The Histopathological Spectrum of Primary Human Glioblastomas with Relations to Tumour Biology
Received: 10-Feb-2012 / Accepted Date: 24-Mar-2012 / Published Date: 26-Mar-2012 DOI: 10.4172/2161-0681.1000110
Abstract
Glioblastoma is the most common primary brain tumour in humans. Diagnostic challenges can occur as glioblastomas are highly heterogeneous tumours. The aim of this study was to investigate the frequencies and correlations between several histological features in primary glioblastomas and describe any link to tumour biology.
Two hundred consecutively diagnosed adult patients with primary glioblastoma located supratentorally were included after revision according to the 2007 World Health Organization criteria. Several histological features were examined. All tumours contained necrosis and/or microvascular proliferation, with frequencies of 98.5% (197/200) and 84.5% (169/200) respectively. Most tumours had detectable mitoses (192/200, 96%), with a median value of 8.5 per 10 high power fields. Further, pseudopalisades (158/200, 79%), thromboses (157/200, 78.5%), atypical mitoses (119/200, 59.5%), and haemorrhages (106/200, 53%) were other common features. Among relevant correlations necroses were positively associated with pseudopalisades, thromboses, small cells and mitoses.
In conclusion, glioblastomas present a variety of cell types and histological features, important to know and be aware of for the diagnostic pathologist. Interestingly, microvascular proliferation and necroses were frequently found in co-existence. Further, several of these features are closely linked to tumour biology, making histopathology fundamental for increased understanding of gliomagenesis.
Keywords: Glioma; Astrocytoma; Brain tumour; Classification; Morphology; Diagnosis
307545Introduction
Glioblastoma (diffuse astrocytoma, WHO grade IV) is the most frequent and malignant primary brain tumour in humans with a dubious prognosis despite intense research and new treatment modalities [1,2].
Light microscopy is still the gold standard for making diagnoses of astrocytic tumours and constitutes the basis for prognostications and treatment options. Various histopathological classification schemes have existed, and the diagnostic criteria have been regularly revised, much due to progress in immunohistochemistry and molecular genetics [1]. Further, new entities, variants, and morphological patterns are steadily being recognized. Actually, glioblastoma was not regarded as an astrocytic tumour before the WHO classification of 1993 [3,4]. Based on histology and molecular genetic studies glioblastoma subtypes include primary and secondary glioblastoma, giant cell glioblastoma, and gliosarcoma [1,5,6]. Other glioblastoma subsets are recognized as well [1,6]. Notwithstanding, the essential diagnostic criteria (necroses and microvascular proliferation) have been accepted for decades.
Histologically, glioblastomas are very heterogeneous tumours that may cause diagnostic difficulties. Even so, reports have shown satisfactorily interobserver agreement for glioblastomas, because of clear diagnostic criteria [7,8]. Few studies have, however, described the frequencies of various histopathological features of glioblastomas in the light of the latest WHO classification [1]. Actually, Kros [9] recently discussed this topic, inquiring more panel reviews and investigations of histopathological features of prognostic significance.
The histopathological features and growth patterns have formed the basis for understanding the biology of glioblastomas. For instance, hypoxic pseudopalisading cells can induce glioma angiogenesis and serve as a link between necrosis and microvascular proliferation [10-12]. Further, the secondary features of Scherer have been related to the infiltrative properties of neoplastic astrocytes [13,14].
In a recent study we investigated the prognostic significance of various histological features found in primary human glioblastomas [15]. Among the significant correlations, the presence of large necroses was independently connected to shorter overall survival, which reflects the need for continued evaluation of histological parameters.
The aim of this study was to examine the frequencies and correlations between the different histological features in these tumours, and to describe relevant relations to tumour biology.
Materials and Methods
Selection of tumour specimens
The inclusion criteria and patient selection are described elsewhere [15]. Briefly, the material represents adult patients with primary glioblastomas diagnosed at St. Olavs Hospital, Trondheim, Norway, over a ten-year period (1997 – 2006).
