Research Article Open Access
The Effects of Iron Chelation and Hypoxic or Hyperoxic Oxygen Manipulation on the Accumulation/Photobleaching of Protoporphryin IX and Cytotoxcity in Human Glioma Cells
Emma Blake, James PC Allen and Alison Curnow*
Clinical Photobiology, European Centre for Environment and Human Health, University of Exeter Medical School, University of Exeter, UK
- *Corresponding Author:
- Alison Curnow
Clinical Photobiology
European Centre for Environment and Human Health
University of Exeter Medical School
University of Exeter, Knowledge Spa
Royal Cornwall Hospital, Truro
Cornwall, TR1 3HD, UK
Tel: +44(0)1872256433
Fax: +44(0)1872256497
E-mail: alison.curnow@exeter.ac.uk
Received date: May 07, 2013; Accepted date: July 23, 2013; Published date: July 26, 2013
Citation: Blake E, Allen JPC, Curnow A (2013) The Effects o f Iron Chelation and Hypoxic or Hyperoxic Oxygen Manipulation on the Accumulation/Photobleaching of Protoporphryin IX and Cytotoxcity in Human Glioma Cells. J Anal Bioanal Tech S1:005. doi: 10.4172/2155-9872.S1-005
Copyright: © 2013 Blake E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Background and objective: Gliomas remain the most common and problematic primary brain tumors to treat given their mobile and invasive characteristics, resulting in a 5 years survival rate of less than 5%. Recently the administration of photosensitive fluorescent drugs has been utilized to aid in the identification and resection of gliomas. In this experimental investigation of human glioma cells the effect of iron chelation and oxygenation manipulation, firstly on the accumulation of the clinically useful photosensitizer protoporphyrin IX (PpIX) and subsequently on PpIX photobleaching and cytotoxicity on irradiation, has been examined with the aim of improving fluorescence-guided resection/photodynamic therapy (PDT).
Materials and methods: Cells were incubated at concentrations of 5%, 20% or 40% oxygen for 24 hours prior to and for 3 hours following the administration of the PpIX precursors aminolevulinic acid (ALA), methyl levulinate (MAL) or hexyl aminolevulinate (HAL) with or without the iron chelator 1,2-diethyl-3-hydroxypyridin-4-one hydrochloride (CP94). PpIX levels were monitored using a fluorescence plate reader (excitation filter 400 ± 30 nm; emission filter 645 ± 40 nm). Cells were irradiated with 15 J/cm2 red lights and viability measured using the neutral red uptake assay.
Results: Manipulation of the oxygen environment and/or co administration of CP94 with PpIX precursors resulted in significant changes in both PpIX accumulation and photobleaching. Incubation with ALA/ MAL at 5% or 40% oxygen produced the greatest levels of PpIX and photobleaching respectively. Both these parameters were further elevated through co administration of CP94. The combination of hyperoxygenation and CP94 administration significantly increased photobleaching (a marker of PDT effect) but not cytotoxicity in this experimental system.
Conclusions: PpIX accumulation was greatest when the cells were grown in hypoxic (5% oxygen) conditions (mimicking the in vivo situation) with ALA/MAL+CP94. Fluorescence-guided resection of glioma may therefore be improved through the addition of the iron chelating adjuvant CP94.
Keywords
CP94; Fluorescence guided resection; Glioma; Iron chelation; Oxygen manipulation; Photodynamic therapy; Protoporphyrin IX
Introduction
Malignant glial tumors (gliomas) originate in glial cells, of which there are several types including astrocytes, oligodendrocytes and ependymal. Gliomas remain the most common [1] and problematic primary brain tumors to treat given their mobile and invasive characteristics. As a result the 5 years survival rates remain dismal at less than 5% [2]. The conventional treatments for primary brain tumors include surgery, radiotherapy and chemotherapy with the ultimate goal being to reduce the tumor as much as possible. These treatments can be used alone but more commonly they are used in combination. Although potentially curative, recurrence rates are high with almost 80% of all cases recurring within 2 cm of the resected margin [2]. Unsurprisingly, there is a strong drive to improve the prognosis and quality of life of patients diagnosed with glioma.
Photodynamic therapy (PDT) is an additional treatment modality that can be utilized for the treatment of glioma. PDT is based on the interaction of a photosensitizing drug, molecular oxygen and light of a specific wavelength which when combined results in the production of cytotoxic reactive oxygen species (ROS) causing sensitized cells to undergo cell death [3]. An additional feature of the activated photosensitizer is its ability to fluoresce and as a result photodiagnosis (PD) can be utilized to help in the detection of tumor cells, which accumulate the fluorescent drug to a greater extent and more rapidly than surrounding normal cells. Fluorescence-guided resection (FGR), as the name suggests, utilizes this selective fluorescence to enable surgeons’ greater precision in the surgical removal of tumors and is particularly useful when operating in the brain [4]. In a study of 9 patients administered with ALA 3 hours prior to surgical excision of their malignant glioma, the biopsies of normal brain tissue revealed no porphyrin fluorescence, whereas tumor tissue was distinguished by bright red fluorescence [4]. For a total of 89 tissue biopsies, sensitivity was 85% and specificity was 100% for the detection of malignant tissue. For seven of the nine patients, visible porphyrin fluorescence led to further resection of the tumor [4]. As an adjuvant therapy, PDT can also be used to destroy tumor cells unreachable by surgical resection. Kaneko [5] treated 250 patients with PD during surgery and 63 patients with PDT. The rate of total removal of tumor tissue increased when using PD during surgery and the time-to-tumor-recurrence was also increased when PDT was employed.
