The Gastric Precancerous Cascade
Received: 02-Sep-2013 / Accepted Date: 31-Oct-2013 / Published Date: 04-Nov-2013 DOI: 10.4172/2161-0681.1000147
Abstract
Although most gastric cancers are first detected after the fifth decade of life, they represent the end product of a prolonged precancerous process initiated during childhood as a consequence of the infection of the mucosa with Helicobacter pylori. The initial phase of the process consists of active non-atrophic gastritis. The following stages are: multifocal atrophic gastritis, intestinal metaplasia (initially of complete type, then incomplete type), dysplasia and finally invasive carcinoma. The progression of the lesions is modulated by other factors such as the diet: excessive salt intake accelerates the process while fresh fruits and vegetables play a protective role. In addition, the geographic location influences the process. In Colombia, the high altitude Andes mountains dwellers display more advanced lesions than the residents of the Pacific coast. It appears that the ancestral origin of the infecting H. pylori strains have a determining influence on their capacity to induce precancerous lesions. The H. pylori strains infecting the mountain dwellers are of predominantly European phylogeographic origin. The H. pylori strains infecting the Pacific coast dwellers are predominantly of African origin, probably less virulent. Chemoprevention trials have been partially effective, when the precancerous lesions are not too advanced. Such trials may not eliminate the infection but have a tendency to eliminate preferentially the most virulent bacterial strains.
Keywords: Gastric Precancerous Lesions; Gastric Cancer; Gastric Adenocarcinoma; Helicobacter pylori; Gastric Atrophy; Multifocal Atrophic Gastritis; Intestinal Metaplasia; Dysplasia; Surveillance
311135Abbreviations
CAG PAI: Cytotoxin-Associated Gene Pathogenicity Island; IM- Intestinal Metaplasia; PG- Pepsinogen; SPEM- Spasmolytic Polypeptide Expressing Metaplasia
Introduction
Although gastric cancer incidence and mortality rates have been slowly declining during the last six decades in many countries, this cancer is still a major health burden worldwide. Gastric cancer represents the second most frequent cause of cancer-related deaths and ranks 4th in cancer incidence [1]. More than 95% of gastric cancer cases are adenocarcinoma, which were classified mainly into intestinal and diffuse types by Lauren and Jarvi, two Finnish pathologists who also proposed that at least half of the gastric carcinomas originate in islands of intestinal epithelium in the gastric mucosa [2,3]. Further observations led to propose that gastric adenocarcinoma of the intestinal type is the final stage of a decades-long inflammatory process characterized by a series of histological changes in the gastric mucosa, as follows: non-atrophic gastritis → multifocal atrophic gastritis without intestinal metaplasia→ intestinal metaplasia, complete type →intestinal metaplasia, incomplete type → dysplasia → adenocarcinoma [4,5]. This precancerous sequence is triggered by the infection with Helicobacter pylori (H. pylori), which usually occurs during childhood in developing countries [6-8]. It has been estimated that 5.5% of the total number of cancer cases and 63% of gastric cancer cases worldwide are attributable to H. pylori infection [9]. For this reason, gastric cancer is considered an infectious disease. There is a wide variety of factors that may interact to determine the outcome of H. pylori infection and the rate of progression of precancerous lesions, including H. pylori virulence, host responses and environmental and dietary factors.
Multiple studies have demonstrated that populations at higher risk of gastric cancer have higher prevalence of precancerous lesions [10-12]. Likewise, patients with premalignant gastric lesions are at considerable risk of gastric cancer. A large study including 92,250 subjects with premalignant gastric lesions at baseline showed that within 1, 5, and 10 years of follow-up after initial diagnosis, gastric cancer was diagnosed in 0.3%, 0.6%, and 0.8% of patients with atrophic gastritis; 0.7%, 1.2 %, and 1.8% of patients with intestinal metaplasia; 2.1%, 3.1%, and 3.9% of patients with mild-to-moderate dysplasia; and 24.9%, 29.5%, and 32.7% of patients with severe dysplasia [13].
The Infectious Agent
The main recognized etiologic factor for gastritis, peptic ulcers and gastric cancer is H. pylori, a bacterium that infects the gastric mucosa of more than half of the world’s population, with higher prevalence rates in the developing world [9]. The isolation of H. pylori for the first time in 1982 and the identification of its role in causing gastritis and peptic ulcers was achieved in Australia by Marshall and Warren [14], who were awarded the Nobel Prize in 2005 for their discovery. Several studies then associated the development of gastric cancer with the H. pylori infection [15-17] and in 1994 the international Agency for Research in Cancer recognized the infection with H. pylori as a type I human carcinogen [18].
