The Balance between Life and Death: Defining a Role for Apoptosis in Aging
Received: 27-Jan-2012 / Accepted Date: 18-Feb-2012 / Published Date: 22-Feb-2012 DOI: 10.4172/2161-0681.S4-001
Keywords: Aging; Apoptosis; Caspases; Mitochondria; Oxidative stress
316969Introduction
The process of human aging is associated with a general decline in the function of several organ systems, where age-related changes represent the sum of many different cellular and molecular changes over-time. Phenotypically, the progressive decline in physiological function is often associated with cell loss suggesting that cell death plays an important role in the aging process. Over the last decade, much attention has been focused on the progressive dysregulation of apoptotic cell death as a possible causal factor in aging and age-related pathologies.
Apoptosis is an evolutionarily conserved cell death program that is strictly regulated and executed through finely controlled signaling pathways. In multicellular organisms, apoptosis is essential for embryogenesis, development and tissue homeostasis (i.e. cell turnover, removal of damaged and oncogenic cells, immune-reactive cells and pathogen infected cells) [1,2]. Given the importance of apoptosis for normal tissue function, it is not surprising that defects in the regulation of apoptosis have been linked to a number of degenerative and hyperproliferative diseases [3]. Alterations in apoptotic potential, due to perturbations in cell signaling cascades, could conceivably underlie any number of age-related pathologies. An increase in apoptosis, particularly in post-mitotic tissues, may lead to excessive cell loss and a functional decline in tissues, as is observed in neurodegenerative diseases. On the other hand, a loss of apoptotic competence may lead to the accumulation of damaged cells and be a significant factor leading to the development of cancers. This article reviews the biochemistry of apoptosis and discusses what is currently known about apoptotic signaling as it relates to normal aging and age-related pathologies, using neurodegenerative tissues, the aging immune system and the aging heart as examples.
Apoptosis
Apoptosis is an intrinsic form of death that is regulated by a variety of cellular signals and defined by a set of characteristic morphological and biochemical features. Apoptotic cells classically exhibit nuclear condensation and fragmentation, DNA laddering (200 bp fragments), blebbing or rounding of the cell, and the externalization of phosphatidylserine [1,2,4]. Cells dying by apoptosis fragment into membrane-bound apoptotic bodies that are readily phagocytosed and digested by macrophages or by neighboring cells without generating an inflammatory response.
Rather than a passive fate, apoptosis is an active process that is initiated by the cell and governed by gene activities that either induce or inhibit cell death. Pro-death stimuli can either be generated by neighbouring cells or can originate from within doomed cells. If the decision is cell death, the end point of the signaling cascade is the activation of an evolutionarily conserved family of cysteine proteases called caspases [4,5]. Caspases are present in cells as inactive zymogens and are triggered by proteolytic processing. Once activated, caspases are involved in the downstream processing of the substrates required to dismantle the cell, including protein kinases, signal transduction proteins, cytoskeletal and nuclear matrix proteins, chromatin modifying enzymes PARP (poly (ADP ribose) polymerase), DNA repair proteins, and the inhibitory subunits of certain endonucleases [1].
Activating apoptosis
Two major pathways have been described that regulate apoptosis: the extrinsic pathway and the intrinsic pathway [2]. During extrinsic cell death signaling, the binding of extracellular ligands to receptors located in the plasma membrane induces trimerization of proteins known as death receptors, namely CD95 (Fas/Apo-1), TNFR1 and 2. Ligands that activate these receptors are structurally related molecules that bind to the TNF superfamily. The CD95 ligand (CD95L) binds to CD95, TNF binds to TNFR1, etc. Activation of these receptors induces trimerization and the recruitment of cytoplasmic adaptor molecules including FADD (Fas associated death domain containing protein), TRADD (TNFR-associated death domain), or RIP (receptorinteracting protein). Recruitment of these adaptor proteins results in the recruitment of pro-caspase-8 to a protein complex known as the Death-Inducing Signaling Complex (DISC). Caspase-8 is activated within this complex and may induce cell death through the cleavage of caspase-3/-7 or through the cleavage of Bid and the subsequent activation of the intrinsic signaling pathway (Figure 1a) [1,6-8].