Protoporphyrin IX (PpIX)-induced PDT manipulates the heme biosynthesis pathway to accumulate an excessive, and as a result a clinically useful amount of the endogenous photosensitizer PpIX. Aminolevulinic acid (ALA) naturally undergoes a series of enzymatic conversions to produce PpIX, which following the insertion of ferrous iron (Fe2+) under the action of ferrochelatase [6] is converted into heme. The presence of free heme acts as a negative feedback mechanism inhibiting ALA synthesis [7]. The exogenous administration of large amounts of ALA bypasses this endogenous negative feedback loop. Also, as the conversion of PpIX to heme by ferrochelatase is relatively slow, making this the secondary rate limiting step of the pathway, there is a resultant temporary accumulation of PpIX within the cell. To optimize the level of fluorescence produced by PpIX, as well as to maximize the level of cell ablation produced by PDT following subsequent irradiation, protocol modifications have been investigated. These have included modifying light dosimetry parameters [8], use of different prodrugs [9], iron chelation [10], altering the local oxygen environment [11] and finally optimizing monitoring techniques [12,13] with a view to individualizing treatment strategies.
Iron chelators enhance PpIX-induced PDT by temporarily inhibiting the final stage of the haem biosynthesis pathway, which requires iron to convert PpIX into haem. Direct comparision of the iron chelator 1,2-diethyl-3-hydroxypyridin-4-one hydrochloride (CP94) and desferrioxamine (DFO; an established iron chelator administered clinically by long infusion) in cultured human lung fibroblasts and epidermal carcinoma cells has established [14] that CP94 is a better enhancer of ALA/MAL-induced PpIX fluorescence than DFO (and it has already been demonstrated that DFO is a better enhancer of PpIX-PDT than EDTA [15]), probably as a result of its lower molecular weight, greater lipophilicity and neutral charge enabling it to access intracellular iron pools more rapidly [16]. Further in vitro experimentation has also indicated that CP94 is also a better enhacer of ALA/MAL/HAL-PDT than the already clinically established iron chelator dexrazoxane [17] and works in a number of different cell lines [10]. It has been established in vivo [18] in a rat colon model that CP94 can be used to significantly enhance PpIX levels. Furthermore on irradiation, CP94+ALA has been found to produce three times the area of necrosis produced by ALA alone when delivered at the same time point [18]. Other investigations of CP94 as an enhancer of PDT have taken place in rat bladder [19] and rabbit uterus [20]. In addition, the iron chelating capacity and toxicity of CP94 have been investigated in a number of iron overloaded and non-overloaded animal models including mice [21,22], rats [23,24], guinea pigs [23], rabbits [25] and Cebus monkeys [24]. Rat studies were found to be predictive of the iron chelating capacity recorded in primates [24] and the metabolism of CP94 in guinea pigs was noted to be more similar to that of humans [23] than studies undertaken in rats as this compound is glucuronidated by the liver and excreted in urine. Topical CP94 administration has been investigated in humans as way of improving PpIX-induced PDT for the treatment of certain non-melanoma skin cancers and precancers. Dose escalating pilot studies in nodular basal cell carcinoma (one treatment cycle without lesion debulking) with simultaneous topical ALA [26] or MAL administration [27] have already been conducted and determined that CP94 administration was a safe, effective and feasible treatment modification which did not produce any additional adverse reactions. Histological analysis also indicated a significant, increased trend towards complete clearance with increased concentrations of CP94 (up to 40% w/w).
A number of studies have utilized a variety of different techniques to monitor oxygen saturation (or the partial pressure of oxygen, pO2) in the tissue before, during and after the light irradiation phase of PDT. These studies have highlighted two main conclusions; i) significant changes in oxygenation occur during and after PDT, with markedly different responses noted for the different PDT photosensitising agents employed [9,11,28-30] and ii) a rapid decline in pO2 has been observed immediately on the initiation of light treatment [30-34], which has been attributed to the photochemical consumption of oxygen and damage to the microvasculature reducing the capacity of the circulation to replenish the tissue with oxygen [29,35]. It has therefore been postulated that if this latter oxygen depletion could be prevented (via oxygen supplementation or reduced treatment parameters) or reversed (via light dose fractionation; temporarily pausing the light delivery allowing reoxygenation to occur prior to restarting and completing the irradiation period [14]) it may be possible to improve PDT outcomes.
This study has investigated the effect of oxygen manipulation and the iron chelator CP94, on the PpIX fluorescence produced within human glioma cells in vitro using the porphyrin-inducing precursors ALA and its esters methyl aminolevulinate (MAL) and hexyl aminolevulinate (HAL). Additionally, the effect this treatment had on PpIX photobleaching and cytotoxicity following irradiation has also been investigated.
Methods
Chemicals and cells
All reagents and chemicals were purchased from Sigma-Aldrich Chemical Company (Poole, UK) unless otherwise stated. The U-87 MG (human glioblastoma-astrocytoma, epithelial-like) cell line was purchased from the European Collection of Cell Cultures (ECACC, Wiltshire, UK). Under aseptic conditions in a class II laminar flow cabinet, cells were cultured in Eagle’s minimum essential medium (EMEM) with 10% fetal calf serum (FCS, standardized to give an iron concentration between 450 and 600 µg/100 g), 2% (200 mM) L-glutamine (2 mM final concentration) and 2% penicillin (200 U mL-1 final concentration) and streptomycin solution (200 µg mL-1 final concentration). Stock solutions of ALA/MAL/HAL were prepared in phosphate buffered saline (PBS), adjusted to physiological pH (pH 7.4) using NaOH (0.5 mM), filter sterilized (0.22 µm; Millipore) and stored at -20°C for up to one month. Cells were grown in 5% CO2 and 20% O2 at 37°C until 70% confluent at which time cells were routinely passaged (every 3-5 days).