H. pylori are a spiral, gram-negative bacterium equipped with a series of mechanisms that guarantee its survival within the acid gastric niche. H. pylori strains have an extraordinary genetic diversity [19] which partially explains the fact that less than 1% of people infected with H. pylori will ever develop gastric cancer. There are multiple recognized H. pylori virulence factors, including cagA, vacA, babA2, iceA and sabA [20-22]. Probably the most intensively studied virulence determinant is the Cytotoxin-Associated Gene Pathogenicity Island (cag PAI). One of the genes within the cag PAI encodes CagA, an oncoprotein that is Trans located into host epithelial cells by a type IV secretion system [23,24]. CagA interacts with numerous intracellular effectors resulting in pro inflammatory and mitogenic responses, disruption of cell-cell junctions, loss of cell polarity, and morphologic transformation of the epithelial cells [20]. In addition, H pylori CagA induces the production of the enzyme spermine oxidase, which induces oxidative damage in epithelial cells, creating a subpopulation of cells that are resistant to apoptosis and thus at high risk for malignant transformation [25]. Subjects infected with strains that contain the cagA gene are associated with more severe gastric pathology and gastric adenocarcinoma than subjects infected with cagA-negative strains [26-29]. It is estimated that the cag PAI is present in approximately 60-70% of Western H. pylori strains and virtually 100% of East-Asian H. pylori strains [30]. In Colombia, a country with high incidence of gastric cancer, it is estimated that more than 80% of the adult population is infected with H. pylori and 70-80% of the strains obtained from symptomatic patients are cagA-positive [31,32].
Despite somewhat contradictory results and an unexplained biologic mechanism, blood group A has been considered a risk factor for gastric cancer. A recent study in Venezuela found that in carriers of cagA-positive H. pylori strains an increased risk of intestinal metaplasia and dysplasia was associated with blood group A [33].
Besides the mentioned virulence determinants, the phylogeographic origin of the H. pylori strains seems to play a role in the presence and severity of gastric precancerous lesions. Multilocus sequence typing of seven housekeeping genes was used to identify the ancestral origin of 64 cagA-positive H. pylori isolates from two populations in Colombia with contrasting gastric cancer risks. In the southern state of Nariño, the inhabitants of Tuquerres in the Andes Mountains (3,000 meters above the sea level), are predominately of Amerindian extraction and have an elevated risk of gastric cancer. The town of Tumaco, on the Pacific coast and approximately 200 kilometers from Tuquerres, is inhabited by African descendants that have a low risk of gastric cancer. All H. pylori isolates from the habitants of the mountains displayed a predominantly European phylogeographic origin. By contrast, two thirds of the H. pylori isolates from African descendants living on the coast displayed predominantly African origin and one third displayed European origin. Overall, subjects carrying H. pylori strains of European origin showed more advanced gastric precancerous lesions and greater oxidative damage in the gastric mucosa than subjects harboring strains of African genotype [34]. Further studies revealed that Colombian strains of European phylogeographic origin expressed higher levels of the oncoprotein CagA than did strains of African origin [35] and a bacterial DNA motif was associated with more advanced precancerous lesions [35].
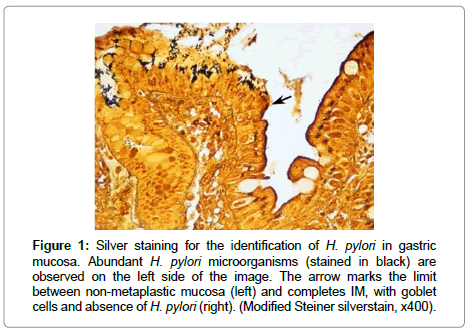
It is important to note that retrospective studies on the risk of gastric cancer in relation to H. pylori infection underestimate the true risk, due to loss of the infection with the onset of precancerous lesions. H. pylori usually do not colonize gastric mucosa with intestinal metaplasia, dysplasia or cancer, and there is evidence that with the development of advanced gastric lesions the organism can be lost from the stomach (Figure 1).
Figure 1: Silver staining for the identification of H. pylori in gastric mucosa. Abundant H. pylori microorganisms (stained in black) are observed on the left side of the image. The arrow marks the limit between non-metaplastic mucosa (left) and completes IM, with goblet cells and absence of H. pylori (right). (Modified Steiner silverstain, x400).
The Gastric Precancerous Cascade
The stages of the precancerous cascade for gastric adenocarcinoma are a series of histologically recognizable changes in the gastric mucosa that follow the sequence: non-atrophic gastritis, multifocal atrophic gastritis, complete intestinal metaplasia, incomplete intestinal metaplasia, and dysplasia. Long-term observations in populations at high risk of gastric cancer have shown that this process may take decades before malignant transformation is observed [36,37]. The gastric mucosa in patients with gastric adenocarcinoma frequently shows the presence of all the steps in this cascade.