Histopathological evaluation
Haematoxylin-eosin-saffron (HES) stained sections from both formalin-fixed and paraffin-embedded frozen and unfrozen tissue were retrieved. New sections were made when staining had faded or sections could not be found. All sections were examined by an experienced neuropathologist (SHT) for revision of the histopathological diagnosis according to the latest WHO criteria (2007) [1]. Recording of histological features were made in collaboration between two of the authors (AHH and SHT) without knowledge of patient outcome. Histological analyses were performed using a Nikon 80i light microscope. High power fields (HPFs) were defined using the 40x objective. Descriptions of the histological features are presented in Table 1. Noteworthy, necroses were classified as either large infarct-like necroses or small necroses where tumour cells frequently formed a pseudopalisading pattern [1].
| Histological features | Classification | Description | Frequencies, n (%) |
|---|---|---|---|
| Necroses | 0) None 1) Small 2) Large 3) Large and small |
Areas with necrotic morphology | 0) 3/200 (1.5 %) 1) 34/200 (17.0 %) 2) 56/200 (28.0 %) 3) 107/200 (53.5 %) |
| Apoptoses | Apoptotic figures | 200/200 (100.0 %) | |
| Mitoses | Number of mitotic figures in 10 consecutive high power fields (HPFs) | Counted in the most proliferative active areas with the 40x objective (HPF) | Median: 8.5 Mean: 12.8 Range: 0 – 64 ≥1 mitosis: 192/200 (96 %) None: 8/200 (4.0 %) |
| Microvascular proliferation | 1) Glomeruloid tufts 2) Endothelial proliferation 3) Both 4) None |
Present or not present, in the tumour centre and the infiltrating front | 1)130/200 (65.0 %) 2) 164/200 (82.0 %) 3) 136/200 (63.0 %) 4) 31/200 (15.5 %) |
| Cell types | 1) Gemistocytes 2) Small cells 3) Spindle cells 4) Epitheloid cells |
When present in more than 20% of the tumour | 1) 67/200 (33.5 %) 2) 42/200 (21.0 %) 3) 19/200 (9.5 %) 5) 4/200 (2.0 %) |
| Subtypes | 1) Small cell glioblastoma 2) Giant cell glioblastoma 3) Gliosarcoma |
Tumours consisting predominantly of one cell type | 1) 14/200 (7.0 %) 2) 2/200 (1.0 %) 3) 0/200 (0.0 %) |
| Giant cells | 0) None 1) Sparsely 2) Moderate 3) Common |
Large, often multinucleated, highly atypical cells | 0) 112/200 = (56.0 %) 1) 53/200 = (26.5 %) 2) 27/200 = (13.5 %) 3) 8/200 = (4.0 %) |
| Oligodendroglioma component | 0) None 1) Present in < 20 % 2) Present in > 20 %. |
Areas of oligodendroglioma-like morphology | 0)187/200 (93.5 %) 1) 8/200 (4.0 %) 2) 5/200 (2.5 %) |
| Perivascular lymphocyte infiltration | 73/200 (36.5 %) | ||
| Macrophage infiltration | 52/200 (26.0 %) | ||
| Haemorrhages | Blood degradation or haemosiderine-laden macrophages | 106/200 (53.0 %) | |
| Thromboses | Thrombotic occluded vessels | 157/200 (78.5 %) | |
| Calcification | 24/200 (12.0 %) | ||
| Pseudopalisades | 158/200 (79.0 %) | ||
| Atypia | 1) Mild 2) Moderate 3) Severe |
1) 15/200 (7.5 %) 2) 134/200 (67.0 %) 3) 51/200 (25.5 %) |
|
| Cell density | 1) Low 2) Moderate 3) High |
1) 7/200 (3.5 %) 2)141/200 (70.5 %) 3) 52/200 (26.0 %) |
|
| Pseudorosettes | 93/200 (46.5 %) | ||
| Desmoplasia | 88/200 (44.0 %) | ||
| Nucleoli | 23/200 (11.5 %) | ||
| Atypical mitoses | 119/200 (59.5 %) | ||
| Microcysts | 58/200 (29.0 %) | ||
| Mucin | 66/200 (33.0 %) | ||
| Leptomeningeal infiltration | 45/200 (22.5 %) | ||
| Secondary structures of Scherer | 1) Perineural growth 2) Angiocentric growth 3) Subpial cell-clustering 4)One or more phenomena |
Assessment of Scherer phenomena was possible in 147 and 127 cases, respectively | 1) 52/147 (34.5 %) 2) 40/147 (27.2 %) 3) 20/124 (16.1 %) 4) 73/152 (48.0 %) |
Table 1: Histopathological characteristics in 200 primary glioblastomas.