Oxygen manipulation
U-87 MG cells were seeded into 96-well plates at a density of 1×105 cells per mL (104 cells per well) and left to adhere in 5% CO2 and 20% O2 at 37°C for 24 hours. Following this, cells were incubated for a further 24 hours at 37°C in 5% CO2 and 5, 20 or 40% O2 with the desired oxygen environment achieved using a Tri-gas incubator (MCO-18M, Sanyo, San Diego, USA). Following this and under dark room conditions, all medium was aspirated from the wells and replaced with 100 µl of appropriate test solution, which was modified EMEM including 250 µM ALA, 1000 µM MAL or 10 µM HAL ± 150 µM CP94 (5 wells per test solution). These doses were chosen based on results from previous work within the group which showed at the doses employed maximum PpIX fluorescence could be achieved with minimal dark toxicity [14]. Both plates were incubated for a further 3 hours to allow PpIX accumulation to occur.
PpIX fluorescence monitoring in vitro
Following 3 hours the plates were removed from the incubator and the lids taped to minimize any change to the oxygen environment. Plates were then read for ‘pre’ PpIX fluorescence levels (prior to light irradiation) using a fluorescence plate reader (Synergy HT; BIO-TEK, Germany). Measurements were taken from the bottom of the wells with a 400 ± 30 nm excitation filters and a 645 ± 40 nm emission filter. Following the ‘pre’ fluorescence measurements the plate was subjected to red light irradiation (Aktilite, Galderma, UK), 15 J/cm2, 635 ± 2 nm. The most efficacious light dose and fluency rate to be utilized for the treatment of glioma brain tumors with PpIX-induced PDT has yet to be discovered and further light dosimetry optimization is required. In our study a total fluence dose of 15 J/cm2 was chosen as we believed from preliminary experimentation that this dose would result in a sufficient PDT effect and the delivery of the total light dose at a fluence rate of 70 mW/cm2 would be feasible for a “one-shot” intraoperative PDT treatment. In vitro studies have found PDT treatment with low fluence doses and low fluence rates to be more effective than low fluence doses with high fluence rates [36]. Finally, plates were measured for ‘post’ PpIX fluorescence levels (following light irradiation).
Neutral red uptake (NRU) assay
Following ‘post’ PpIX fluorescence measurements the medium containing the test solutions was removed and the cells washed three times with PBS. A volume of 100 µl serum free medium per well containing neutral red (40 µg ml-1) was then added. The plate was returned to the incubator for a further 2 hours, and following this the neutral red medium was removed, cells washed with 150 µl of PBS and 150 µl neutral red destain solution (50% (v/v) 96% ethanol; 49% (v/v) deionised water; 1% (v/v) glacial acetic acid) was added to each well. Finally, plates were rapidly shaken for a minimum of 10 minutes and the level of neutral red uptake measured on the Synergy HT plate reader using a 530 nm excitation wavelength and 645 nm emission wavelength [37].
Data analysis and statistics
The first 5 wells contained control cells incubated with modified EMEM only (blank controls). The PpIX fluorescence measurements from these wells were used to remove natural cellular PpIX autofluorescence from all subsequent measurements made from the same plate. For the cell viability data, the viability in each test group was recorded and cell viability was displayed as a percentage of the blank control group. All data points in the figures represent mean values from experiments carried out in triplicate. Statistical significance between individual groups was determined using the Student’s t-test and an ANOVA was employed when considering any differences between data sets.
Results
Pre and post PpIX fluorescence
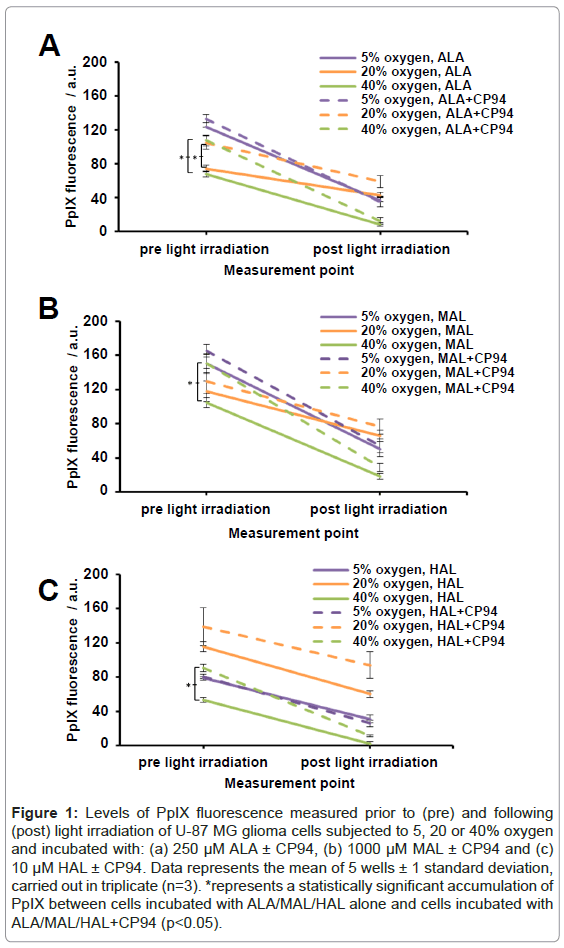
Human glioma cells (U-87 MG) were incubated with ALA/MAL/ HAL ± CP94 whilst being subjected to an oxygen environment of 5, 20 or 40% and were irradiated with red light. Prior to light irradiation and following light irradiation ‘pre’ and ‘post’ PpIX fluorescence levels were recorded, respectively. As anticipated the observed trend was a decrease in PpIX fluorescence following light irradiation (Figures 1A-1C) indicating that PpIX photobleaching had occurred during light delivery. The greatest levels of PpIX fluorescence prior to light irradiation were observed in cells incubated with 1000 µM MAL ± CP94 (in the range of 104-165 arbitrary units (a.u.); Figure1B). These PpIX fluorescence values were found to be significantly greater (ANOVA, p<0.05) when compared to cells incubated with 250 µM ALA ± CP94 or 10 µM HAL ± CP94 (ALA, 68-133 a.u., Figure 1A; HAL, 53-139 a.u., Figure 1C). Following incubation with ALA or MAL and subjection to 5/20/40% oxygen the observed trend for the level of ‘pre’ PpIX fluorescence achieved was in the order 5>20>40%. When either of these pro-drugs were combined with CP94 and subjected to 5/20/40% oxygen (Figures 1A and 1B), levels of ‘pre’ PpIX produced were increased and this increase reached significance for the cells incubated with ALA+CP94 at both 20 and 40% oxygen (p<0.005) and for the cells incubated with MAL+CP94 at 40% oxygen (p<0.005).