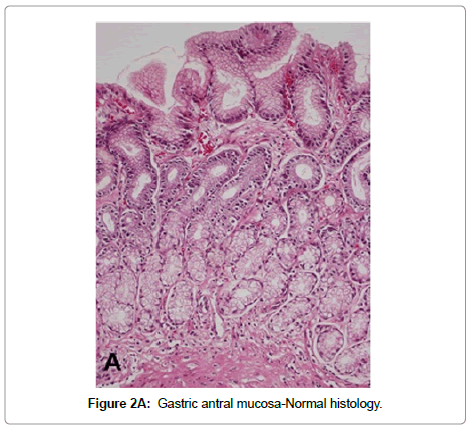
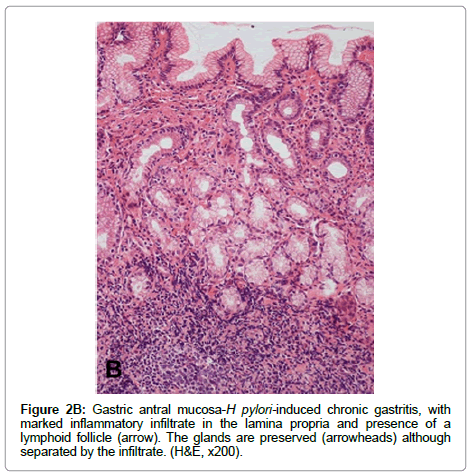
Non-atrophic gastritis H. pylori infection is the most common condition causing chronic gastritis. Upon colonization of the gastric mucosa, H. pylori elicit an acute and chronic inflammatory response characterized by infiltration of polymorpho nuclear neutrophils and mononuclear cells, predominantly lymphocytes and plasma cells in the lamina propria. The term “active gastritis” is used to denote the presence of neutrophils, which is a very sensitive marker of the presence of H. pylori. Lymphoid aggregates with germinal centers are frequently observed and are considered characteristic of H. pyloriinduced chronic gastritis (Figure 2). Other histological changes include intraepithelial neutrophilic or lymphocytic infiltrate, pit abscesses, mucus depletion, epithelial detachment, and foveolar hyperplasia. When H. pylori is eradicated, the neutrophilic infiltrate disappears within days but the chronic infiltrate may remain for months or years [38]. The Updated Sydney System for the classification of gastritis has been a useful tool for the understanding between pathologists and gastroenterologists. This classification combines topographical, morphological and etiological information for non-atrophic and atrophic (non-metaplastic and metaplastic) gastritis and provides a visual scale for grading of histological variables [38].
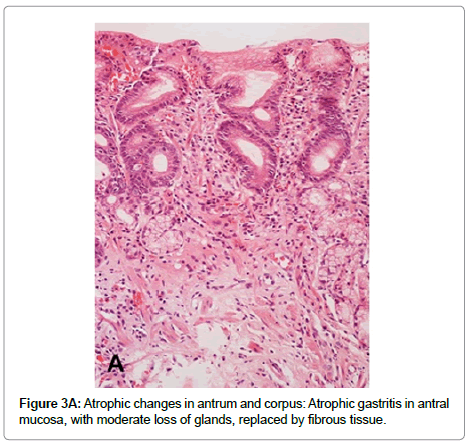
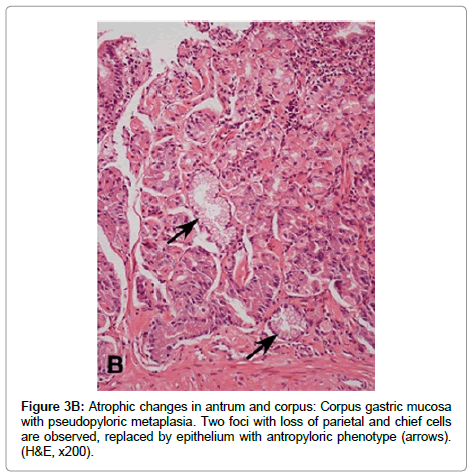
Multifocal atrophic gastritis Prolonged and severe chronic active inflammation may lead to permanent damage of epithelial cells and subsequent loss of glands (atrophy). In addition, as a result of the sustained inflammatory process, deposit of fibrous tissue may be observed in the lamina propria (Figure 3A). Because H. pylori colonize initially the gastric antrum, both inflammatory and atrophic changes are initially and more frequently observed in the antral mucosa. Over time, the bacterium may disseminate proximally to the oxyntic mucosa, sometimes favored by the decrease in gastric acid secretion caused by the use of proton pump inhibitors. H. pylori colonization and its subsequent inflammatory process in the oxyntic mucosa may result in loss of parietal and chief cells, which are frequently replaced by cells with mucous phenotype similar to antro pyloric epithelial cells. This lesion is called pseudopyloric metaplasia or Spasmolytic Polypeptide Expressing Metaplasia (SPEM) (Figure 3B). In pseudopyloric metaplasia the number of glands may not be reduced, but the loss of native glandular epithelium is considered an atrophic change. Recent evidence supports the importance of pseudopyloric metaplasia in gastric carcinogenesis (through Tran’s differentiation from mature chief cells following parietal cell loss) [39,40]. The loss of native glandular epithelium is usually observed in multiple small foci, which are more frequently found along the lesser curvature of the stomach, but over time they may be scattered throughout the entire mucosa.
Intestinal Metaplasia (IM) is the replacement of gastric epithelium with epithelium displaying intestinal phenotypes, implying adaptation to environmental stimuli.
Identified risk factors for IM include H. pylori infection, high salt intake, smoking, alcohol consumption, and chronic bile reflux [41-45]. It is not clear how metaplastic cells emerge, or what cell they emerge from, but the new cell type is presumably better suited to withstand the altered environment. IM usually develops in islands within areas of chronic atrophic gastritis, more frequently along the lesser curvature of the stomach. Over time, the metaplastic foci increase in size and may coalesce, covering large areas of antral and oxyntic mucosa. The prevalence of IM increases with advanced age [11,36,46].