The study was conducted in accordance with the Helsinki declaration of 2008, and approved by the Regional Committee for Medical and Health Research Ethics.
Statistical analyses
Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL). Associations between dichotomous variables were tested with 2 x 2 contingency tables and p-values were computed by Fisher’s exact or Pearson’s chi-square tests. For non-dichotomous categorical data r x c contingency tables and Pearson’s chi-square tests were assessed, while relations between ordinal and continuous variables were examined by linear regression. To investigate relations between dichotomous and continuous variables, independent sample T-tests were used. P value < 0.05 was regarded statistically significant.
Results
Frequencies
This study comprised of 200 patients with primary glioblastoma (114 males and 86 females, ratio 1.3/1) with a median age of 62 years (range 21-84 years). The histological features and their frequencies are presented in Table 1. In addition to the essential diagnostic criteria (necroses and/or microvascular proliferation), apoptosis were observed in all the tumours. Mitoses were recorded in 192/200 cases (96 %) with a median value of 8.5 per 10 HPFs, and 119/200 cases (59.5 %) presented atypical ones. Further, pseudopalisades (158/200, 79 %), thromboses (157/200, 78.5 %), and haemorrhages (106/200, 53 %) were the most frequent features.
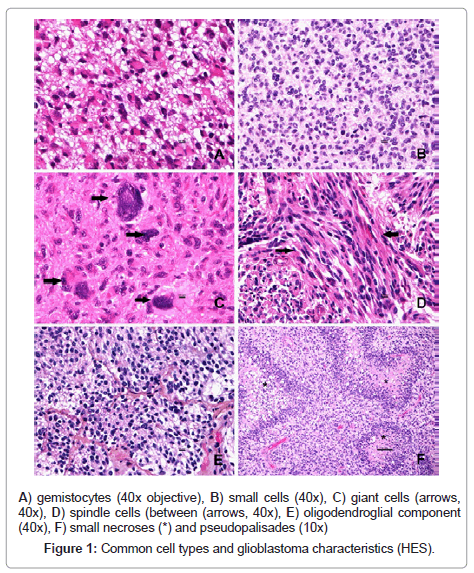
14/200 tumours (7 %) were classified as small cell glioblastoma, 2/200 (1 %) as giant cell glioblastoma and none as gliosarcoma. When including tumours composed of more than 20 % of a cell type, we found 67/200 (33.5 %) with gemistocytes, 42/200 (21 %) with small-cells, and 19/200 (9.5 %) with spindle (sarcomatous) cells. Giant cells and oligodendroglial components were seen in 88/200 (44 %) and 13/200 (6.5 %) tumours respectively. Some typical glioblastoma characteristics and major cell types are shown in Figure 1.
Relations
Major significant correlations are shown in Table 2. Large necroses correlated significantly with pseudorosettes (p < 0.001), thromboses (p < 0.001), haemorrhages (p = 0.018), pseudopalisades (p = 0.026), and perineural growth (p = 0.031). Small necroses correlated significantly with pseudopalisades (p < 0.001), cell density (p < 0.001), small cells (p = 0.001), higher number of mitoses (p = 0.001), desmoplasia (p = 0.03), and centrally located microvascular proliferation (p = 0.033). Pseudopalisades were also seen in significant relation to higher number of mitoses (p < 0.001), thromboses (p = 0.006), glomeruloid tufts (p = 0.006), pseudorosettes (p = 0.009), higher age (p = 0.032), and endothelial proliferation (p = 0.045). Additionally, significant higher mitotic values were seen in tumours with pseudorosettes (p < 0.001), thromboses (p = 0.006), atypical mitoses (p < 0.001), centrally located microvascular proliferation (p = 0.02), and tumours with small cells (p < 0.001). Higher mitotic activity also correlated with increasing atypia (p < 0.001), giant cells (p < 0.001), and cell density (p < 0.001). Apart from correlations between perineural and angiocentric growth (p < 0.001) and subpial cell-clustering and leptomeningeal infiltration (p = 0.018), secondary structures of Scherer and age correlated poorly to most of the histological features. Gemistocytes were frequently observed together with perivascular lymphocytes (p = 0.002), and these tumours had lower number of mitoses as well (p = 0.001).