Figure 1: Levels of PpIX fluorescence measured prior to (pre) and following (post) light irradiation of U-87 MG glioma cells subjected to 5, 20 or 40% oxygen and incubated with: (a) 250 μM ALA ± CP94, (b) 1000 μM MAL ± CP94 and (c) 10 μM HAL ± CP94. Data represents the mean of 5 wells ± 1 standard deviation, carried out in triplicate (n=3). *represents a statistically significant accumulation of PpIX between cells incubated with ALA/MAL/HAL alone and cells incubated with ALA/MAL/HAL+CP94 (p<0.05).
Following incubation with HAL and subjection to 5/20/40% oxygen the observed trend for the level of ‘pre’ PpIX achieved was in the order 20>5>40% (Figure 1C). When co-incubated with CP94 levels of ‘pre’ PpIX produced were again observed to increase and this increase reached significance for the cells incubated with HAL+CP94 at 40% oxygen only (p<0.005).
Measurement of PpIX photobleaching
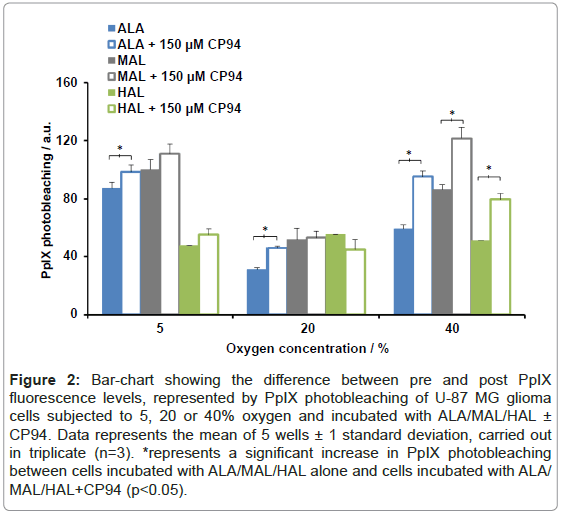
The difference between the ‘pre’ and ‘post’ PpIX fluorescence readings can be attributed to PpIX photobleaching and these measurements have been quantified (Figure 2). Excluding HAL+CP94 at 20% oxygen the observed trend was an increase in PpIX photobleaching when each pro-drug was co-incubated with CP94. Using a Student’s t-test between the pro-drug alone and pro-drug+CP94 data sets, at an oxygen environment of 40%, a significant increase in PpIX photobleaching was observed for all three pro-drugs co-incubated with CP94 (p<0.05). In addition, a significant increase in PpIX photobleaching was observed for ALA+CP94 at oxygen concentrations of 5 and 20% when compared to incubation with ALA alone (p<0.05).
Figure 2: Bar-chart showing the difference between pre and post PpIX fluorescence levels, represented by PpIX photobleaching of U-87 MG glioma cells subjected to 5, 20 or 40% oxygen and incubated with ALA/MAL/HAL ± CP94. Data represents the mean of 5 wells ± 1 standard deviation, carried out in triplicate (n=3). *represents a significant increase in PpIX photobleaching between cells incubated with ALA/MAL/HAL alone and cells incubated with ALA/ MAL/HAL+CP94 (p<0.05).
With the exception of 10 µM HAL ± CP94, comparison of the different oxygen concentration data sets indicated that greater levels of PpIX photobleaching were observed in the 5% and 40% groups compared to the levels detected in the 20% group (ANOVA, p<0.05). When the levels of PpIX photobleaching between the 5 and 40% oxygen concentration data sets were compared using ANOVA to detect any significant difference, none was found.