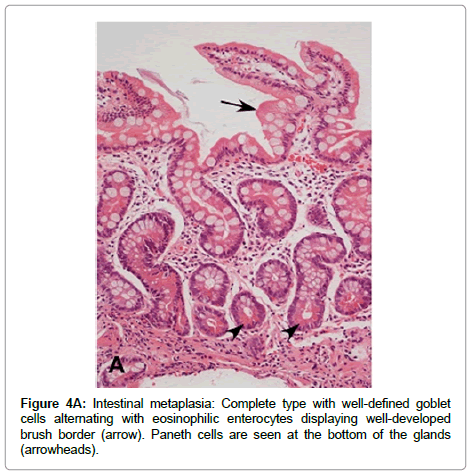
IM is classified into two main types: complete (type I or small intestinal) and incomplete (types II and III, or colonic) [47]. Complete IM is characterized by the presence of mature goblet cells, alternating with enterocytes that display well-developed brush border (microvilli). Paneth cells may be observed, usually at the bottom of the glands (Figure 4A). Sialomucins are observed in goblet cells and enterocytes do not show any mucin content. Complete IM is the most frequently observed type in gastric biopsies and is the type that carries the lowest risk of gastric cancer.
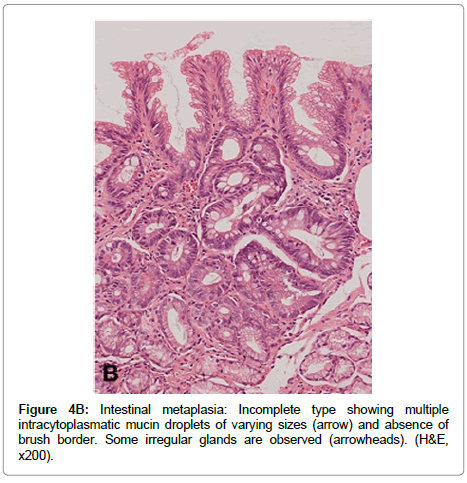
Incomplete IM is characterized by presence of columnar mucous and goblet cells in various stages of differentiation displaying mucus vacuoles of different sizes, and by absence of absorptive cells (Figure 4B). Incomplete IM may display mild to moderate alteration of the glandular architecture, and is frequently found mixed with complete IM. Incomplete IM is sub classified into types II and III according to the type of mucin expressed [47]. In type II, neutral and Sialomucins are expressed in column arc ells, and Sialomucins in goblet cells. Type III expresses predominantly sulfomucins in columnar cells and sialoor sulfomucins in goblet cells.
Multiple studies have demonstrated that the incomplete type of IM, especially the type III [47-53] and the extension of the metaplastic changes [48,54-56] are associated with higher risk of progression to gastric cancer. Furthermore, a strong correlation has been documented between IM extension and IM type, with incomplete or type III IM occurring more frequently among more extensive metaplastic lesions [48,56]. In addition, on the basis of quantitative morphometric analyses, it has been observed that type III IM may be classified as lowgrade dysplasia [57].
It is not clear to what extent the metaplastic changes can be reversed if the normal cellular environment is restored, but it does not seem to happen frequently, especially when the metaplastic changes are extensive.
Dysplasia (synonyms: intra epithelial neoplasia, non-invasive neoplasia). Dysplasia in the stomach is a term to define architectural and cytological abnormalities of the epithelium that are considered an intermediate step between no neoplasia and invasive neoplasia. Dysplastic lesions are usually observed arising in areas of IM, especially of the incomplete type, and also surrounding adenocarcinoma. Only a small fraction of individuals with IM develops dysplasia.
Multiple systems of classification for dysplasia have been developed. The Padova international classification [58] was proposed to facilitate the communication between pathologists and clinicians and for a better understanding among pathologists around the world. The term noninvasive neoplasia was introduced in this classification to replace the term dysplasia. The categories proposed were: 1) negative for dysplasia, 2) indefinite for dysplasia, 3) non-invasive neoplasia (sub classified into low grade and high grade), 4) suspicious for invasive carcinoma, and 5) invasive adenocarcinoma. Shortly after, another system was developed: the Vienna classification of gastrointestinal epithelial neoplasia. In this system and its subsequent modified version, a nomenclature was proposed to be used not only for gastric dysplasia but also for esophageal and colonic dysplastic lesions [59]. More recently, the classification of the World Health Organization recommends the term slow-grade and high-grade intra epithelial neoplasia/dysplasia [60].