| Histological features | Associated with (p value) | ||||
|---|---|---|---|---|---|
| Large necroses | Thromboses (<0.001) | Pseudorosettes (<0.001) | Haemorrhages (0.018) | Pseudopalisades (0.026) | Perineural growth (0.031) |
| Small necroses | Pseudopalisades (<0.001) | Small cells (0.001) | Central microvascular proliferation (0.033) | Desmoplasia (0.030) | |
| Microvascular proliferation | Microcysts (0.001) | Small cells (0.031) | Small necroses (0.038) | ||
| Pseudopalisades | Small necroses (<0.001) | Thromboses (0.001) | Glomeruloid tufts (0.006) | Pseudorosettes (0.009) | Large necroses (0.026) |
| Perivascular lymphocytes | Gemistocytes (0.002) | Nucleoli (0.005) | Macrophages (0.012) | ||
| Cell density | Small cells (<0.001) | Small necroses (<0.001) | Atypical mitoses (0.001) | Gemistocytes (0.005)1 | Giant cells (0.026) |
| Atypia | Giant cells (<0.001) | Atypical mitoses (<0.001) | Small cells (0.001)1 | Nucleoli (0.005) | Perivascular lymphocytes (0.041) |
| Giant cells | Atypia (<0.001) | Atypical mitoses (<0.001) | Nucleoli (0.008) | Small cells (0.019)1 | Perivascular lymphocytes (0.023) |
| More mitoses2 | Cell density (<0.001) | Atypia (<0.001) | Pseudopalisades (<0.001) | Giant cells (<0.001) | Thromboses (0.006) |
| Small cells (<0.001) | Small necroses (<0.001) | Atyical mitoses (<0.001) | Pseudorosettes (<0.001) | ||
| Fewer mitoses2 | Gemistocytes (0.001) | ||||
Table 2: Major significant associations between histological features.
Discussion
The present study has clearly confirmed the variegated histopathology of primary human glioblastomas. Additionally, we have demonstrated associations between various histological features and discussed some relations to tumour biology.
Primary glioblastoma is the most common glioblastoma type and differs from secondary glioblastoma by developing in older patients without a previous lesion (de novo) [1,5]. The median age of 62 years in this material is consistent with that of primary glioblastomas [5]. As the age ranged from 21 to 84 years, this type of glioblastoma is not only restricted to higher age groups. Among other glioblastoma subtypes we recorded two cases with giant cell glioblastoma (2/200, 1%). Compared to previous studies this percentage is low, however, the reported frequencies are varying [6,16,17]. This may be due to the poor definition of these tumours as to what extent such tumour cells are required. Sampling errors and small biopsies could also contribute to misclassification. Additionally, giant cell glioblastomas are reported to be more frequent in secondary glioblastomas, as they are suggested to represent a combination of primary and secondary glioblastomas [1,6,17]. Concerning gliosarcomas, we did not encounter this variant in our material. This subtype is rare, constituting about 2% of all glioblastomas [1,6]. As for giant cells, sarcomatous changes were frequently encountered (19/200, 9.5%), so this entity is a definition issue as well. We defined 14 out of 200 (7%) tumours as small cell glioblastomasthis which is in accordance with others [6,18]. We cannot, however, exclude the possibility that some of these tumours were anaplastic oligodendrogliomas. To distinguish between these two entities, analyses of epidermal growth factor receptor (EGFR) amplification and loss of heterozygosity (LOH) 1p/19q should be performed [6,18,19]. Glioblastomas with oligodendroglial component occurred in 6.5% (13/200) in parallel with others [6,16]. This entity is reported to have a more favourable prognosis than the conventional glioblastomas [6,16,20], however, it is hampered by poor histological definitions [9,20]. In addition to the above mentioned subtypes, glioblastomas present a cytological heterogeneity [1,16,21]. Most common in our study were gemistocytes (67/200, 33.5%), small cells (42/200, 32%), giant cells (35/200, 17.5%), and sarcomatous cells (19/200, 9.5%). These frequencies relate to the various subtypes, suggesting that the different tumour cell types are present in a continuum. All in all, the subsets of glioblastomas represent controversies in diagnostic neuropathology. Actually, the diverse cellular composition of glioblastomas may be explained by acquisition of various genetic alterations [5,16-17,22], underlining the coming need for molecular genetics for improving the glioblastoma diagnosis.
