Review Article Open Access
The History of Modern Biotechnology in Iran: A Medical Review
Ramin Mazaheri Nezhad Fard1*, Masoumeh Moslemy2 and Hannaneh Golshahi31Rastegar Reference Laboratory, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
2National Nutrition and Food Technology Research Institute, Faculty of Nutrition Science, Food Science and Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Department of Pathology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
- Corresponding Author:
- Ramin Mazaheri Nezhad Fard
Rastegar Reference Laboratory, Faculty of Veterinary Medicine
University of Tehran, PO Box: 14155- 6453, Tehran, Iran
Tel: +98-21-61117055/Mobile: +98-9392788833
Fax: +98-21-66933229
E-mail: raminmazaheri@ut.ac.ir
Received date: April 21, 2013; Accepted date: May 15, 2013; Published date: May 20, 2013
Citation: Nezhad Fard RM, Moslemy M, Golshahi H (2013) The History of Modern Biotechnology in Iran: A Medical Review. J Biotechnol Biomater 3:159. doi:10.4172/2155-952X.1000159
Copyright: © 2013 Nezhad Fard RM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The origin of biotechnology goes back to millenniums ago. The ancient China and Egypt were the first countries that used biotechnology in the form of food fermentation. The modern biotechnology especially in the field of medicine is introduced as “Technology of Hope” as many people are favored from the medical biotechnology products in therapeutic or prophylaxis form worldwide. Interestingly, Iran is one of the pioneers in many fields of biotechnology especially medical biotechnology in the Asia-pacific region. About nine decades ago, vaccine production was commenced in Pasteur and Razi Institutes in Iran by the emphasis on human vaccines and animal studies, respectively. It was a start point in development of modern pharmaceutical industries in Iran. Continued national research has led to investigate and launch various medical products at both national and international scales. Now, results of incoming research in medical biotechnology have various promising advances at different views from experimental to clinical trials. One of these surprising approaches includes animal cloning for the basic research and therapeutic agent recovery means. Other applications are related to genomics, proteomics, tissue engineering, stem cell techniques and diagnostic tools. However, any efforts in this area must be supported financially and educationally by the government and private sectors to continue the rich history of biotechnology in Iran.
Keywords
Biotechnology; Medicine; Health; History; Iran
Introduction
Progresses in science make a good opportunity for the revolution of human welfare activities through improvements in the quality and quantity of healthcare. One of these valuable progresses has occurred in biotechnology [1]. The history of biotechnology starts with the development of a wide variety of novel techniques and procedures particularly in relation to viable organisms in terms of their potencies and possible risks [2]. In 1919, Karl Ereky, a Hungarian engineer, defined the term of biotechnology. Based on his opinion, biotechnology comprised upstream and downstream processes producing substances from raw materials by living organisms. However, the origin of biotechnology is not quite novel. Fermentation was the earliest form of biotechnology that initially used in food process in China and brewing and bakery in ancient Egypt [3,4]. In 1992, a standard definition was published in the Convention on Biological Diversity in Rio de Janeiro. The definition described biotechnology as “any technological application that uses biological systems, living organisms or derivatives thereof, to make or modify products and processes for specific use”. This general explanation was agreed by 168 member nations and verified by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) of the United Nations [5]. Applied biotechnology has been acknowledged by the public in the late 1970s, introducing the recombinant DNA technology.
Biotechnology is classically divided into four major categories, including agricultural (Green), environmental (Blue), industrial (White) and biomedical/healthcare (Red) biotechnologies [3]; from which the latter one is the major focus of this review. The modern (orthodox) medicine uses many products of biotechnology and genetic engineering including diagnostic and therapeutic monoclonal antibodies, therapeutic proteins produced by recombinant DNA technology, genetic testing and analysis kits, and gene therapy and infertility services products [6,7]. Use of biotechnology and genetic engineering in medical applications has resulted in the production of important vaccines against canine Lyme disease, whooping cough, diphtheria, tetanus and influenza [8].
The History of Modern Biotechnology in Iran
Historical data reveals that the modern biotechnology has a relatively long background in Iran. Substitution of natural components by the alternative medicines was the basis of modern biotechnology in Iran. Following the primary changes to use biotechnology products, research centers were established and developed. The aim of establishing institutes for the microbiology and immunology research in Iran was conceived as an outcome of the 1918–19 influenza pandemic in Persian regions which killed hundreds of thousands of people [9]. The modern biotechnology has been established nearly nine decades ago in Iran, started with vaccine production in Pasteur and Razi Institutes in 1920 and 1925, respectively. Today, local manufacture of biotechnology products is a novel investment in the Iranian pharmaceutical section. Interferon, growth hormone and erythropoietin are amongst biopharmaceuticals produced in Iran over the past years [10].