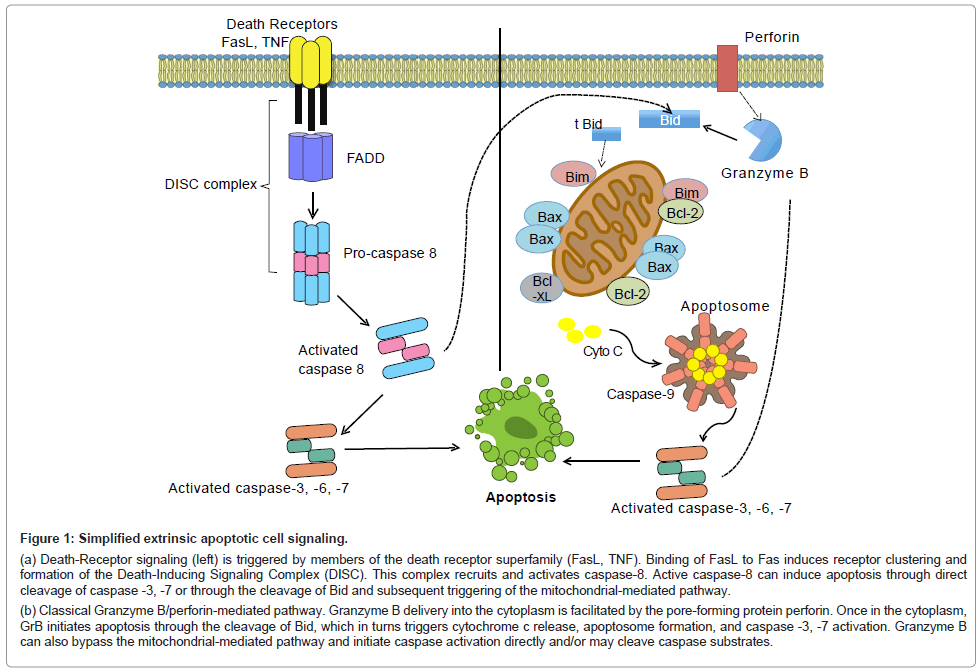
Figure 1: Simplified extrinsic apoptotic cell signaling.
(a) Death-Receptor signaling (left) is triggered by members of the death receptor superfamily (FasL, TNF). Binding of FasL to Fas induces receptor clustering and formation of the Death-Inducing Signaling Complex (DISC). This complex recruits and activates caspase-8. Active caspase-8 can induce apoptosis through direct cleavage of caspase -3, -7 or through the cleavage of Bid and subsequent triggering of the mitochondrial-mediated pathway.
(b) Classical Granzyme B/perforin-mediated pathway. Granzyme B delivery into the cytoplasm is facilitated by the pore-forming protein perforin. Once in the cytoplasm, GrB initiates apoptosis through the cleavage of Bid, which in turns triggers cytochrome c release, apoptosome formation, and caspase -3, -7 activation. Granzyme B can also bypass the mitochondrial-mediated pathway and initiate caspase activation directly and/or may cleave caspase substrates.
The Granzyme B-perforin pathway is a mechanism by which cytotoxic T cells and Natural Killer (NK) cells eliminate target. Upon target cell engagement, immune cells release granules containing Granzyme B and the pore-forming protein, perforin. Once in the cytoplasm, Granzyme B will induce cell death primarily through cleavage of the protein Bid and the subsequent activation of the intrinsic apoptotic pathway (Figure 1b). Granzyme B will also target other intracellular substrates to induce apoptosis, including caspases-10 and caspase-3, and several caspase substrates such as the inhibitor of caspase-activated deoxyribonuclease (ICAD), reviewed in [9,10].
Cell death signals originating from within the cell signal through the intrinsic or mitochondrial-mediated pathway. In mammals, internal death stimuli directly or indirectly change mitochondrial membrane permeability causing the release of cytochrome c from the mitochondrion into the cytosol where it binds to the adaptor molecule, Apaf-1 (apoptotic protease activating factor-1) [11-13]. This binding triggers the formation of a protein complex known as the apoptosome, which contains Apaf-1 molecules, cytochrome c, and procaspase-9 molecules [7,14]. The procaspase-9 molecules bound to the apoptosome are activated and subsequently activate downstream caspases such as caspase-3 (Figure 2) [6,7].