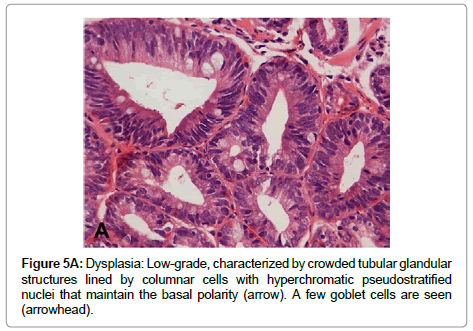
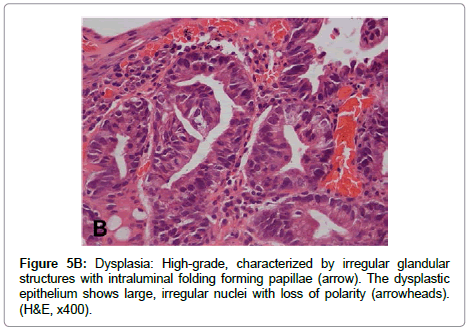
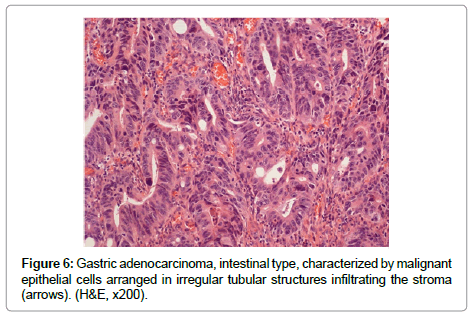
The term “indefinite for dysplasia” is used in the presence of atypical lesions of the gastric mucosa in which the pathologist cannot differentiate with certainty between reactive epithelial changes and dysplasia. It is a temporary diagnosis and a follow-up including a new endoscopic procedure and biopsy sampling should be scheduled. Low-grade dysplasia is characterized by crowded tubular glands lined by column arc ells displaying elongated, hyper chromatic and pseudo stratified nuclei, with cigar-like appearance. The nuclei preserve their basal orientation and polarity. The dysplastic epithelium shows little or no mucin secretion. There is loss of interglandular stroma displaying back to back orientation (Figure 5A). In high-grade dysplasia, the architectural changes become prominent, showing irregular glands with budding and branching. Intraluminal folding of the dysplastic epithelium may develop into complex forms such as cribriform pattern. Cytologically, the nuclei are larger and show rounded shape rather than the cigar-shaped nuclei seen in low-grade dysplasia. Prominent nucleoli are observed and the basal nuclear orientation is lost (Figure 5B). Mitotic figures are frequent and atypical forms may be present. The dysplastic epithelium is confined to the epithelial compartment and there is no evidence of invasion through the basement membrane into the lamina propria. Disagreement between Japanese and Western pathologists is present in this category [61]. For Western pathologists invasion to the lamina propria or beyond is the hallmark of adenocarcinoma (Figure 6). For Japanese pathologists definitive neoplastic epithelia, even in the absence of proven invasion, are grounds for a diagnosis of adenocarcinoma.
Management of Gastric Precancerous Lesions
Under current standards, it is advisable to eradicate H. pylori infection in any patient with gastritis with or without precancerous lesions. Evidence supports that eradication of H. pylori has a preventive effect on progression to gastric cancer by reducing or eliminating mucosal inflammation and by reversing molecular events induced by this bacterium [62-69]. However, the beneficial effect of H. pylori eradication is greater in subjects with non-atrophic gastritis or atrophic gastritis without IM. In patients with extensive IM or dysplasia, the eradication may have little or no beneficial effect in prevention of gastric cancer [70, 71]. Ample evidence suggests that the progression from IM to dysplasia and carcinoma is an H. Pylori-independent process. However, the reduced colonization density in the gastric mucosa with such lesions may impair the sensitivity of histology, culture, and urea breath test for the detection of the bacterium. Although H. pylori are generally absent from areas of complete IM, there are a small percentage of cases with incomplete IM that show adherence of the bacterium to the metaplastic epithelium [72,73].
Large-scale H. pylori eradication as a preventive measure for gastric cancer has been the object of controversy. This measure is not recommended due to the possibility of appearance of antimicrobialresistant strains, not only H. pylori but other bacteria as well. In addition, virtually all treatment schemes present some margin of failure and the reinfection/recrudescence rates are often high. Moreover, since H. pylori has accompanied human beings since ancient times [74], strong arguments exist that consider this bacterium as part of the human gastric microbiome and its elimination as the cause of the increase in prevalence of diseases such as gastro esophageal reflux, Barrett’s esophagus, and esophageal and cardiac adenocarcinoma [75].
Besides eradicating the infection, the clinical management of patients with precancerous gastric lesions is controversial and the guidelines are still evolving. The identification and surveillance of patients at high risk of progressing to gastric cancer represents the most effective way of reducing the burden of this disease. Subjects at the highest risk are those with: 1) Extensive atrophic gastritis and/or IM, 2) Presence of incomplete type IM (usually found in a background of extensive IM), 3) Dysplasia, including “indefinite for dysplasia”, 4) Family history of gastric cancer. Additional risk factors to consider are: history of tobacco smoking, ethnicity (Hispanics, African Americans, Asians, and Native Americans in the United States are at higher risk) and origin of the patient in a geographic area with high risk of gastric cancer.