In 1924, cattle plague (rinderpest) was prevailed extensively. The Public Health Department of the League of Nations requested the Iranian government to establish a research institute for the prevention of animal diseases under the sponsorship of Pasteur Institute of Iran. The main task of this institute was the production of sera and vaccines needed in animal husbandry. The institute became soon independent in 1927. In 1930, a research laboratory was constructed in the institute in Karaj to carry out diagnostic research on animal diseases [11]. In 1932, Paul Delpy Louis, a French veterinarian and vaccine and serum specialist, assisted the institute (later renamed as Razi Institute in 1948) to produce vaccines to contest the microbes causing cattle epidemics [12]. Consequently, vaccination strategies against fatal diseases such as diphtheria, tetanus and pertussis (known as DTP vaccination) were commenced in Iran in 1950 using vaccines produced by the institute [11]. Decades later, the institute established a biotechnology department in 1992. Until 1997, the department was improved significantly using advanced biotechnology facilities and a specialized laboring scheme [12]. However, the Pasteur Institute of Iran, as a branch of Pasteur Institute of Paris, was the first research center involved in the research of domestic infectious diseases, including relapsing fever, smallpox, rabies, plague, tuberculosis, cholera, diphtheria, typhoid fever and typhus (Figure 1).
Founded in 1919, Pasteur Institute of Iran is the oldest scientific center for the medical research in Iran. The first director of Pasteur Institute of Iran, Dr. Joseph Mesnard, was a French medical doctor came from France to head the institute in 1920. The mission of the institute was mainly limited to vaccine production for the initial decades. However, the history of vaccination in Iran turns back to the Reign of Qajar in 18th century and was reinforced in 19th century when the Prime Minister Amir Kabir ruled an obligatory public vaccination against smallpox [13,14]. Before the establishment of Pasteur Institute of Iran, smallpox vaccine was imported from Paris branch of Pasteur Institute. During the delivery, vaccines lost a part of their viability due to problems such as the long transport distance and excessive heat. Surprisingly, the Iranian branch produced more than 100 smallpox vaccine doses during 19 months of commence of vaccine production. Furthermore, 1,638 cases of vaccinations against livestock anthrax were recorded. However, the majority of vaccines were still imported from Paris. Later, various vaccines were manufactured in Iran by the Pasteur Institute in bundle factories. In 1947, Dr. Ghodssi (the first Iranian director of the institute) supervised the manufacture of BCG vaccines in Iran [15]. The Institute started its novel activities against fatal infections in 1950 but the laboratories had previously been established in 1924 [11,15].
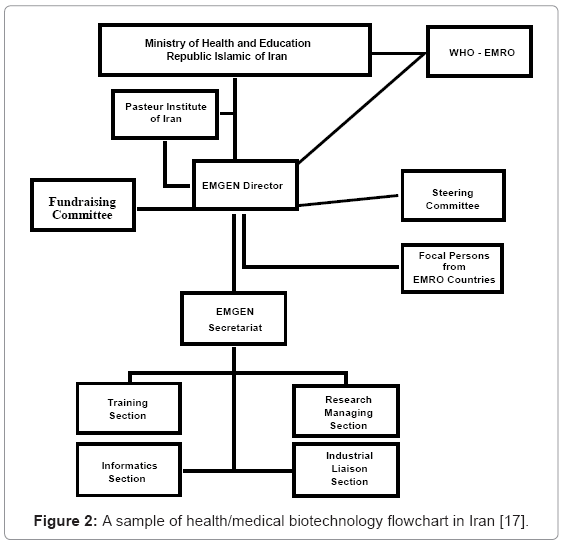
One of the modern sections of the Pasteur Institute of Iran, the Biotechnology Department, has been a pioneer in molecular biotechnology investment over the past years. The investment in molecular biotechnology was then continued by the National Research Centre for Genetic Engineering and Biotechnology (NRCGEB). In 1988, the Iranian Ministry of Science, Research and Technology supervised the establishment of NRCGEB association. Gene based research studies were the major activity of NRCGEB association in medical biotechnology by focusing on human genetic disorders, recombinant proteins and heterologous gene expressions. In 1997, the Biotechnology Department of the Iranian Pasteur Institute was officially entitled “Biotechnology Research Centre” (BRC). In 1997, a national organization named the Iranian Biotechnology Society (IBS) was established in Iran. Other important governmental bodies associated with biotechnology research in Iran include the Supreme Council in Biotechnology (est. 2005; under the direct supervision of the president), the Technology Cooperation Office of the Presidency, the Medical Biotechnology Committee (est. 1998; the Ministry of Health and Medical Education), the National Medical Biotechnology Network, (est. 2002; Deputy of Research and Technology, Ministry of Health and Medical education), the High-tech Industry Centre (est. 2001; Ministry of Industry), the Iranian Molecular Medicine Network (est. 2001; 34 joined research institutes and centers) and the Regional Health Genomics and Biotechnology Network (est. 2004; Eastern Mediterranean region, including centers from Jordan, Kuwait, Morocco, Oman, Pakistan, Saudi Arabia, Sudan, Syrian Arab Republic and Tunisia) (Figure 2).