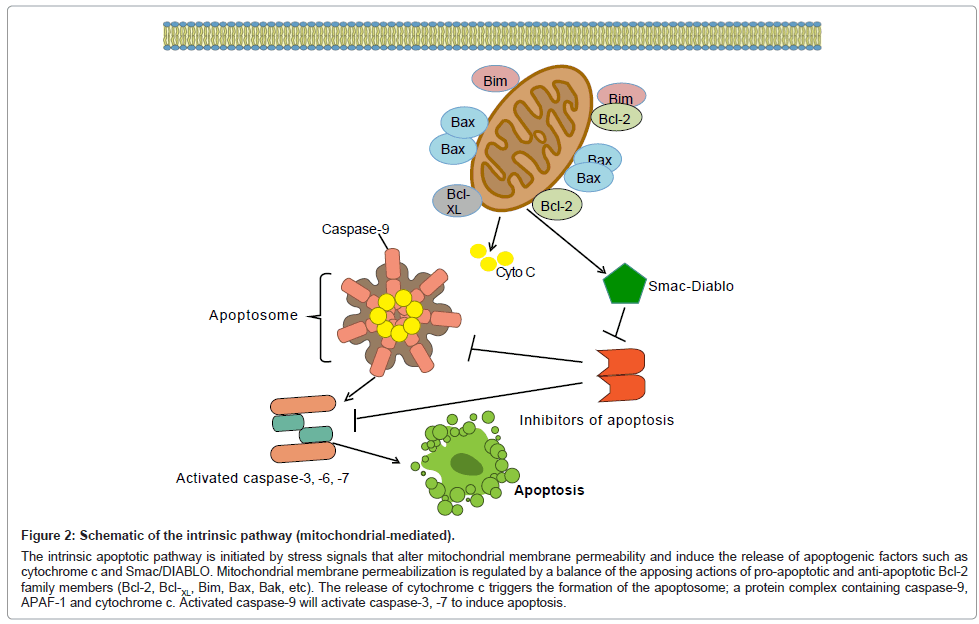
Figure 2: Schematic of the intrinsic pathway (mitochondrial-mediated).
The intrinsic apoptotic pathway is initiated by stress signals that alter mitochondrial membrane permeability and induce the release of apoptogenic factors such as cytochrome c and Smac/DIABLO. Mitochondrial membrane permeabilization is regulated by a balance of the apposing actions of pro-apoptotic and anti-apoptotic Bcl-2 family members (Bcl-2, Bcl-XL, Bim, Bax, Bak, etc). The release of cytochrome c triggers the formation of the apoptosome; a protein complex containing caspase-9, APAF-1 and cytochrome c. Activated caspase-9 will activate caspase-3, -7 to induce apoptosis.
Regulating apoptosis
Most cells constitutively express caspase zymogens at levels sufficient to bring about cell death and the key to cell fate depends on the levels of active caspases in the cell. The triggering of death pathways depends not only on the activation of effector molecules but also on the mechanisms that counteract the pro-death signals. In mammals, the molecules that counteract the pro-death signals are the Bcl-2 and IAP families of proteins.
Bcl-2 proteins: Proteins of the Bcl-2 family are critical regulators of apoptosis, whose primary functions include governing mitochondrialdependent cell death [11,12]. Bcl-2 proteins directly regulate the permeability of mitochondrial membranes by either permitting or inhibiting the release of apoptogenic proteins such as cytochrome c. The Bcl-2 protein family has at least 20 members that have been identified in mammals [15,16], and that can be broken down into three groups; the pro-apoptotic multi-domain proteins (Bax, Bak, Bok); the pro-apoptotic BH3-only proteins (Bad, Noxa, Puma, and Bim), and the anti-apoptotic multi-domain protein proteins that include Bcl-2, Bcl- XL and Bcl-W. [17]
Inhibitors of apoptosis proteins (IAPs): Given the irreversible nature of most proteolytic reactions, the most critical checkpoints for cell death regulation occur at the level of caspase activation. IAPs are a conserved family of caspase suppressors [18,19]. The characteristic structural motif of all IAP family members is the presence of one to three Cys/His regions known as baculovirus IAP repeats, (BIRs) [20,21]. BIR motifs mediate interactions with multiple death proteins, including the caspases. Many IAP proteins also contain a C-terminal Zinc RING finger. RING domains can act as E3 ubiquitin ligases and catalyze the transfer of ubiquitin to target proteins, including themselves, for ubiquitination. Five human IAPs have been characterized: NAIP1, XIAP, c-IAP1, c-IAP2, and survivin [18,22]. In general, IAPs suppress apoptosis by negatively regulating the activity of caspases [23,24]. In mammals, caspase-9 is primarily inhibited by XIAP while caspase-3 and -7 are inhibited by XIAP and to a lesser extent c-IAP1, c-IAP2, and NAIP [24,25].
Smac/Diablo and Omi/HtrA2: These two proteins negatively regulate XIAP and therefore regulate caspase activation. Both proteins contain an IAP binding motif through which they bind to IAPs and release IAP bound caspases. Smac/Diablo and Omi/HtrA2 are located in the intermembrane space of mitochondria and are released into the cytosol during apoptosis, where they bind to IAPs releasing them from their caspases and thereby enabling the activation of caspases [26,27].