To assess the extension of the atrophic (non-metaplastic and metaplastic)changes in a patient with diagnosis of atrophy or IM in a pathology report, accepted guidelines recommend sampling of the gastric mucosa as follows: antrum (two biopsy samples), incisura angularis (at least one sample), and corpus mucosa (two samples) [38,76]. Two systems have been proposed to classify the risk of gastric cancer based on histological scoring of the extension of atrophicmetaplastic lesions: 1) Operative Link On Gastritis Assessment (OLGA) which includes scoring of non-metaplastic atrophy, pseudopyloric metaplasia and IM [76], and 2) Operative Link On Gastric Intestinal Metaplasia Assessment (OLGIM) [77], which only scores extension of IM. Both systems classify the risk of cancer into stages 0 to IV, and consider that patients in stages III and IV are at high risk and should enter surveillance programs for gastric cancer prevention. Both systems ignore the type of IM as a risk factor, although a later study reported a strong association between incomplete IM and the high-risk OLGA stages [78].
In 2012, a consensus on guidelines for the management of precancerous gastric lesions [79] recommended taking “at least four biopsies of the proximal and distal stomach, on the lesser and greater curvatures” using magnification chromoendoscopy or narrow-band imaging endoscopy for proper evaluation of the lesions. Besides the fact that these tools for endoscopy are time-consuming, costly, and not widely available in clinical practice, their actual benefit for improving diagnosis of gastric atrophy and intestinal metaplasia are mains to be consistently proved. In addition, these guidelines do not emphasize sampling the incisura angularis, which is widely considered necessary [38,76,80,81].
The above mentioned consensus guidelines recommend that patients with extensive atrophy and/or extensive IM should be followed-up with endoscopy every three years and that those with mild to moderate atrophy and/or intestinal metaplasia limited to the antrum do not need follow-up [79]. Controversy exists regarding these recommendations. First, it has been questioned that the same recommendations be applied to patients with either non-metaplastic atrophic gastritis or IM, since these two lesions show different risks of progression to cancer [81]. Second, due to the poor survival of gastric cancer in the Western world and to the fact that most of these tumors are diagnosed in an advanced stage, it has been proposed that subjects with IM that have an additional risk factor for gastric cancer should be followed with annual endoscopy [81,82]. In the remaining patients with IM, a less intensive surveillance (every 2-3 years) has been proposed [81, 82].
The presence of dysplasia is an indicator of high risk for gastric cancer and it should be confirmed and classified by two gastrointestinal pathologists, due to the high inter-observer variability. The management of gastric dysplasia is also a matter of current debate and several approaches have been used/proposed [79]. However, the finding of dysplasia of any grade in a non targeted biopsy sample must be followed-up immediately (with endoscopy and extensive mapping) and between6 and12 months later to rule out a coexisting gastric cancer missed at the initial endoscopy. If there is dysplasia with a visible lesion at endoscopy, it should be classified according to stage and resection of the lesion must be carried out [79].
Non-invasive screening tests that can be applied to large populations to assess cancer risk or detect early gastric cancer cases are needed. In Japan and Korea, where gastric cancer is highly prevalent, national programs for mass screening and early detection exist.In Japan, a 40 to 60% decrease in gastric cancer mortality has been observed in cases detected under screening programs. Serum Pepsinogen (PG) levels have shown some potential for establishing the extent of gastric atrophy in various populations. PGI is secreted only in the oxyntic mucosa, and PGII is secreted in both oxyntic and antro pyloric mucosa. In presence of atrophy of the oxyntic mucosa, there may be decrease in both PGI and PGII, but PGI usually shows a more marked decrease. In Japan, severe gastric atrophy is diagnosed when serum PGI levels are <70 μg/l and a PGI/PGII ratio<3; these levels have proven useful for the identification of subjects at high risk of gastric cancer, to whom follow-up should be offered. The combination of PG serum levels and H. pylori serology has also demonstrated to be useful in gastric cancer risk stratification in some populations. However, multiple studies around the world have shown inconsistent results for the validity of the serum PG levels on the detection of precancerous lesions. Sensitivity values ranging between 9 and 92% and specificities between 10 and 100% have been found for the detection of gastric corpus atrophy [79]. Similarly, sensitivity values between 15 and 75% have been reported for the detection of IM [79]. The use of different methods for PG levels assessment, different cutoff values, and variable endoscopic biopsy protocols for histological diagnosis makes it difficult to compare studies. Other serological markers for the detection of precancerous gastric lesions such as trefoil factor 3, hypermethylation of genes and micro RNAs are subject of current investigation.
Acknowledgements
This work was supported by the National Institutes of Health of the United States grants P01CA28842 and P30DK058404.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917.
- JARVI O, LAUREN P (1951) On the role of heterotopias of the intestinal epithelium in the pathogenesis of gastric cancer. Acta Pathol Microbiol Scand 29: 26-44.
- Lauren p (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta pathol microbiol scand 64: 31-49.
- Correa P (1992) Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52: 6735-6740.
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M (1975) A model for gastric cancer epidemiology. Lancet 2: 58-60.
- Camargo MC, Yepez MC, Ceron C, Guerrero N, Bravo LE, et al. (2004) Age at acquisition of Helicobacter pylori infection: comparison of two areas with contrasting risk of gastric cancer. Helicobacter 9: 262-270.
- Goodman KJ, Correa P (1995) The transmission of Helicobacter pylori. A critical review of the evidence. Int J Epidemiol 24: 875-887.
- Goodman KJ, Correa P, Tenganá Aux HJ, RamÃrez H, DeLany JP, et al. (1996) Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. Am J Epidemiol 144: 290-299.
- Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118: 3030-3044.
- Bonne C, Hartz PH, Klerks JV, Posthuma JH, Radsma W, et al. (1938) Morphology of the stomach and gastric secretion in Malays and Chinese and the different incidence of gastric ulcer and gastric cancer in these races. Am J Cancer 33:265-269.
- Correa P, Cuello C, Duque E (1970) Carcinoma and intestinal metaplasia of the stomach in Colombian migrants. J Natl Cancer Inst 44: 297-306.
- Fennerty MB, Emerson JC, Sampliner RE, McGee DL, Hixson LJ, et al. (1992) Gastric intestinal metaplasia in ethnic groups in the southwestern United States. Cancer Epidemiol Biomarkers Prev 1: 293-296.
- de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, et al. (2008) Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 134: 945-952.
- Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1: 1311-1315.
- Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, et al. (1991) Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 302: 1302-1305.
- Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, et al. (1991) Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 325: 1132-1136.
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, et al. (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325: 1127-1131.
- IARC Monographs on the evaluation of carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori (1994) International Agency for Research on Cancer. IARC Press, Lyon, pp. 177-240.
- Achtman M, Azuma T, Berg DE, Ito Y, Morelli G, et al. (1999) Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol Microbiol 32: 459-470.
- Wroblewski LE, Peek RM Jr (2013) Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am 42: 285-298.
- Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, et al. (1996) cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A 93: 14648-14653.
- Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, et al. (1993) Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A 90: 5791-5795.
- Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, et al. (2011) Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141: 1696-1708.
- Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, et al. (1995) Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 55: 2111-2115.
- Parsonnet J, Friedman GD, Orentreich N, Vogelman H (1997) Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40: 297-301.
- Peek RM Jr, Vaezi MF, Falk GW, Goldblum JR, Perez-Perez GI, et al. (1999) Role of Helicobacter pylori cagA(+) strains and specific host immune responses on the development of premalignant and malignant lesions in the gastric cardia. Int J Cancer 82: 520-524
- Plummer M, van Doorn LJ, Franceschi S, Kleter B, Canzian F, et al. (2007) Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J Natl Cancer Inst 99: 1328-1334.
- Noto JM, Peek RM Jr (2012) The Helicobacter pylori cag Pathogenicity Island. Methods Mol Biol 921: 41-50.
- Bravo LE, van Doom LJ, Realpe JL, Correa P (2002) Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma? Am J Gastroenterol 97: 2839-2842.
- Piazuelo MB, Camargo MC, Mera RM, Delgado AG, Peek RM Jr, et al. (2008) Eosinophils and mast cells in chronic gastritis: possible implications in carcinogenesis. Hum Pathol 39: 1360-1369.
- de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, et al. (2011) Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut 60: 1189-1195.
- Loh JT, Shaffer CL, Piazuelo MB, Bravo LE, McClain MS, et al. (2011) Analysis of cagA in Helicobacter pylori strains from Colombian populations with contrasting gastric cancer risk reveals a biomarker for disease severity. Cancer Epidemiol Biomarkers Prev 20: 2237-2249.
- Correa P, Cuello C, Duque E, Burbano LC, Garcia FT, et al. (1976) Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst 57: 1027-1035.
- Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, et al. (1990) Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res 50: 4737-4740.
- Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20: 1161-1181.
- Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, et al. (2010) Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028-2037.
- Weis VG, Goldenring JR (2009) Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer 12: 189-197.
- Camorlinga-Ponce M, Flores-Luna L, Lazcano-Ponce E, Herrero R, Bernal-Sahagun F, et al. (2008) Age and severity of mucosal lesions influence the performance of serologic markers in Helicobacter pylori-associated gastroduodenal pathologies. Cancer Epidemiol Biomarkers Prev17:2498-2504.
- de Vries AC, Haringsma J, de Vries RA, ter Borg F, Nagtzaam NM, et al. (2009) The use of clinical, histologic, and serologic parameters to predict the intragastric extent of intestinal metaplasia: a recommendation for routine practice. Gastrointest Endosc 70: 18-25.
- Fontham E, Zavala D, Correa P, Rodriguez E, Hunter F, et al. (1986) Diet and chronic atrophic gastritis: a case-control study. J Natl Cancer Inst 76: 621-627.
- Kneller RW, You WC, Chang YS, Liu WD, Zhang L, et al. (1992) Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J Natl Cancer Inst 84: 1261-1266.
- Leung WK, Lin SR, Ching JY, To KF, Ng EK, et al. (2004) Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 53: 1244-1249.
- Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, et al. (1990) Gastric precancerous process in a high risk population: cross-sectional studies. Cancer Res 50: 4731-4736.
- Filipe MI, Muñoz N, Matko I, Kato I, Pompe-Kirn V, et al. (1994) Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer 57: 324-329.
- Cassaro M, Rugge M, Gutierrez O, Leandro G, Graham DY, et al. (2000) Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol 95: 1431-1438.
- González CA, Pardo ML, Liso JM, Alonso P, Bonet C, et al. (2010) Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer 127: 2654-2660.
- González CA, Sanz-Anquela JM, Gisbert JP, Correa P (2013) Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer 133: 1023-1032.