An Overview of Current Medical Biotechnology in Iran
Biotechnology is one of the seven key technologies to achieve economy, food and public hygiene security [16]. Amongst Eastern Mediterranean regions, Iran is currently one of the most contributing countries to biotechnology [18]. Despite a negative concern in past years, there is now an outstanding interest for the biotechnology research and application in routine life within the society. In comparison, general acceptance of medical biotechnology including genetic experiments, pharmaceuticals research and environmental biotechnology (bioremediation) is very promising [19]. In general, Iran is one of the fast-growing innovators in biotechnology in Asia-pacific region. Various centers are now participated in biotechnological research. National Institute for Genetic Engineering and Biotechnology, Pasteur Institute, Razi Institute, Sharif University, Persian Gulf Biotechnology Research Center, major universities (Tarbiat Modares, Tehran, Shiraz, Mashhad, Isfahan and Tabriz universities) and Agriculture Biotechnology Research Center are some example of institutes which work in biotechnology field in Iran.
It has been predicted that more than 27 biotechnology products will be launched by 2013. Interestingly, Iran’s total local market value will estimate nearly 800 million US dollars while potential world’s market value is expected up to two billion US dollars annually. Now, the most potent Iranian organizations in medical and conventional biotechnology areas are Pasteur and Razi Institutes with more than nine decades of experience [18]. Biopharmacy, diagnosis, cell therapy and regenerative medicine are the current issues included in medical biotechnology in Iran. In the last decade, 12 biotechnology products have been registered in Iran. Furthermore, it is expected that more than 15 products of recombinant proteins and monoclonal antibodies are approved within a few years [5]. Fabricating a number of recombinant proteins in bio-pharmaceutics is now finished or given certification. Private companies manufactured a wide variety of recombinant proteins in molecular biology and molecular diagnostic kits. These biotechnology companies extensively export their products to Europe, South America, India, Egypt and Pakistan [18].
As a key player in biotechnology derived products, Razi Institute produces 21 human and veterinary vaccines in commercial scales and several other biological products. Over 16 countries (mostly Islamic countries) import human and animal vaccines produced by the Razi Institute. Medicine export scheme is one of humanitarian aid programs in Iran. According to Razi’s director, the institute manufactures 1.7 billion doses of 57 types of vaccines, serums and antigens every year [12]. Thus, Razi has become one of the biggest vaccine producers in the Middle East. The major products include medical antisera such as monovalent and polyvalent snake antivenom, monovalent and polyvalent scorpion antivenom, anti-tetanus and anti-diphtheria sera, bacterial vaccines such as trivalent DTP (diphtheria, tetanus, pertussis), bivalent dT (for adults), bivalent Td (for children) and tetanus, viral vaccines such as measles, mumps, rubella, polio, MMR, MR and MM, various poultry vaccines, veterinary viral vaccines and anti-parasite vaccines. Other main achievements include improvements in infertility treatment, cancer detection and anticancer drug synthesis [20]. Epizootological and ecological studies in fields of animal diseases and biology, as well as human biology, are some other examples of the main activities within the institute. The range of research conducted by the institute varies from clinical cytogenetics and chromosome studies in carcinomatous cells to molecular techniques for the clinical diagnosis [12]. Under the direct supervision of the head of the institute, current main activities of the Razi’s Biotechnology Department include synthesis of a new generation of livestock, bird and human vaccines, diagnostic studies of livestock and bird diseases based on the genetic assessments, and production of transgenic animals. Creation of recombinant vaccines using genetic engineering techniques and development of antigens and diagnostic kits for medical and veterinary laboratories are some other projects currently performed in the Biotechnology Department of the Razi Institute [12].