Apoptosis and aging
The prevalence of apoptosis has been shown to increase with age in many different cell types, reviewed in [28]. To identify the molecular events associated with aging, a number of investigators have examined genome-wide changes associated with aging in various human and animal tissues [29-35]. Collectively, these studies have suggested that aging results in a differential gene expression pattern indicative of altered apoptotic signaling and stress responses, as well as changes to metabolic and biosynthetic signaling. In support of the genetic data, several in vitro and in vivo studies have provided strong evidence that changes in age-dependent apoptosis can be directly linked to changes in caspase expression and caspase activity. For example, age-dependent alterations in caspase activity have been observed for multiple caspases in rodent models of aging, including an increase in caspase-3, -6, -7, -9, -2 activity in the spleen, lung, and liver of old (24-26 months) compared to young (6 months) or middle aged (12-14 months) Fisher 344 rats [36]. Similarly, increased caspase-3 activity was reported in the hippocampus of aged (22 months) compared to young (4 months) rats [37]. In addition to changes in caspase activity, other markers indicative apoptotic activity have been detected in aging rats including elevated levels of cytoplasmic cytochrome c, and poly(ADP-ribose) (PARP) cleavage and terminal dUTP nick end staining [38,39]. Collectively, these studies provide a strong link between increased apoptotic activity and the normal aging process in several different tissues in aging animals.
Mitochondria, apoptosis and aging
The concept that mitochondria play a key role in the aging process is not new. In 1952, Harman et al. [40] proposed the free radical theory of aging where oxygen free radicals are the cause of cumulative cellular damage that eventually results in aging and age-related pathologies in humans and animals. This theory was later refined to include mitochondria as key players in this aging process as they are the major source of free radicals in animal cells [41]. In 1989, Linnane et al. [42] proposed the mitochondrial theory of aging where the accumulation of somatic mutations in mitochondrial DNA is a major contributor to the aging process. Here the progressive accumulation of mutations in mtDNA over an individual’s lifetime leads to a decline in the bioenergetic function of mitochondria, dysfunction of the respiratory chain, increased production of reactive oxygen species (ROS) and the subsequent accumulation of more mutations in mtDNA.
In addition to being key mediators in the initiation and regulation of intrinsic apoptotic signaling in animal cells, mitochondria are also the major energy producers of the cell. Moreover, they are both the major source of reactive oxygen species (ROS) and free radicals under normal physiologic conditions, and are the major target for ROS and free radical damage within the cell. Studies from human and animal models have shown a wide spectrum of alterations in mitochondrial function and mtDNA during the normal aging process that include; a decline in mitochondrial respiratory function, an increase in ROS production, an increase in the resulting damage to cellular constituents resulting from increased ROS production, enhanced mutations to mtDNA, and enhanced apoptosis, reviewed in [43].
Bioenergetic studies in human and animal models indicate that the respiratory functions of mitochondria decline with age in postmitotic tissues. This is supported by studies reporting cytochrome c oxidase negative cardiomyocytes and muscle fibres in heart, limb muscles and diaphragm of elderly patients [44-46]. Further support is provided from observations that electron transport activities decline with age in the brain, skeletal muscle and liver of normal aging human subjects [47-49] and experimental animals [50,51]. The above changes can be accompanied by a decline in ATP synthesis and increased ROS production in various human tissues [52]. Under normal physiologic conditions, ROS and free radicals are generated and maintained at a steady-state by mitochondria in animal tissues [53,54] and the rates of ROS production from mitochondria are reported to increase with age in mammalian cell lines and tissues [55-57]. Importantly, the oxidative damage associated with increased ROS production has been associated with damage to cellular constituents including lipids, proteins, and nucleic acids [58].
Oxidative damage can cause modifications to both nuclear and mtDNA and many of these modifications have been shown to increase with age [58-60]. MtDNA is particularly susceptible to oxidative damage because of its close proximity to sites of ROS production, lack of protective histones and limited capacity for DNA repair, reviewed in [43]. The age-associated increase in mtDNA mutations has been associated with reduced life span, and premature onset of aging-related phenotypes including alopecia, kyphosis, osteoporosis, anemia, and reduced fertility [61]. Furthermore, oxidative damage to mtDNA is reported to be major cause of instability and mutations in mitochondria that are associated with respiratory dysfunction, an increase in ROS production and a general decline in the efficiency of energy metabolism [42,62]. In aging cells and tissues, these factors have been proposed to influence susceptibility to apoptosis and promotion of the aging process.
