swimming crab Portunus segnis. Metals concentrations were calculated in microgram per gram wet basis (μgg-1). In order to check the validity of the measurements, reference material (Multi-4, Merck) was used. The mean concentration and standard error of studied heavy metals are given in Table 2.
| Scientific name | Sex | Mean Weight (g) | Food type |
|---|---|---|---|
| Portunussegnis | Female (n = 122) | 170 ± 0.10 | Shrimp; Fish; Mollusca; Plant; Detritus |
| Male (n = 107) | 151 ± 0.02 |
Table 1: Sex, food type, and weight (Mean ± SE) of the blue swimming crab Portunussegnis.
| Metal | Sex | Gill | Hepatopancreas | Muscle |
|---|---|---|---|---|
| Ni | Male | 15.4 ± 0.05 | 20.4 ± 0.02 | 14.5 ± 0.06 |
| Female | 20.2 ± 0.01 | 31.9 ± 0.01 | 17.3 ± 0.05 | |
| Pb | Male | 1.1 ± 0.02 | 2.2 ± 0.02 | 0.43 ± 0.03 |
| Female | 1.5 ± 0.05 | 2.5 ± 0.05 | 0.71 ± 0.02 | |
| Co | Male | 0.63 ± 0.06 | 0.91 ± 0.01 | 0.53 ± 0.06 |
| Female | 0.71 ± 0.02 | 1.2 ± 0.03 | 0.62 ± 0.01 | |
| Cd | Male | 0.43 ± 0.01 | 0.61 ± 0.01 | 0.15 ± 0.04 |
| Female | 0.51 ± 0.03 | 0.88 ± 0.02 | 0.38 ± 0.02 |
Table 2: Metal concentrations (μg g-1) in the gill, hepatopancreas and muscle of male and female of blue swimming crab P. segnis.
Ni
Nickel concentration in the hepatopancreas tissue of crabs ranged from (09.2 μg g-1) to (20.5 μg g-1) for male and from (11.2 μg g-1) to (31.1 μg g-1) for female, while concentrations in the gill were in the range of (07.5 μg g-1) to (15.2 μg g-1) for male and from (10.4 μg g-1) to (20.6 μg g-1) for female crabs. The nickel concentrations in the muscle of the male and the female crabs were observed to be between (7.6 μg g-1) to (14.1 μg g-1) and between (8.1 μg g-1) to (17.5 μg g-1), respectively.
Co
Cobalt concentration in the hepatopancreas tissue of crabs ranged from (0.41 μg g-1) to (0.9 μg g-1) for male and from (0.45 μg g-1) to (1.2 μg g-1) for female, while concentrations in the gill were in the range of (0.19 μg g-1) to (0.6 μg g-1) for male and from (0.24 μg g-1 2) to (0.7 μg g-1) for female crabs. The nickel concentrations in the muscle of the male and the female crabs were observed to be between (0.16 μg g-1) to (0.5 μg g-1) and between (0.21 μg g-1) to (0.6 μg g-1), respectively.
Pb
Lead concentration in the hepatopancreas tissue of crabs ranged from (0.75 μg g-1) to (2.2 μg g-1) for male and from (0.93 μg g-1) to (2.5 μg g-1) for female, while concentrations in the gill were in the range of (0.65 μg g-1) to (1.1 μg g-1) for male and from (0.74 μg g-1) to (1.5 μg g-1) for female crabs. The nickel concentrations in the muscle of the male and the female crabs were observed to be between (0.36 μg g-1) to (0.54 μg g-1) and between (0.53 μg g-1) to (0.81 μg g-1), respectively.
Cd
Cadmium concentration in the hepatopancreas tissue of crabs ranged from (0.31 μgg-1) to (0.61 μg g-1) for male and from (0.48 μg g-1) to (0.88 μg g-1) for female, while concentrations in the gill were in the range of (0.19 μg g-1) to (0.43 μg g-1) for male and from (0.24 μg g-1 2) to (0.55 μg g-1) for female crabs. The nickel concentrations in the muscle of the male and the female crabs were observed to be between (0.11 μg g-1) to (0.25 μg g-1) and between (0.15 μgg-1) to (0.38 μg g-1), respectively.