Cell viability
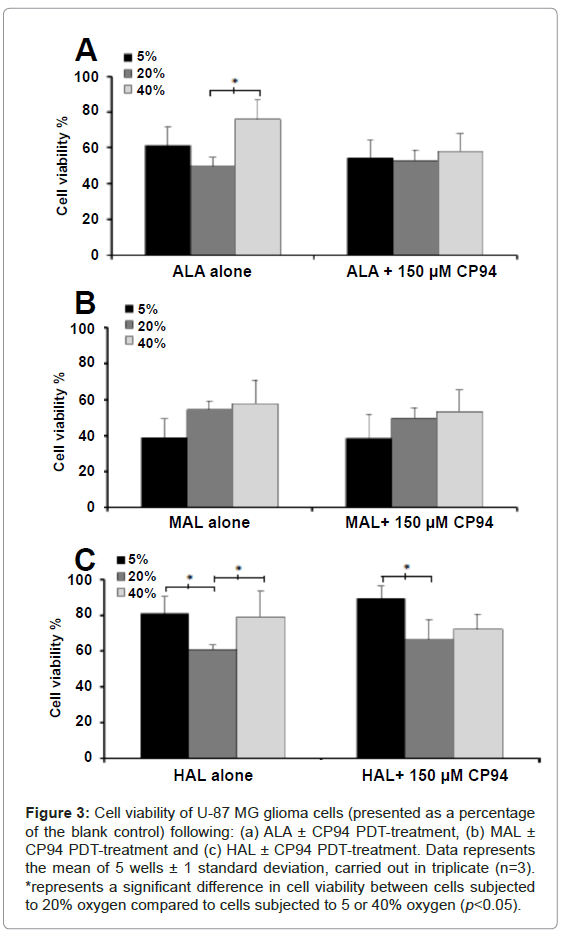
Following irradiation, the viability of the cells was assessed using the NRU assay. As this experimental procedure was conducted on cells contained within 96 well plates, it was not possible to confirm the following findings via light microscopy in this current investigation. For cells incubated with ALA ± CP94 at 5/20/40% oxygen concentration the observed trend was a decrease in cell viability in the order of 40>5>20% (Figure 3A). The only significant difference, however, was observed when the cells incubated with ALA alone were subjected to either 20 or 40% oxygen (p<0.05). For cells incubated with MAL ± CP94 the observed trend was a decrease in cell viability in the order of 40>20>5% oxygen concentration (Figure 3B). Nevertheless, no significant difference in cell viability was observed between the different oxygen concentrations. For cells incubated with HAL ± CP94 the observed trend was a decrease in cell viability in the order of 5>40>20% oxygen concentration (Figure 3C). When the cells were incubated with HAL alone a significant difference in cell viability between the 5 and 20% oxygen concentrations, and between the 40 and 20% oxygen concentrations was detected (p<0.05). In addition, when the cells were incubated with HAL+CP94 a significant difference in cell viability was detected between the 5 and 20% oxygen concentrations (p<0.05). Furthermore, comparison of the cell viability observed between the different pro-drugs indicated significantly less cell ablation was achieved when cells were incubated with 10 µM HAL ± CP94 compared to cells incubated with 250 µM ALA or 1000 µM MAL ± CP94 (ANOVA, p<0.05). Finally, when considering the effects of iron chelation on cell viability, for each pro-drug when co-incubated with CP94 compared to each pro-drug alone, no significant difference was found (Student’s t-test, p>0.05). This indicated that the elevated PpIX accumulation induced by CP94 prior to irradiation and the increased PpIX photobleaching produced by CP94 during irradiation were not translated into increased cell kill in this particular experimental system using these specific treatment parameters.
Figure 3: Cell viability of U-87 MG glioma cells (presented as a percentage of the blank control) following: (a) ALA ± CP94 PDT-treatment, (b) MAL ± CP94 PDT-treatment and (c) HAL ± CP94 PDT-treatment. Data represents the mean of 5 wells ± 1 standard deviation, carried out in triplicate (n=3). *represents a significant difference in cell viability between cells subjected to 20% oxygen compared to cells subjected to 5 or 40% oxygen (p<0.05).
Discussion
Despite improvements to treatment therapies, the prognosis of patients with a malignant glioma remains dismal. The survival time for patients with a low-grade glioma is 3-8 years and with a high-grade glioma is 6-12 months. These figures are thought to be attributed to the hypoxic areas of the tumor, which lead to increased aggressiveness and result in malignant gliomas being notoriously resistant to treatment [38].
In the last 15-20 years PD and FGR have been included in the surgical resection of primary brain tumors [39] and PDT may offer an adjuvant treatment modality alongside other conventional therapies. In this in vitro study the U-87 MG glioma cells were incubated at 5, 20 or 40% oxygen concentration with the porphyrin-precursors ALA/MAL/ HAL and the effect the addition of an iron chelator (CP94) had on the level of PpIX fluorescence prior to and following light irradiation was also investigated. In addition, the effect these various PDT treatment parameters had on cell viability was subsequently determined.
Using a tri-gas incubator hypoxic and hyperoxic environments were created by delivering nitrogen or oxygen into the chamber, respectively. Under hypoxic (5% oxygen) conditions the glioma cells incubated with either ALA or MAL ± CP94 produced the greatest level of PpIX fluorescence prior to light irradiation (Figure 1). This is in contrast to another study which incubated bladder cells with ALA at various oxygen tensions and found PpIX production was reduced at 0.0%, 2.5% and 5.0% oxygen compared to that observed with 21.0% oxygen (0.15, 0.28 and 0.40 ng µg-1 protein compared with 0.68 ng µg-1, respectively; p<0.05) [40]. Our findings could be explained by the fact that glioma cells naturally exist in a hypoxic tumor microenvironment in vivo [41]. Taking into account the high metabolic rates of glioma cells [42] it is therefore unsurprising that at 5% oxygen the greatest accumulation of PpIX was observed as this is the environment that best represents the in vivo situation. This could also explain why at 40% oxygen, when cells were incubated with ALA/MAL/HAL, the least amount of PpIX fluorescence was observed because the activity of the cells could have been compromised by subjecting them to a hyperoxic environment. However, when CP94 was introduced significantly greater levels of PpIX were produced with all three congeners, indicating that intracellular iron is also playing an important role in this intricate biochemistry. There may therefore be clinical benefit to be derived through the coadministration of CP94 with a PpIX prodrug when conducting FGR of gliomas and this possibility warrants further investigation. This is particularly important as to our knowledge we are the only group to have so far considered the use of an iron chelating agent to enhance FGR/PDT of glioma [17]. The investigation of a range of iron chelating agents for the enhancement of PpIX-induced fluorescence and/or cell kill in a range of applications in the literature over a number of years [14-17] has left us to conclude that CP94 is the iron chelating agent of greatest interest for this particular application. It has a number of advantages over more established clinical iron chelators including being orally available unlike DFO [14], which is already known to be more effective itself for this purpose than EDTA [15]. In addition, CP94 has been established to be more effective than dexrazoxane [17] and has a high specificity for iron, whilst also possessing low molecular weight as well as neutral charge, which enables it to enter intracellular iron pools rapidly and effectively [16]. We postulate that it these properties that are responsible for the significantly enhanced levels of fluorescence observed here.