Lastly, phospholipids of mitochondrial membranes are extremely sensitive to oxidation. Alterations to these phospholipids can impact mitochondrial inner membrane barrier properties, maintenance of mitochondrial membrane permeability (MMP) and mitochondrial calcium buffering capacity [63-65]. Factors that affect (MMP), the regulation of pro- and anti-apoptotic Bcl-2 proteins, and the release of cytochrome c into the cytosol will lead to the activation of caspase-9- induced apoptosis. Studies in aging mice have shown that mitochondria from brain, liver and lymphocytes of older animals exhibit increased MMP activation. Similarly, the threshold for calcium-induced MMP has been shown to decrease with age in the lymphocytes, brain and liver in older mice, which in turn was found to lower the threshold for the release of apoptogenic factors such as cytochrome c into the cytosol and the subsequent induction of apoptosis [66]. In aging humans and animals, increased oxidant production is also associated with elevated levels of calcium in the cardiomyocytes [67] and elevated levels of cytosolic cytochrome c have been observed in the heart cells of aged (24 months) compared to young (6 months) male Fisher 344 rats [68].
Aging and Age-related Pathologies
Neurodegenerative disorders
Apoptosis plays a pivotal role in the progression of a variety of neurodegenerative diseases. Despite the many causes of such diseases, caspase activity has been reported across a broad spectrum of neurodegenerative diseases; however, the trigger for aberrant caspase activation is not well understood. The first evidence of a role for caspases in neurodegenerative diseases came from experiments in mouse models of ALS, where prolonged caspase activation was detected in ALS-transgenic mice. In this model, caspase-1 and caspase-3 transcripts were up-regulated as these mice aged [69-71].
Alzheimer’s disease (AD) is the most common form of dementia and is characterized by progressive impairment of cognitive function. Histologically, AD is associated with senile plaques containing amyloid-β peptide (Aβ) and neurofibrillary tangles composed of hyperphosphorylated tau proteins and paired helical fragments [72]. Several studies have reported a link between multiple caspases and AD, reviewed in [73]. Both in vitro and in vivo studies have reported elevated expression and activation of caspases in animal models of AD [74-78]. Moreover, elevated levels of caspases are found in the brain of severe definitive cases of AD [78-84]. Pompl et al. [85] showed elevated expression of caspases-1, -2, -3, -5, -6, -7, -8 and -9 in the brain of patients with AD compared with controls. Pyramidal neurons from vulnerable regions of the brain are reported to show an increase in activated caspase-3 and caspase-6 [84], and synaptosomes from AD brain frontal cortices have shown an enrichment in caspase-9 compared with non-demented controls [81,83]. Strong evidence linking caspase activity to the development of AD are provided by studies showing that amyloid precursor protein (APP) can be cleaved by caspase-3, -6, -7 and -8 [74,83] and amyloid plaques are enriched in caspase-cleaved APP [86]. Further evidence is provided from studies in caspase-2 and caspase-12 knockout mice that demonstrate neurons resistant to Aβ [77,87]. In addition to APP, caspases will also cleave the protein tau, thereby enhancing tau filament polymerization in vitro [88,89]. Taken together, these data demonstrate that caspases are involved in the pathogenesis of AD but that further studies are required to refine the roles played by each specific caspase. It should also be noted there is evidence linking caspase activity to the development of AD, Parkinson’s and dementia resulting from human immunodeficiency virus [90,91].
Lastly, there is growing evidence for a link between the Bcl-2 proteins and the development of AD. Lu et al. [92] showed Bcl-2 and Bax are found to co-localize in the frontal cortex of patients with AD. Further support for the involvement of Bcl-2 proteins in AD pathogenesis comes from in vitro studies showing that Aβ induces an up-regulation of pro-apoptotic molecules such as Bax and a downregulation of anti-apoptotic molecules such as Bcl-2, Bcl-XL, and Bcl-W [93]. One additional pro-apoptotic Bcl-2 family member, Bim (Bcl- 2-interacting mediator of cell death) has been linked to AD. Bim is induced in both corticol and hippocampal neuronal cultures after Aβ exposures and its induction is essential for the neurotoxic effects of Aβ [94]. In conclusion, there is strong evidence to provide support for the hypothesis that apoptosis plays an important role in the neuronal loss observed in dementia and Alzheimer’s disease.