Correlation
Correlation analysis also showed relationship between individual elements including Co and Ni (r=0.65) and between Cd and Co (r=0.76). Pearson correlation showed that there was no significant correlation among these metals (p>0.05).
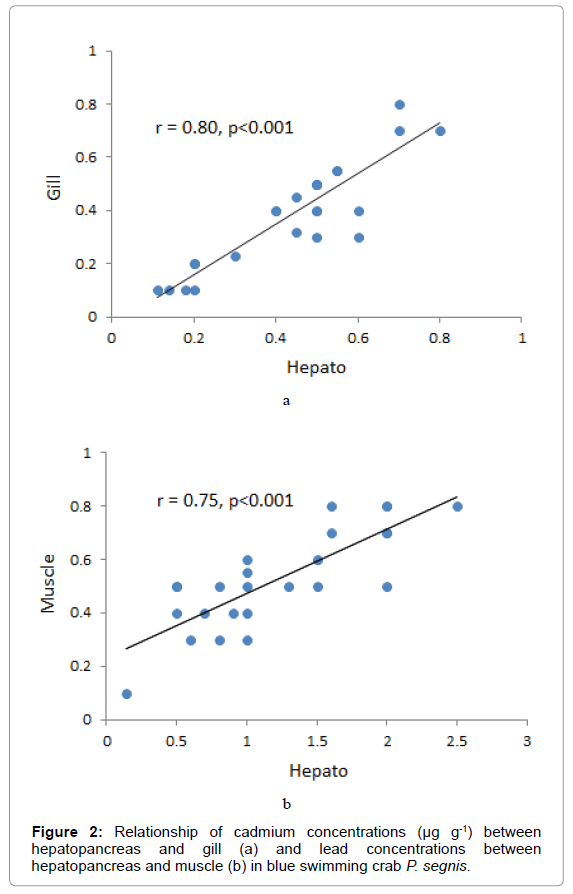
In our samples, hepatopancreas cadmium concentrations were positively correlated with those in gill of the blue swimming crab (r = 0.80 P<0.002, Figure 2a). Also, there was a positive correlation between lead concentrations in hepatopancreas with those in muscle (r = 0.75 P<0.001, Figure 2b)
Discussion
This area is surrounded by many petrochemical units and petroleum refinery. Also, metals concentration may be due to discharge of sewage and urban effluents and related to the oil tankers traffic in the area. The fact that total concentration of some petrochemicalrelated metals such as Ni, Co and Cd in the northwest of Persian Gulf decreased with distance from Boushehr coasts is strong evidence reflects that the metals in this area were sourced from petrochemical activities, and not from background geological sources. Besides, some main creeks of this estuary flow into two crowded and industrialized cities and consequently receive huge amounts of domestic effluents and urban wastewaters [8].
It is likely that this high concentration of nickel was related to the oil tankers traffic and petrochemical complex in this area [8]. This area is surrounded by some petrochemical complex and petroleum refinery. The high concentration of lead was situated at the boat stations where high boating activities takes place and in located where the pH of the sediment was the lowest. the highest concentration of lead was found in the Turlok Lake when there were high boating activities.
The result demonstrated that metals concentrations in the different organs followed the hierarchical pattern H > G > M (Figure 3). Heavy metal accumulation in shrimp organs depend on the physiological role of the organs [14]. Some tissues such as hepatopancreas are considered as target organs for metals accumulation [15]. The very high levels metals in the hepatopancreas in comparison to other tissues may be related to the content of metallothionein protein in hepatopancreas tissue. Metallothionein protein that plays a significant role in the regulation and detoxification of metals is produced in high levels in hepatopancreas tissue [16]. This protein contains a high percentage of amino group, nitrogen and sulphur that sequester metals in stable complexes. In general, the accumulation of metals in the hepatopancreas could be resulted from the abundance of metallothioneins proteins in these tissues in comparison to gill and muscle.