One study which investigated the effect of hyperoxia on PDT and lipid peroxidation in two human colon carcinoma cell lines (SW480 and WiDr) and one rat bladder carcinoma cell type (AY-27) found normoxic conditions outperformed hyperoxic exposure. The group suggested a normoxic environment is sufficient to produce an optimal PDT effect [43]. However, the cells were exposed to hyperoxia following light irradiation. This may be too late to replenish oxygen levels depleted via the photochemical reactions which take place during light irradiation and or miss the opportunity to oxygenate preexisting hypoxic cells prior to light irradiation. Therefore it was hypothesized that exposing cells to a hyperoxic environment prior to, and post light irradiation, would address these issues and have an effect on the amount of PpIX photobleaching measured and subsequently the level of cell ablation observed. The only previous study where both iron chelation with CP94 and oxygen manipulation was studied in conjunction PpIX-induced PDT found that light dose fractionation and CP94 were both equally effective methods of enhancing ALA-PDT in normal rat colon and furthermore that the techniques were not mutually exclusive and could therefore be combined to produce even greater treatment effects [44].
In this study, the level of PpIX photobleaching observed (Figure 2) was increased when each prodrug was coincubated with CP94, and this was attributed to the increased accumulation of PpIX observed preirradiation. This finding (increased photobleaching) was also found to be statistically significant when the cells were grown in hyperoxic conditions (40%) when compared with normoxic conditions (20%). This is likely to be due to the fact that PpIX photobleaching is an oxygen dependent process and this process may therefore have been limited within the lower oxygen environment. Interestingly with ALA and MAL, hypoxic conditions (5%) were also found to produce statistically greater levels of PpIX photobleaching (Figure 2) than normoxic conditions (20%). This finding is most likely due to the large levels of PpIX observed in these groups pre-irradiation (Figure 1).
When the cells were subjected to ALA/MAL/HAL-PDT at varying concentrations of oxygen the level of cell ablation generally observed in most cases was similar with or without the iron chelator CP94, at least in this particular experimental system when using these specific treatment parameters. This observation warrants further investigation to determine if further alteration of the treatment parameters could remedy this and thus translate the increased PpIX accumulation and photobleaching observed here into increased cell kill when conducting PDT. The NRU assay is one of the most sensitive and commonly used cytotoxicity tests based on the ability of viable cells to incorporate and bind dye in the lysosomes [37]. This offers advantages over the colorimetric tetrazolium assays such as those which use the reagents 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]- 2H-tetrazolium hydroxide (XTT) as these rely on the metabolism of MTT and XTT which is inefficient in some human cell lines [45]. Notably in this study a significant percentage of viable cells remained following incubation with HAL ± CP94 compared to cells incubated with ALA/ MAL ± CP94. This could be explained by the lack of initial PpIX accumulation for cells incubated with HAL ± CP94 and thus a noteworthy lack of PpIX photobleaching irrespective of the oxygen concentration. As a result the cell ablation observed within this group was limited. The decreased ability of cells to produce PpIX whilst incubated with HAL ± CP94 could be due to the fact that HAL is the more lipophilic derivative of ALA (longer-chain). As a result the highly amphiphilic properties of HAL may induce membrane disruption [46], therefore decreasing the accumulation of PpIX. In addition, due to its dark toxicity, a low dose of only 10 µM HAL was selected for experimentation. Taken together these data suggest that of the three pro-drugs tested HAL would be the least effective at inducing detectable fluorescence for PD or FGR, as well as only producing a limited cytotoxic effect for use as a PpIX precursor in PDT treatment.
The glioma cells tested in this study were subjected to PpIXinduced PDT under atmospheric conditions which could be considered in tissue culture terms as normal (20% oxygen), hypoxic (5% oxygen) and hyperoxic (40% oxygen). Interestingly, it has been suggested that using a cell incubator at 20% is already subjecting cells to hyperoxic conditions [47]. Although it is agreed that the air we breathe is ~21% oxygen, Roy et al. [47] report mammalian organs experience O2 concentrations ranging from 12% to <0.5%. They therefore consider that working with 21% oxygen levels experimentally is thus equivalent to hyperoxic conditions.
In this study, the greatest level of PpIX fluorescence was observed when cells were incubated with ALA or MAL+CP94 at an oxygen concentration of 5%. CP94 has previously been shown to cross the blood-brain barrier (BBB) [48] and thus future in vivo studies should consider the use of ALA or MAL+CP94 for FGR during surgery. Furthermore, our results suggest that for the maximal production of PpIX during, for instance FGR glioma procedures, it doesn’t appear to be necessary to increase oxygen concentration, however for maximal photobleaching on irradiation (a marker of PDT effect) hyperoxgenation does appear to be a beneficial modification of the PDT treatment process. We therefore conclude that although further research is necessary to determine whether the enhancements produced by the coadministration of CP94 and hyperoxygenation (40% oxygen) on PpIX accumulation and photobleaching can be translated into improved PDT cytotoxicity, simple co administration of the iron chelator CP94 as an adjuvant with the PpIX-inducing precursors ALA or MAL utilized during FGR of glioma may confer substantial clinical benefit.