Caspase activity has also been linked to the development of Huntington’s disease. Huntington’s disease is an autosomal dominant disorder in which specific cell death occurs in the neostriatum and cortex [38]. Two features commonly associated with this neurodegenerative disease are mutations associated with the protein Huntingtin, and neuronal dysfunction associated with the down-regulation of neurotransmitter receptors [95,96]. One of the earliest events in the progression of Huntington’s is the up-regulation of caspase-1 gene expression [96]. As the disease progresses, caspase-3 transcription is up-regulated and elevated levels of caspase-3 activity are detected [97]. Moreover, caspase-8 and caspase-9 activation, as well as the release of cytochrome c have also been demonstrated in Huntington’s disease [98,99]. While the direct cause of aberrant caspase activity is not fully understood in many neurologic disorders, there is strong evidence directly linking caspase activity to the progression of Huntington’s disease. Here, Huntingtin, a protein believed to be central to the development of Huntington’s disease, is a substrate for both caspase-1 and caspase-3 [100,101]. As the disease progresses, increased caspasemediated cleavage of Huntingtin increases the levels of Huntingtin fragments and depletes the levels of wild-type Huntingtin [96]. Both are thought to play a role in Huntington’s disease progression. Lastly, caspase activity is believed to play a role in the development of neuronal dysfunction as the inhibition of caspase activity also inhibits the down-regulation of neurotransmitter receptors in a mouse model of Huntington’s disease [96]. Transgenic mice have been used as a tool for evaluating the efficacy of caspase inhibitors in animal models of Huntington’s disease [90,96,97].
The aging immune system
The deterioration of the immune system is believed to contribute to morbidity and mortality in aging humans. Certainly, older animals are more susceptible to microbial infections and demonstrate an increased prevalence of specific cancers and certain autoimmune diseases with advancing aging. Aging is associated with thymic involution, lymphopenia, and a progressive deterioration in T cell function, reviewed in [102]. This decline in T cell function is believed to play a role in age-enhanced susceptibility to infection, autoimmunity and cancer [103-105]. Apoptosis is vital in controlling T cell number, deleting self-reactive cells and maintaining immune surveillance during development and tissue homeostasis. Age-dependent increases in apoptotic activity and caspase activity have been reported in both human and murine T cells and B cells, reviewed in [106]. Lacelle et al. [107] used quantitative RT-PCR to screen the PBMCs from human subjects ranging from 2-102 yrs of age where they observed an increase in caspase-1 and caspase-3 mRNA levels in old (70-89 years) and extremely old (>90 years) humans compared to those in younger age groups. In this same study, old individuals were also reported to express higher levels of caspase-8 mRNA. Similarly, Aggrawal et al. [108] reported increased expression of caspase-8 and caspase-3 protein has been reported in T lymphocytes of aging human subjects. Moreover, CD4+ and CD8+ T cells from older human subjects are reported to display increased expression of TNFR1 and TRADD, and increased caspase-8 and caspase-3 activity upon stimulation with TNF-α. Given that TNF-α levels are reported to increase with age, this suggests that T cells from older individuals may be more sensitive to apoptotic stimuli [103,109]. In addition to increased caspase activity, lymphocytes from older subjects have been found to express higher levels of Fas and Fas ligand, increased expression of FADD, increased expression of the pro-apoptotic protein Bax and decreased expression of anti-apoptotic protein Bcl-XL [103,110]. While these data suggest that apoptotic activity increases with age, it is unclear if the increased apoptotic activity is the result of altered cell signaling during the aging process or an increased sensitivity to apoptosis. Either scenario in lymphocytes may help to explain a general decline in immunity with aging humans.
In addition to the observed increase in peripheral lymphocyte apoptosis, hypocellularity of primary lymphoid organs is a distinctive characteristic of aging humans. The thymus is known to atrophy with progressive aging resulting in a significant loss in its capability to generate new T cells for export into the peripheral T cell pool [111]. There is a clear increase in the number of apoptotic cells in the thymic cortex with aging. Histologically, Bar-Dayan et al. [112] showed an increase in the apoptotic index of the thymic cortex in 7-month old mice compared to 1-month old mice. The noted increase in apoptotic activity was accompanied by a marked decline in proliferation suggesting that apoptosis may account for the reduction of thymic cortical cellularity during aging. Support for this hypothesis is provided by other studies showing an age-associated increase in thymocyte loss associated with enhanced expression of the pro-apoptotic genes Bax and p53, and with increased the cleavage of poly (ADP-ribose) polymerase (PARP) and increased caspase-3 activation [39,113]. Collectively, these alterations in apoptosis-related components create a scenario where the balance between survival and death is altered in aged lymphocytes and lymphoid organs and may provide a mechanism for reduced immunity in aging animals.