- Rokkas T, Filipe MI, Sladen GE (1991) Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut 32: 1110-1113.
- Silva S, Filipe MI (1986) Intestinal metaplasia and its variants in the gastric mucosa of Portuguese subjects: a comparative analysis of biopsy and gastrectomy material. Hum Pathol 17: 988-995.
- Sipponen P, Seppälä K, Varis K, Hjelt L, Ihamäki T, et al. (1980) Intestinal metaplasia with colonic-type sulphomucins in the gastric mucosa; its association with gastric carcinoma. Acta Pathol Microbiol Scand A 88: 217-224.
- Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, et al. (1992) A prospective study of atrophic gastritis and stomach cancer risk. Jpn J Cancer Res 83: 1137-1142.
- Stemmermann GN, Hayashi T (1968) Intestinal metaplasia of the gastric mucosa: a gross and microscopic study of its distribution in various disease states. J Natl Cancer Inst 41: 627-634.
- Tava F, Luinetti O, Ghigna MR, Alvisi C, Perego M, et al. (2006) Type or extension of intestinal metaplasia and immature/atypical "indefinite-for-dysplasia" lesions as predictors of gastric neoplasia. Hum Pathol 37: 1489-1497.
- Tosi P, Filipe MI, Luzi P, Miracco C, Santopietro R, et al. (1993) Gastric intestinal metaplasia type III cases are classified as low-grade dysplasia on the basis of morphometry. J Pathol 169: 73-78.
- Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, et al. (2000) Gastric dysplasia: the Padova international classification. Am J Surg Pathol 24: 167-176.
- Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, et al. (2000) The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47: 251-255.
- Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. (2010) WHO Classification of tumours of the digestive system. (4th edn), International Agency for Research on Cancer, IARC Press, Lyon.
- Lauwers GY, Shimizu M, Correa P, Riddell RH, Kato Y, et al. (1999) Evaluation of gastric biopsies for neoplasia: differences between Japanese and Western pathologists. Am J Surg Pathol 23: 511-518.
- Ma JY, Borch K, Sjostrand SE, Janzon L, Mardh S (1994)Positive correlation between H,K-adenosine triphosphatase autoantibodies and Helicobacter pylori antibodies in patients with pernicious anemia. Scand J Gastroenterol29:961-965.
- Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, et al. (2005)Long term follow up of patients treated for Helicobacter pylori infection. Gut54:1536-1540.
- Rugge M, Capelle LG, Cappellesso R, Nitti D, Kuipers EJ (2013) Precancerous lesions in the stomach: from biology to clinical patient management. Best Pract Res Clin Gastroenterol 27: 205-223.
- Shiotani A, Cen P, Graham DY (2013) Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol .
- Tan VP, Wong BC (2013) Gastric cancer chemoprevention: the current evidence. Gastroenterol Clin North Am 42: 299-316.
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, et al. (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345: 784-789.
- Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, et al. (2009) Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 137: 1641-1648.
- Yanaoka K, Oka M, Ohata H, Yoshimura N, Deguchi H, et al. (2009) Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer 125: 2697-2703.
- de Vries AC, Kuipers EJ, Rauws EA (2009) Helicobacter pylori eradication and gastric cancer: when is the horse out of the barn? Am J Gastroenterol 104: 1342-1345.
- Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, et al. (2004) Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291: 187-194.
- Bravo JC, Correa P (1999) Sulphomucins favour adhesion of Helicobacter pylori to metaplastic gastric mucosa. J Clin Pathol 52: 137-140.
- Genta RM, Gürer IE, Graham DY, Krishnan B, Segura AM, et al. (1996) Adherence of Helicobacter pylori to areas of incomplete intestinal metaplasia in the gastric mucosa. Gastroenterology 111: 1206-1211.
- Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R (1999) Helicobacter pylori virulence and genetic geography. Science 284: 1328-1333.
- Cover TL, Blaser MJ (2009) Helicobacter pylori in health and disease. Gastroenterology 136: 1863-1873
- Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, et al. (2008) OLGA staging for gastritis: a tutorial. Dig Liver Dis 40: 650-658.
- Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, et al. (2010) The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 71: 1150-1158
- Rugge M, Fassan M, Pizzi M, Pennelli G, Nitti D, et al. (2011) Operative Link for Gastritis Assessment gastritis staging incorporates intestinal metaplasia subtyping. Hum Pathol 42: 1539-1544.
- Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, et al. (2012)Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy44:74-94.
- Correa P, Piazuelo MB, Wilson KT (2010) Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 105: 493-498.
- Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, et al. (2008) Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 9: 279-287.
- Iijima K, Abe Y, Kikuchi R, Koike T, Ohara S, et al. (2009) Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol 15: 853-859.
- Miki K (2006) Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 9: 245-253.
- Miki K (2011) Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - "ABC method". Proc Jpn Acad Ser B Phys Biol Sci 87: 405-414
- Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, et al. (2004) Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 109: 138-143.
Citation: Correa P, Blanca Piazuelo M (2013) The Gastric Precancerous Cascade. J Clin Exp Pathol 3:147. DOI: 10.4172/2161-0681.1000147
Copyright: © 2013 Correa P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 19994
- [From(publication date): 11-2013 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 15168
- PDF downloads: 4826