Acknowledgements
This work was supported in part by KILLING cancer. The European Centre for Environment and Human Health (part of the University of Exeter Medical School) is part financed by the European Regional Development Fund Programme 2007 to 2013 and European Social Fund Convergence Programme for Cornwall and the Isles of Scilly.
References
- Muller PJ, Wilson BC (2006) Photodynamic therapy of brain tumors--a work in progress. Lasers Surg Med 38: 384-389.
- Madsen SJ, Sun CH, Tromberg BJ, Wallace VP, Hirschberg H (2000) Photodynamic therapy of human glioma spheroids using 5-aminolevulinic acid. Photochem Photobiol 72: 128-134.
- Pass HI (1993) Photodynamic therapy in oncology: mechanisms and clinical use. J Natl Cancer Inst 85: 443-456.
- Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, et al. (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42: 518-525.
- Kaneko S (2008) Recent Advances in PDD and PDT for Malignant Brain Tumors. Rev Laser Eng 36: 1351-1354.
- Liu HF, Xu SZ, Zhang CR (2004) Influence of CaNa2 EDTA on topical 5-aminolaevulinic acid photodynamic therapy. Chin Med J (Engl) 117: 922-926.
- Szeimies RM, Calzavara-Pinton P, Karrer S, Ortel B, Landthaler M (1996) Topical photodynamic therapy in dermatology. J Photochem Photobiol B 36: 213-219.
- Henderson BW, Busch TM, Vaughan LA, Frawley NP, Babich D, et al. (2000) Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res 60: 525-529.
- Pogue BW, O'Hara JA, Goodwin IA, Wilmot CJ, Fournier GP, et al. (2002) Tumor PO(2) changes during photodynamic therapy depend upon photosensitizer type and time after injection. Comp Biochem Physiol A Mol Integr Physiol 132: 177-184.
- Bech O, Phillips D, Moan J, MacRobert AJ (1997) A hydroxypyridinone (CP94) enhances protoporphyrin IX formation in 5-aminolaevulinic acid treated cells. J Photochem Photobiol B 41: 136-144.
- Chen Q, Huang Z, Chen H, Shapiro H, Beckers J, et al. (2002) Improvement of tumor response by manipulation of tumor oxygenation during photodynamic therapy. Photochem Photobiol 76: 197-203.
- McIlroy BW, Curnow A, Buonaccorsi G, Scott MA, Bown SG, et al. (1998) Spatial measurement of oxygen levels during photodynamic therapy using time-resolved optical spectroscopy. J Photochem Photobiol B 43: 47-55.
- Tyrrell J, Campbell SM, Curnow A (2011) Monitoring the accumulation and dissipation of the photosensitizer protoporphyrin IX during standard dermatological methyl-aminolevulinate photodynamic therapy utilizing non-invasive fluorescence imaging and quantification. Photodiagnosis Photodyn Ther 8: 30-38.
- Pye A, Curnow A (2007) Direct comparison of delta-aminolevulinic acid and methyl-aminolevulinate-derived protoporphyrin IX accumulations potentiated by desferrioxamine or the novel hydroxypyridinone iron chelator CP94 in cultured human cells. Photochem Photobiol 83: 766-773.
- Berg K, Anholt H, Bech O, Moan J (1996) The influence of iron chelators on the accumulation of protoporphyrin IX in 5-aminolaevulinic acid-treated cells. Br J Cancer 74: 688-697.
- Curnow A, Pye A (2007) Biochemical manipulation via iron chelation to enhance porphyrin production from porphyrin precursors. J Environ Pathol Toxicol Oncol 26: 89-103.
- Blake E, Allen J, Curnow A (2011) An in vitro comparison of the effects of the iron-chelating agents, CP94 and dexrazoxane, on protoporphyrin IX accumulation for photodynamic therapy and/or fluorescence guided resection. Photochem Photobiol 87: 1419-1426.
- Curnow A, McIlroy BW, Postle-Hacon MJ, Porter JB, MacRobert AJ, et al. (1998) Enhancement of 5-aminolaevulinic acid-induced photodynamic therapy in normal rat colon using hydroxypyridinone iron-chelating agents. Br J Cancer 78: 1278-1282.
- Porter JB, Morgan J, Hoyes KP, Burke LC, Huehns ER, et al. (1990) Relative oral efficacy and acute toxicity of hydroxypyridin-4-one iron chelators in mice. Blood 76: 2389-2396.
- Porter JB, Hoyes KP, Abeysinghe RD, Brooks PN, Huehns ER, et al. (1991) Comparison of the subacute toxicity and efficacy of 3-hydroxypyridin-4-one iron chelators in overloaded and nonoverloaded mice. Blood 78: 2727-2734.
- Bergeron RJ, Streiff RR, Wiegand J, Luchetta G, Creary EA, et al. (1992) A comparison of the iron-clearing properties of 1,2-dimethyl-3-hydroxypyrid-4-one, 1,2-diethyl-3-hydroxypyrid-4-one, and deferoxamine. Blood 79: 1882-1890.
- Porter JB, Abeysinghe RD, Hoyes KP, Barra C, Huehns ER, et al. (1993) Contrasting interspecies efficacy and toxicology of 1,2-diethyl-3-hydroxypyridin-4-one, CP94, relates to differing metabolism of the iron chelating site. Br J Haematol 85: 159-168.