Cardiovascular system
There is increasing evidence of a relationship between apoptosis and cardiovascular disease, particularly for the heart diseases common to the elderly populations, including ischemic heart disease and congestive heart failure. The aging process in the heart is characterized by a significant loss of cardiac myocytes with the base-line level of apoptosis higher in older compared to younger animals [114]. This age-associated elevation in apoptotic activity is clinically relevant, as the adult heart cannot repair damaged tissue due to the fact that mature cardiomyocytes are terminally differentiated and therefore unable to divide. The loss of cardiomyocytes with age may ultimately lead to impaired cardiac function with [115]. Evidence of age-associated elevations in cardiomyocyte apoptosis has been noted across different several different species (humans, mouse, rabbit, dog, sheep and pig) and is observed in different experimental models including myocardial infarction (MI) [116-121]. Kajstura et al. [120] estimated the initial ventricular cardiomyocyte population to decline by as much as 30% as the heart ages. Moreover, increased levels of cardiomyocyte apoptosis are detected following ischemic attacks suggesting that apoptosis in the aging heart is a contributing factor for the elevated MI-related morbidities and mortalities observed in elderly patients [121-123].
The underlying mechanisms for apoptosis in the aging heart are not fully understood; however, there is strong evidence for alterations in mitochondrial oxidative stress in aging cardiomyocytes, including elevated levels of diastolic Ca2+ and elevated levels of cytochrome c [66-68,114]. Interestingly, while several studies have shown progressive changes in intracellular Ca2+ levels and cytoplasmic cytochrome c levels, studies that have examined other markers of mitochondrial-mediated apoptosis, namely the Bcl-2 family of proteins, have shown interesting but occasionally conflicting results. Phaneuf et al. [68] have shown an increase in cytosolic cytochrome c in 16-24 month animals compared to 6-month animals, accompanied by an age-dependent decrease in mitochondrial Bcl-2 levels but no alteration in mitochondrial Bax levels. In contrast, studies in spontaneously hypertensive rats (SHR) have shown significantly elevated ratios of Bax/Bcl-2 that correlate with an increase in apoptosis that begins at 4 weeks and is maintained at high levels throughout aging [124]. Similarly, in ischemia-reperfusion models of cardiac injury, Liu et al. [125] reported significantly increased levels of Bax in aged compared to young hearts. It is interesting to note that transcriptional analysis of apoptotic genes in the aging heart have shown that caloric restriction can down-regulate the expression of pro-apoptotic factors, such as Bax and caspase-9, as well as up-regulate the expression of pro-apoptotic genes such as IAPs and Bcl-2 [32]. Taken together, these studies suggest an age-dependent elevation in mitochondrial oxidative stress may lead to alterations to the relative amounts of pro- and anti-apoptotic proteins, which can be modified by caloric restriction. Whether this imbalance is involved in altered apoptotic signaling that directly impacts the aging process or whether these changes simply represent an increased sensitivity to apoptosis in aging tissues requires further elucidation. Strategies aimed at promoting cardiomyocyte survival and proliferation will provide new therapies to reduce the susceptibility of aging hearts to ischemic injury and end-stage heart failure.
It is also worth noting that in addition to age-related cell loss, apoptosis has been linked to other age-related vascular diseases, including atherosclerosis. Atherosclerosis is a lipid-driven inflammatory disease responsible for the majority of heart attacks, and lower limb loss [126-128]. T cells and macrophages are abundant in atherosclerotic lesions where they are known to participate in the inflammatory response and the induction of apoptotic cell death. Here, apoptosis has been observed during the early stages of lesion development where smooth muscle cell apoptosis may be a beneficial way to reduce intimal hyperplasia [129]. However, in more advanced lesions, apoptosis may decrease plaque stability through decreased cellularity and extracellular matrix degradation, reviewed in [9,130].
Other cells and organ systems
The prevalence of apoptosis has been shown increase with age in many other cell types and organ systems. Age-associated increases in chondrocytes have been observed on all articular surfaces of the knee joints of C57BL/6 mice and Wistar rats [131], suggesting that apoptosis plays a role in the maintenance and remodeling of mature articular cartilage. Granzyme B-induced apoptosis has been implicated in cartilage matrix destruction and is thought to be an important mediator in the pathogenesis of reactive arthritis [132,133], as well as contribute to cartilage degeneration over time, reviewed in [9]. There is also strong evidence that the age-associated loss of skeletal muscle mass is related to apoptosis in both human and animal models, reviewed in [134]. Finally, age-associated increases have also been observed hepatocytes [135-138], β cells in pancreatic islets [139-141] and oocytes [139,140].