Further Examples of the Advances in Biotechnology Research in Iran
Recombinant proteins
Recombinant proteins have a wide variety of applications in medical biotechnology especially in disease prevention and immunology research. The Mtb32 is a nucleotide based structure as a fusion partner to improve the expression of fused recombinant proteins such as serine protease of M. tuberculosis which can lead to immune responses stimulation against the bacterium. The clone and expression of a vector containing Mtb32C fragment was constructed in a study by Nabavinia et al. [21]. The constructed vector was suitable for the design of fusion proteins and recombinant vaccines since it could enhance the expression of fused proteins and improve the immune responses against the protein [21].
Recombinant medicines
Production of recombinant medicines is the major experimental research carried out in animal biotechnology studies. A surprising improvement in drug biotechnology (recombinant medicines) in Iran is Interferon-beta 1a synthesis, one of the most expensive recombinant drugs in bio-pharmaceutics. Iranian researchers have succeeded in synthesis and fabrication of this drug with commercial name “CinnoVex®”. The product has been launched in three countries and has an annual sale of nearly 18 million US dollars [5].
Animal cloning
In 2006, an experimental reproductive animal cloning was conducted in Iran. This was the first successful experience in Middle East with the major aim of drug synthesis/fabrication. The study was carried out based on two different procedures and has led to the birth of the first Iranian cloned male lamb namely Royana. Another recent significant biotechnology achievement in Iran included the creation of two transgenic goats (Shangool and Mangool) which were able to secrete the human coagulation factor IX in their milk [22].
Alternative to Helicobacter pylori antibiotics
UreC protein is a subunit of urease enzyme expressed by H. pylori and is responsible for the neutralization of high acidic condition in the human stomach. It is presumed that the ability of IgY in urease neutralization is an appropriate potency in treatment of H. pylori infection. Indeed, it acts as an anti-UreC for H. pylori urease and hence could be a good substitution in antibiotic therapies. In the study carried out by Amiri Javid et al. [23], H. pylori UreC gene was cloned into eukaryotic pCI expression vector. Experimental results showed that DNA vaccination was an effective protocol in facilitating the IgY production against the UreC antigens of H. pylori and could be used in over expression of active and efficient antibodies instead of antibiotic therapy [23].
Stem cell techniques
Stem cell technology, as a field of medical biotechnology, had been experimentally used to cure a wide range of diseases during the last years. The Royan Institute of Iran has expanded a framework to investigate how ESCs differentiate into various cell types, including cardiomyocytes. In 2003, the Royan Institute constructed Iran’s first human ESC line from blastocytes, named Royan. With such an improvement, Iran became the 10th country worldwide in production, cultivation and freezing of human ESCs. A year later, Royan Institute had established five human ESC lines, named Royan H2–H6. Royan H2, H5 and H6 had normal karyotypes (46,XX and 46,XY) while Royan H3 and H4 cell lines had a triploid karyotype (69,XXY) which could differentiate to various cell types in vitro. In parallel, other Iranian research organizations were involved in regenerative medicine. These organizations included the Iranian Molecular Medicine Network, the Iran Polymer and Petrochemical Institute and Shahid Beheshti University of Medical Sciences [24].
Tissue engineering
Patients may need tissue and organ substitution or repair in the case of degenerative diseases and accidents such as traumas, age related disorders and end-stage organ failures. In a recent study, the condition of tissue engineering knowledge in various countries was assessed and classified according to different criteria. Iran was classified as a country with the existence of tissue engineering academic institutions and/or publications. In the Middle East, Iran has adopted similar approaches to this field (mainly academics) as well as Turkey, Saudi Arabia and Pakistan [25]. Bone tissue engineering is a relative technology developed in Iran. Kazemzadeh et al. [26] used a composite of hydroxyapatite and gelatin to stimulate the mineral and organic components of the natural bones. At the final stage, a good compatibility with fibroblast cells was observed. Results showed that the prepared scaffold had an open, interconnected porous structure which was appropriate for the osteoblast cell proliferation and possibly a suitable candidate for the trabecular bone tissue engineering. Therefore, cells cultured by this method could attach and proliferate very well [26].