Gills usually reflect the concentrations of metals in surrounding water. This organ is directly in contact with water and suspended materials thus could absorb different substances from the surrounding environment. They also serve a variety of physiological functions such as osmoregulation and gas exchange. Due to these functions, gills have remarkable influences on the exchange of toxic metals between a fish and its environment. However, the muscle tended to accumulate less mercury in comparison to the liver and gills. This finding may reflect the low concentration of metallothioneins in the muscle tissue.
The result also showed that there are differences in metal concentration in the samples collected from different station. The indicated variability of metal concentration in the same species depends on their habitats [15] because chemical reactions, bioavailability of metals, and sources of pollution that potentially could affect the accumulation of metals are different between stations.
There have been several studies on accumulation of heavy metals in crustacean species, yet few studies have taken into account the effect of sexual changes with respect to the metal accumulation and distribution among tissues. We found that metals values were larger in tissues of female of the species than the males. Differences in accumulation between the genders have been mainly attributed to differences in diet or differences in habitat [17].
The male crab more feed on fish and bivalvia and females crab more feed on shrimp, plant and detritus [4]. Plants have relationship with sediment and receive more sediment associated metals. The roots of plants have an important role in depurating the water and the sediment, retaining large quantities of organic material and trace metals brought by the tides [17]. Metals are closely bound to the plant cell wall, slowing its translocation from roots to buds. Because plants are salt-excluder, it hinders the entry of metals through its root system [18].
It is known that certain forms of metals can readily accumulate within crustacean tissues at much higher levels. Shrimp have been reported as a vector of the transfer of mercury element to top marine predators of the food chains. Therefore, female crabs more feed on shrimp and plant and receive high levels of metals (Figure 3).
Since larger organisms generally exhibit higher contaminant level in their bodies [8] and crabs that are higher on the food chain also accumulate more contaminants when comparing to crabs that eat a range of different foods or eat smaller organisms. We expected to see higher metals levels in tissues of female crabs because they are larger and can eat larger food items. In general metal levels have been shown to increase with size and age of the ingested crab and it tends to be higher in species that occupy higher trophic levels [19], based on this logic we predicted that there should be higher levels of metals in the larger predators. Gewurtz et al. [20] have shown that higher metals levels in female fish were due to the increased consumption of food, relative to males, to meet the increasing demands of reproduction.
Conclusion
The chief purposes of current investigation were to determine to be biomagnification of Ni, Co, Cd and Pb in three tissues (muscle, hepatopancreas and gills) of blue swimming crab Portunus segnis and relationship with its food source. There was a positive correlation between metals concentrations in tissues with size of food items. Therefore, we expected to see higher metals levels in tissues of female crabs because they are larger and can eat larger food items. The results of this study show that highest mean metals level were found in the herbivorous crabs, followed by detritivorous, carnivorous and omnivorous. Planteating crab can be used to develop a sensitive bioindicator for ecological and human health parameters. One advantage of studies such as this is that they provide baseline data which can be used in future to evaluate differences within and across specific geographical areas. Without such a database it will be difficult to evaluate and interpret future results from the region, or to identify place with disturbing trends in pollution levels. Finally, there is a need to develop and refine herbivorous crab model to serve as a sentinel of ecosystem health, to help provide early warning indications for possible human exposure.
Acknowledgements
Financial support was carried out by Shahid Beheshti University and Environment Protection institute and Environmental project, Iran.
References
- Beltrame MO, Marco SGD (2010) Influences of sex, habitat, and seasonality on heavy-metal concentrations in the burrowing crab (Neohelice granulate) from a coastal lagoon in Argentina. Arch Environ Contam Toxicol 58: 746-756.
- Karadede Hl, Oymak SA, Unlu E (2004) Heavy metals in mullet, Liza abu, and catfish, Silurustriostegus, from the Ataturk Dam Lake (Euphrates), Turkey. Environ Int 30: 183-188.