Mandatory for the histopathological diagnosis of glioblastoma are serpiginous areas of necrosis and/or microvascular proliferation. Necroses occurred in almost all cases (197/200, 98.5%), confirming their pivotal role as a major diagnostic criterion for glioblastomas. Two separate ways are proposed in the development of necroses [23,24]. Large areas of necroses may form as a consequence of thromboses in large vessels, probably due to coagulation abnormalities in the tumours [23,24], while extensive cell proliferation combined with pathological blood vessels constitute the basis for small necrosis [23,24]. When separating the necroses into these two categories, large necroses correlated significantly to thromboses (p < 0.001), whereas tumours with small necroses showed significantly higher numbers of mitoses (p < 0.001) and increasing cell-density (p < 0.001), supporting the above mentioned hypotheses of necrosis development. According to our recent study on glioblastoma survival [15], the presence of large necroses was independently connected to shorter overall survival, and this may reflect the importance of coagulation abnormalities and infarctions in the survival of glioblastoma patients.
Microvascular proliferation consists of pathologic vessels formerly termed endothelial proliferation and glomeruloid tufts [1,6,20] composed by proliferating endothelial cells, smooth muscle cells, and pericytes [1,20]. This vascular proliferation is often found in relation to hypoxia and is stimulated by proangiogenetic factors including hypoxia inducible factor 1 (HIF 1), vascular endothelial growth factors (VEGFs), IL 8, and platelet-derived growth factor (PDGF) [11,12,25]. In our series of glioblastomas microvascular proliferation was commonly seen (169/200, 84.5%), with endothelial proliferation more common (164/200, 82%) than glomeruloid tufts (131/200, 65.5%). These pathological vessels were irregularly distributed in the tumour tissue occurring both in the centre of the tumour in relation to necroses and in the infiltrative front as described by others [1,23]. We demonstrated that centrally located microvascular proliferation significantly correlated to small necroses (p = 0.033) and higher mitotic activity (p = 0.02), indicating a coupling of these processes and possibly related to the malignant progression of glioblastomas. Contrary, no correlation between pathological vessels located in the periphery to mitoses or necroses were achieved (data not shown), which may reflect that these vessels are not stimulated by the same mechanisms. Formation of new blood vessels is, however, regarded as tightly linked to pre-existing ones and reflects the expansive and infiltrating nature of glioblastomas [12,23,25,26].
Highly characteristic for glioblastomas are also the rows of tumour cells surrounding the necrotic areas, so-called pseudopalisades. We found these structures adjacent to both small (p < 0.001) and large necroses (p = 0.026), as well as in combination with glomeruloid tufts (p = 0.006), endothelial proliferation (p = 0.045), and thromboses (p < 0.001). These observations are in accordance with pseudopalisading cells being formed by hypoxic actively migrating neoplastic astrocytes moving away from a hypoxic region [10,11]. The close association to vascular pathology may be explained by these cells’ expression of HIF 1 and VEGF, and pseudopalisades may be considered as a link between necroses and microvascular proliferation [10-12]. The common occurrence of thrombosed blood vessels within pseudopalisades [10] is also supported by our results, and vascular occlusion may indeed be an initiate event for the development of necroses, pseudopalisades, and microvascular proliferation. We speculate that pseudopalisades may form as a response to hypoxia independently of what causes the hypoxia or necrosis.