- Campbell SM, Morton CA, Alyahya R, Horton S, Pye A, et al. (2008) Clinical investigation of the novel iron-chelating agent, CP94, to enhance topical photodynamic therapy of nodular basal cell carcinoma. Br J Dermatol 159: 387-393.
- Fredenburg AM, Wedlund PJ, Skinner TL, Damani LA, Hider RC, et al. (1993) Pharmacokinetics of representative 3-hydroxypyridin-4-ones in rabbits: CP20 and CP94. Drug Metab Dispos 21: 255-258.
- Pye A, Campbell S, Curnow A (2008) Enhancement of methyl-aminolevulinate photodynamic therapy by iron chelation with CP94: an in vitro investigation and clinical dose-escalating safety study for the treatment of nodular basal cell carcinoma. J Cancer Res Clin Oncol 134: 841-849.
- Epemolu RO, Ackerman R, Porter JB, Hider RC, Damani LA, et al. (1994) HPLC determination of 1,2-diethyl-3-hydroxypyridin-4-one (CP94), its iron complex [Fe(III) (CP94)3] and glucuronide conjugate [CP94-GLUC] in serum and urine of thalassaemic patients. J Pharm Biomed Anal 12: 923-930.
- Kontoghiorghes GJ, Bartlett AN, Hoffbrand AV, Goddard JG, Sheppard L, et al. (1990) Long-term trial with the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1). I. Iron chelation and metabolic studies. Br J Haematol 76: 295-300.
- Busch TM (2006) Local physiological changes during photodynamic therapy. Lasers Surg Med 38: 494-499.
- Busch TM, Hahn SM, Evans SM, Koch CJ (2000) Depletion of tumor oxygenation during photodynamic therapy: detection by the hypoxia marker EF3 [2-(2-nitroimidazol-1[H]-yl)-N-(3,3,3-trifluoropropyl)acetamide ]. Cancer Res 60: 2636-2642.
- Curnow A, Haller JC, Bown SG (2000) Oxygen monitoring during 5-aminolaevulinic acid induced photodynamic therapy in normal rat colon. Comparison of continuous and fractionated light regimes. J Photochem Photobiol B 58: 149-155.
- Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, et al. (2004) Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res 64: 2120-2126.
- Sitnik TM, Hampton JA, Henderson BW (1998) Reduction of tumour oxygenation during and after photodynamic therapy in vivo: effects of fluence rate. Br J Cancer 77: 1386-1394.
- Tromberg BJ, Orenstein A, Kimel S, Barker SJ, Hyatt J, et al. (1990) In vivo tumor oxygen tension measurements for the evaluation of the efficiency of photodynamic therapy. Photochem Photobiol 52: 375-385.
- Tyrrell J, Thorn C, Shore A, Campbell S, Curnow A (2011) Oxygen saturation and perfusion changes during dermatological methylaminolaevulinate photodynamic therapy. Br J Dermatol 165: 1323-1331.
- Busch TM, Wileyto EP, Emanuele MJ, Del Piero F, Marconato L, et al. (2002) Photodynamic therapy creates fluence rate-dependent gradients in the intratumoral spatial distribution of oxygen. Cancer Res 62: 7273-7279.
- Angell-Petersen E, Spetalen S, Madsen SJ, Sun CH, Peng Q, et al. (2006) Influence of light fluence rate on the effects of photodynamic therapy in an orthotopic rat glioma model. J Neurosurg 104: 109-117.
- Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3: 1125-1131.
- Amberger-Murphy V (2009) Hypoxia helps glioma to fight therapy. Curr Cancer Drug Targets 9: 381-390.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. (2007) Cancer statistics, 2007. CA Cancer J Clin 57: 43-66.
- Wyld L, Reed MW, Brown NJ (1998) The influence of hypoxia and pH on aminolaevulinic acid-induced photodynamic therapy in bladder cancer cells in vitro. Br J Cancer 77: 1621-1627.
- Seidel S, Garvalov BK, Wirta V, von Stechow L, Schänzer A, et al. (2010) A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain 133: 983-995.
- MURRAY MR, PETERSON ER, HIRSCHBERG E, POOL JL (1954) Metabolic and chemotherapeutic investigation of human glioblastoma in vitro. Ann N Y Acad Sci 58: 1147-1171.
- Hjelde A, Gederaas OA, Krokan HE, Brubakk AO (2005) Lack of effect of hyperoxia on photodynamic therapy and lipid peroxidation in three different cancer cell lines. Med Sci Monit 11: BR351-356.
- Curnow A, MacRobert AJ, Bown SG (2006) Comparing and combining light dose fractionation and iron chelation to enhance experimental photodynamic therapy with aminolevulinic acid. Lasers Surg Med 38: 325-331.
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, et al. (1988) Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48: 4827-4833.
- Fotinos N, Convert M, Piffaretti JC, Gurny R, Lange N (2008) Effects on gram-negative and gram-positive bacteria mediated by 5-aminolevulinic Acid and 5-aminolevulinic acid derivatives. Antimicrob Agents Chemother 52: 1366-1373.
- Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, et al. (2003) Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1). Circ Res 92: 264-271.
- Fredenburg AM, Sethi RK, Allen DD, Yokel RA (1996) The pharmacokinetics and blood-brain barrier permeation of the chelators 1,2 dimethly-, 1,2 diethyl-, and 1-[ethan-1'ol]-2-methyl-3-hydroxypyridin-4-one in the rat. Toxicology 108: 191-199.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14066
- [From(publication date):
specialissue-2013 - Apr 10, 2025] - Breakdown by view type
- HTML page views : 9501
- PDF downloads : 4565