- Dalman O, Demirak A, Balci A (2006) Determination of heavy metals (Cd, Pb) and trace elements (Cu, Zn) in sediments and fish of the Southeastern Aegean Sea (Turkey) by atomic absorption spectrometry. Food Chemistry 95: 157-162.
- Williams MJ (1981) Methods for analysis of natural diet in portunid crabs (Crustacea: Decapoda: Portunidae). J Experimental Marine Biol Ecol 52: 103-113.
- Prasad PN, Neelakantan N (1988) Food and feeding of the mud crab Scylla serrata (Forskal) (Decapoda: Portunidae) from Karwar waters. Indian Journal of Fisheries 35: 164-170.
- Sheppard C, Al-Husiani M, Al-Jamali F, Al-Yamani F, Baldwin R, et al. (2010) The Gulf: A young sea in decline. Mar Pollut Bull 60: 13-38.
- ROPME (1999) Regional report of the state of the marine environment. Regional Organization for the Protection of the Marine Environment (ROPME), Kuwait 220.
- Abdolahpur MF,PeeryS, Karami O, Hosseini M, Bastami AA, et al. (2012) Distribution of Metals in the Tissues of Benthic, Euryglossaorientalis and Cynoglossusarel., and Bentho-Pelagic, Johniusbelangerii., Fish from Three Estuaries, Persian Gulf. Bull Environ Contam Toxicol 89: 489-494.
- Potter IC, Lestang SD (2000) Biology of the blue swimmer crab Portunuspelagicus in Leschenault Estuary and Koombana Bay, south-western Australia. J R Soc West Aust 83: 443-458.
- Fu CT, Wu SC (2005) Bioaccumulation of polychlorinated biphenyls in mullet fish in a former ship dismantling harbour, a contaminated estuary, and nearby coastal fish farms. Mar Pollut Bull 51: 932-939.
- MourenteG (1996) In vitro metabolism of 14C-polyunsaturated fatty acids in midgut gland and ovary cells from Penaeuskerathurus Forskal at the beginning of sexual maturation. Comp Biochem Physiol Part B Biochem Mol Biol 115: 255-266.
- Athanasopoulos N (1993) Flame methods manual for atomic absorption. GBC Scientific Equipment PTY Ltd, Victoria.
- Basset J, Denney RC, Jeffery GH, Mendhan J (1981) Vogel: AnaliseInorganica Quantitativa, fourthed. Guanabara SA, Rio de Janeiro.
- Uysal K, Emre Y, Köse E (2008) The determination of heavy metal accumulation ratios in muscle, skin and gills of some migratory fish species by inductively coupled plasma-optical emission spectrometry (ICP-OES) in Beymelek Lagoon (Antalya/Turkey). Microchemical J 90: 67-70.
- Yilmaz AB, Yilmaz L (2007) Influences of sex and seasons on levels of heavy metals in tissues of green tiger shrimp (Penaeussemisulcatus). Food Chem 101: 1664-1669.
- Sen A, Semiz A (2007) Effects of metals and detergents on biotransformation and detoxification enzymes of leaping mullet (Liza saliens). Ecotoxicol Environ Saf 68: 405-411.
- Beckvar N, Field J, Salazar S, Hoff R (1996) Contaminants in aquatic habitats at hazardous waste sites: mercury. NOAA, Seattle.
- Bernini E, Silva MAB, Carmo TMS (2006) Composicaoquimica do sediment e de folhas das especies do manguezal do estuario do Rio Sao Mateus, Espirito Santo, Brasil. Rev Bras Bot 29: 689-699.
- Phillips GR, Lenhart TE, Gregory RW (1980) Relation between trophic position and mercury accumulation among fishes from the Tongue River reservoir. Environ Res 22: 73-80.
- Gewurtz SB, Bhavsar SP, Fletcher R (2011) Influence of fish size and sex on mercury/PCB concentration: importance for fish consumption advisories. Environ Int 37: 425-434.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 16699
- [From(publication date):
February-2014 - Oct 18, 2025] - Breakdown by view type
- HTML page views : 11870
- PDF downloads : 4829