Glioblastomas are characterized by high mitotic activity, as shown in this study as well with both high occurrence (≥1 mitosis in 192/200, 96%) and large numbers of mitoses (median: 8.5 per 10 HPFs). We experienced, however, a great range of mitotic figures (0-64 per 10 HPF), and the fact that glioblastomas may have few or no mitoses (8/200, 4%), is important to be aware of in the histopathological examination. Glioblastomas with small cells had higher numbers of mitoses (p < 0.001), while gemistocytic differentiated tumours had fewer than the others (p = 0.001). This is in agreement with gemistocytes being less proliferative and possibly terminally differentiated tumour cells [27], while small cells correlated well with higher cell density (p < 0.001) and less atypia (p = 0.001) in accordance with the description as highly proliferative monotonous cells [1,6,18,19]. Atypical mitoses were frequently found (119/200, 59.5%) which may reflect the high cell turn over and the abnormal and instable genomic material in glioblastomas.
The high frequency of secondary structures of Scherer [14] (73/152, 48%) clearly demonstrates the infiltrative nature of diffuse astrocytomas and represents the main cause for non-radical tumour resection and the high recurrence rate [13,26]. In agreement with Scherer [14] these features commonly occurred in combination with a strong correlation between perineural and perivascular growth (p < 0.001). Further, subpial cell growth and leptomeningeal infiltration correlated significantly (p = 0.018), suggesting that the meninges are only a temporary obstacle for tumour infiltration. Apart from that, the secondary structures correlated poor with other histological features. This may reflect the pronounced heterogeneous population of neoplastic astrocytes with various characteristics, exemplified by the hypothesis that infiltrating tumour cells may transiently arrest from the cell cycle during the migration phase [28]. Accordingly, in glioblastomas there are clones of tumour cells involved in various processes in the gliomagenesis such as angiogenesis, proliferation, and infiltration, pointing to a multimodal therapeutic approach against these tumours.
Which role the immune system plays in the glioma biology is questionable [2]. In our material perivascular lymphocytes were seen in 36.5% (73/200) of the tumours. The lymphocyte infiltration was in general sparse and focal. Anyway, the relation seen between lymphocytes and macrophages (p = 0.012) may suggest an immune response in these tumours. Even though we did not identify macrophages by immunohistochemistry, we observed such cells in a quarter (52/200, 26%); noteworthy in the differential diagnosis to radiation necroses and cerebral infarction where macrophages are commonly presented [29,30]. In accordance with others, lymphocytes were commonly seen in tumours with gemistocytic differentiation (p = 0.002) [1,31]. The cause of this relation remains unclear.
In conclusion, our study confirms the highly heterogeneous histology in glioblastomas, making the diagnosis of these tumours challenging, however, a consistent finding was the frequent co-existence of necroses and microvascular proliferation. Since histopathology is closely associated with various aspects of tumour biology, this constitutes a basis for the pathogenesis of these tumours, and is thus fundamental for the ongoing research on diagnostic and therapeutic aspects. Still histopathology remains the gold standard in making the diagnosis of glioblastomas, however, molecular genetic analyses will gradually be included for a more accurate diagnosis.
References
- Kleihues P, Burger P C, Aldape K D, Brat D J, Biernat W, et al. (2007) Glioblastoma, in: WHO classification of tumours of the central nervous system(4thedn), International Agency for Research on Cancer (IACC), Lyon.
- Vauleon E, Avril T, Collet B, Mosser J, Quillien V (2010) Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol 2010.
- Kleihues P, Burger PC, Scheithauer BW (1993) The new WHO classification of brain tumours. Brain Pathol 3: 255-268.
- Kleihues P, Burger P C, and Scheithauer B W (1993) Histological typing of Tumours of the Central Nervous System. Springer-Verlag, Berlin Heidelberg.