Proteomics studies: Protein folding in biotechnology
Pharmaceutical recombinant proteins such as growth hormone, insulin and anti-hemophilic factor VIII were commonly expressed and overproduced in microorganisms such as E. coli. Since the over expression of genes in foreign hosts may form inclusion bodies, further treatments on the secreted proteins are required, including unfolding and refolding processes. Genetic engineering can also be used to increase protein stability. Studying the structural properties and folding processes, Mirzahoseini and Alibolandi investigated the effect of chaperones as heat shock proteins on human basic fibroblast growth factor stability [27].
Vaccine development
Cholera toxin B-subunit (CTB) is a pentameric structure which binds to its ganglioside receptors. CTB is administered in oral immunization and could be used in vaccine development. Scaling up in the production of CBT encountered any problems such as pathogenesis of bacterial species, high cost and labor dependent procedures. Shah- Hosseiny et al. [28] carried out studies in CTB genomic principles and cloned and expressed genes associated with CTB production in E. coli plasmids.
Bacterial diagnostic tools
Zarei et al. [29] set up an experimental diagnostic protocol for the detection of Mycoplasma genitalium (known as genitally transmitted organism). The approach was based on the production of polyclonal antibodies and their reactions to synthetic peptides derived from bacterial proteins. The researchers reported that the produced antibodies had a desired reaction with the immunogenic peptides [29].
Ethical and political debates
Owed to its promising metabolites, medical biotechnology is also described as “Technology of Hope”. Medical biotechnology products such as approved therapeutic agents and vaccines favor hundreds of millions of people all over the world [5]. However, this useful tool must be adapted with human beliefs and life philosophies to be generally accepted by the nations (ethical issues). The term “ethics” has a different definition at any region. For example, in Asia, it mostly focuses on human attitudes to nature and its relationship with other members of the biological community. In comparison, a modern North American concept describes human as the centered unit which must be respected and benefited anyway [30]. However, it is now clear that approaches used by other countries cannot exactly be employed elsewhere because the cultural difference dictates the application of different approaches. Iranian bioethics organizations currently include the Ministry of Health, Treatment and Medical Education, the Office of Study for Humanistic, Islamic Science on Medicine and Medical Ethics and the Iranian National Commission for UNESCO. The last organization has a national bioethics committee which includes several governmental and nongovernmental associations as membership. Furthermore, other permanent members are involved in the committee, including two philosophy and ethics experts, two lawyers, two biotechnology specialists, two biologists and one expert at each field of immunology, genetics, pharmacology, biochemistry, psychology and epidemiology [31].
Conclusion
Biotechnology has a proud history in Iran. Many achievements have been reached over the past decades. However, many others must be reached to secure the country’s place amongst the biggest players in this area. This requires an immediate action by the governments and private sectors; either alone will be insufficient. These anticipated improvements will be achieved via a precise development scheme, including specifying more funds on biotechnology research; training more experts and researchers and establishing new research centers and academies. Furthermore, protecting biotechnology patents and issuing up-to-date legislations which are matched to new global demands can facilitate this scheme. Another problematic issue which must be solved is the lack of a global plan for the export of biotechnology products to other countries. Nowadays, medical biotechnology products consist a significant portion of the global medicine trade. According to financial news, under-developed countries would dramatically become the major investigators of novel drugs and vaccines. Furthermore, the global biotechnology industries’ income will grow exponentially. The majority of these amounts belong to chemicals and pharmaceuticals. The Pasteur and Razi Institutes are the most potent Iranian organizations in medical and conventional biotechnology with a brilliant history in biotechnology products manufacture and export. Production and development of recombinant proteins and drugs, antibiotic alternatives, stem cell technology, tissue engineering, bacterial diagnostic tools and proteomics studies are some examples of biotechnological research currently carried out in Iran. However, as previously stated, more technical issues are still remained unattended which need seriously urgent actions.
References
- DaSilva EJ, Baydoun E, Badran A (2002) Biotechnology and the developing world. Electron J Biotechnol 5: 1-2.
- Bud R, Cantley MF (1994) The Uses of Life: A History of Biotechnology. Cambridge, Cambridge University Press, UK.
- Colwell RR (2002) Fulfilling the promise of biotechnology. Biotechnol Adv 20: 215-228.