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, et al. (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64: 6892-6899.
- Miller CR, Perry A (2007) Glioblastoma. Arch Pathol Lab Med 131: 397-406.
- Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK (1997) Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer 79: 1381-1393.
- Daumas-Duport C, Scheithauer B, O'Fallon J, Kelly P (1988) Grading of astrocytomas. A simple and reproducible method. Cancer 62: 2152-2165.
- Kros JM (2011) Grading of gliomas: the road from eminence to evidence. J Neuropathol Exp Neurol 70: 101-109.
- Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, et al. (2004) Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res 64: 920-927.
- Rong Y, Durden DL, Van Meir EG, Brat DJ (2006) 'Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol 65: 529-539.
- Wong ML, Prawira A, Kaye AH, Hovens CM (2009) Tumour angiogenesis: its mechanism and therapeutic implications in malignant gliomas. J Clin Neurosci 16: 1119-1130.
- Laws ER Jr, Goldberg WJ, Bernstein JJ (1993) Migration of human malignant astrocytoma cells in the mammalian brain: Scherer revisited. Int J Dev Neurosci 11: 691-697.
- Scherer H J (1938) Structural development in gliomas. The American Journal of Cancer 34: 333-351.
- Habberstad A H, Lind-Landström T, and Torp S H (2012) Primary human glioblastomas - prognostic value of clinical and histopathological parameters. Clinical Neuropathology - Accepted for publication.
- Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, et al. (2006) Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol 65: 846-854.
- Kleihues P, Ohgaki H (2000) Phenotype vs genotype in the evolution of astrocytic brain tumors. Toxicol Pathol 28: 164-170.
- Perry A, Aldape KD, George DH, Burger PC (2004) Small cell astrocytoma: an aggressive variant that is clinicopathologically and genetically distinct from anaplastic oligodendroglioma. Cancer 101: 2318-2326.
- Burger PC, Pearl DK, Aldape K, Yates AJ, Scheithauer BW, et al. (2001) Small cell architecture--a histological equivalent of EGFR amplification in glioblastoma multiforme? J Neuropathol Exp Neurol 60: 1099-1104.
- Scheithauer BW, Fuller GN, VandenBerg SR (2008) The 2007 WHO classification of tumors of the nervous system: controversies in surgical neuropathology. Brain Pathol 18: 307-316.
- Burger PC, Green SB (1987) Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer 59: 1617-1625.
- Fujisawa H, Kurrer M, Reis RM, Yonekawa Y, Kleihues P, et al. (1999) Acquisition of the glioblastoma phenotype during astrocytoma progression is associated with loss of heterozygosity on 10q25-qter. Am J Pathol 155: 387-394.
- Louis DN (2006) Molecular pathology of malignant gliomas. Annu Rev Pathol 1: 97-117.
- Schiffer D (1998) Classification and biology of astrocytic gliomas. Forum (Genova) 8: 244-255.
- Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D (2005) Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 15: 297-310.
- Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21: 1624-1636.
- Watanabe K, Tachibana O, Yonekawa Y, Kleihues P, Ohgaki H (1997) Role of gemistocytes in astrocytoma progression. Lab Invest 76: 277-284.
- Bolteus AJ, Berens ME, Pilkington GJ (2001) Migration and invasion in brain neoplasms. Curr Neurol Neurosci Rep 1: 225-232.
- Prayson R A, Cohen M L (2000) Radiation Change, in: Practical Differential Diagnosis in Surgical Neuropathology.(1stedn), Human Press Inc., Totowa.
- Kochanek PM, Hallenbeck JM (1992) Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke 23: 1367-1379.
- Burger PC, Vogel FS, Green SB, Strike TA (1985) Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer 56: 1106-1111.
Citation: Habberstad AH, Lind-Landström T, Torp SH (2012) The Histopathological Spectrum of Primary Human Glioblastomas with Relations to Tumour Biology. J Clinic Experiment Pathol 2:110. DOI: 10.4172/2161-0681.1000110
Copyright: © 2012 Habberstad AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16864
- [From(publication date): 4-2012 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 12071
- PDF downloads: 4793