- Nout MJR (1992) Upgrading traditional biotechnological processes. In: National Research Council (US). Panel on the Applications of Biotechnology to Traditional Fermented Foods, editors. Applications of Biotechnology to Traditional Fermented Foods. Washington DC, National Academies, pp. 11–19.
- Mahboudi F, Hamedifar H, Aghajani H (2012) Medical biotechnology trends and achievements in iran. Avicenna J Med Biotechnol 4: 200-205.
- Daar AS, Berndtson K, Persad DL, Singer PA (2007) How can developing countries harness biotechnology to improve health? BMC Public Health 7: 346.
- Macer DR (1994) Perception of risks and benefits of in vitro fertilization, genetic engineering and biotechnology. Soc Sci Med 38: 23-33.
- Dennehy PH (2001) Active immunization in the United States: developments over the past decade. Clin Microbiol Rev 14: 872-908.
- Afkhami A (2003) Compromised constitutions: the Iranian experience with the 1918 influenza pandemic. Bull Hist Med 77: 367-392.
- Cheraghali AM (2012) Iran pharmaceutical market. Iran J Pharml Res 1: 1-7.
- Azizi MH, Bahadori M, Raees-Jalali GA (2012) A historical profile of diphtheria in Iran during the 19th and 20th centuries. Arch Iran Med 15: 181-186.
- http://www.nti.org/
- Azizi MH (2010) A brief history of smallpox eradication in Iran. Arch Iran Med 13: 69-73.
- Azizi MH (2007) The historical backgrounds of the ministry of health foundation in Iran. Arch Iran Med 10: 119-123.
- Azizi MH, Nayernouri T (2008) The establishment and the first four decades of the activities of the Pasteur Institute of Iran. Arch Iran Med 11: 477-481.
- http://pasteur.research.ac.ir/
- http://www.vimb.basijmed.ir/
- Larijani B, Zahedi F (2007) Biotechnology, bioethics and national ethical guidelines in biomedical research in Iran. Asian Biotechnol Dev Rev 9: 43-56.
- Gaskell G, Allum N, Bauer M, Durant J, Allansdottir A, et al. (2000) Biotechnology and the European public. Nat Biotechnol 18: 935-938.
- http://www.rvsri.ac.ir/
- Nabavinia MS, Nasab MN, Meshkat Z, Derakhshan M, Khaje-Karamadini M (2011) Construction and Evaluation of an Expression Vector Containing Mtb32C (Rv0125) of Mycobacterium tuberculosis. Avicenna J Med Biotechnol 3: 207-210.
- http://www.royaninstitute.org/cmsen/
- Amiri Javid S, Mousavi SL, Salmanian AH, Basiri M (2011) Production of chicken egg yolk immunoglobulin against Helicobacter pylori UreC protein by DNA vaccination in chicken. Kowsar Med J 15: 197-202.
- Saniei M, De Vries R (2008) Embryonic stem cell research in Iran: status and ethics. Indian J Med Ethics 5: 181-184.
- Samadikuchaksaraei A (2010) Scientific and industrial status of tissue engineering. Afr J Biotechnol 6: 2897-2909.
- Kazemzadeh Narbat M, Orang F, Solati Hashtjin M, Goudarzi A (2006) Fabrication of porous hydroxyapatite-gelatin composite scaffolds for bone tissue engineering. Iran Biomed J 10: 215-223.
- Mirzahoseini H, Alibolandi M (2009) Stability of recombinant proteins in Escherichia coli: the effect of co-expression of five different chaperone sets. J Sci I R Iran 20: 305-310.
- Shah-Hosseiny M, Akbari M, Tabarrai B, Rechinsky V (1998) Basic science in medicine. Med J I R Iran 12: 123-128.
- Zarei O, Irajian GR, Zarnani AH, Chamani-Tabriz L, Emami S, et al. (2011) Peptide-based Polyclonal Antibody Production against P110 Protein of Mycoplasma genitalium. Avicenna J Med Biotechnol 3: 79-85.
- Bhardwaj M, Macer DR (2003) Policy and ethical issues in applying medical biotechnology in developing countries. Med Sci Monit 9: RA49-RA54.
- Zali MR, Shahraz S, Borzabadi S (2002) Bioethics in Iran: Legislation as the main problem. Arch Iran Med 5: 136-140.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 19578
- [From(publication date):
June-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14602
- PDF downloads : 4976