It is important to note to that age-dependent alterations in apoptotic activity are not limited to increases in apoptotic activity as decreased caspase levels and activities have also been observed in aged tissues. For example, decreased levels of caspase-3, caspase-8 and caspase-9 have been reported in the colon mucosa of old (22-24 months) F344 rats compared to young (4-5 months) and middle aged (12-14 months) rats [142]. This observed decrease in caspase activity was accompanied by a decrease in PARP cleavage, as well as decreased levels of Bak, and increased levels of the anti-apoptotic protein Bcl- XL. Moreover, the observed decrease in caspase expression and activity was associated with lower numbers of apoptotic cells in colon mucosa in older rats. Similar results are reported in patients with acromegaly [143]. These alterations may help to explain the increased incidence of colon cancer in older individuals.
Conclusions
Aging is a natural and complex biological process and many studies have shown that aging is associated with altered cell signaling. There is increasing evidence that apoptosis plays a role in the aging processes as age-related changes in apoptotic proteins have been observed in many different cells type and a number of different organ systems. Although an increase in apoptotic cell death has been reported with aging in both human and animal models, it is often difficult to determine if it is altered apoptotic signaling or simply normal apoptotic activity overtime that is contributing to the observed age-related phenotypes. While, apoptosis is, without question, critical for homeostasis, it is conceivable that even normal apoptotic processes might, over-time lead to normal aging phenotypes or age-related pathologies, particularly in postmitotic cells such, as neurons and cardiomyocytes.
Studies of apoptosis and aging are limited by the same difficulties that plague the studies of apoptosis in general, namely the variable specificity of the techniques used to detect apoptosis and the challenges in translating and linking these findings to a particular phenotype or clinical outcome. A deeper understanding of how apoptosis relates to aging and age-related pathologies will be essential if we are to better manage age-related diseases or develop strategies for intervening in the aging process.
Acknowledgements
I thank Kendra Mitchell-Foster for helpful comments on the manuscript and Paul Hiebert for help with the figures.
References
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495-516.
- Hengartner MO (2000) The biochemistry of apoptosis. Nature 407: 770-776.
- Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C (2002) The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann N Y Acad Sci 959: 93-107.
- Thornberry NA (1998) Caspases: key mediators of apoptosis. Chem Biol 5: R97-103.
- Kornbluth S, White K (2005) Apoptosis in Drosophila: neither fish nor fowl (nor man, nor worm). J Cell Sci 118: 1779-1787.
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, et al. (2003) A unified model for apical caspase activation. Mol Cell 11: 529-541.
- Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15: 725-731.
- Boatright KM, Salvesen GS (2003) Caspase activation. Biochem Soc Symp : 233-242.
- Boivin WA, Cooper DM, Hiebert PR, Granville DJ (2009) Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Lab Invest 89: 1195-1220.
- Russell JH, Ley TJ (2002) Lymphocyte-mediated cytotoxicity. Annu Rev Immunol 20: 323-370.
- Green D, Kroemer G (1998) The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol 8: 267-271.
- Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281: 1309-1312.
- Schafer ZT, Parrish AB, Wright KM, Margolis SS, Marks JR, et al. (2006) Enhanced sensitivity to cytochrome c-induced apoptosis mediated by PHAPI in breast cancer cells. Cancer Res 66: 2210-2218.
- Adrain C, Slee EA, Harte MT, Martin SJ (1999) Regulation of apoptotic protease activating factor-1 oligomerization and apoptosis by the WD-40 repeat region. J Biol Chem 274: 20855-20860.
- Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322-1326.
- Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13: 1899-1911.
- Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122: 437-441.
- Altieri DC (2010) Survivin and IAP proteins in cell-death mechanisms. Biochem J 430: 199-205.
- Clem RJ, Miller LK (1994) Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol 14: 5212-5222.
- Goyal L, McCall K, Agapite J, Hartwieg E, Steller H (2000) Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J 19: 589-597.
- Adams JM, Cory S (2002) Apoptosomes: engines for caspase activation. Curr Opin Cell Biol 14: 715-720.
- Silke J, Kratina T, Chu D, Ekert PG, Day CL, et al. (2005) Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc Natl Acad Sci U S A 102: 16182-16187.
- Deveraux QL, Reed JC (1999) IAP family proteins--suppressors of apoptosis. Genes Dev 13: 239-252.
- Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 3: 401-410.
- Shi Y (2002) A conserved tetrapeptide motif: potentiating apoptosis through IAP-binding. Cell Death Differ 9: 93-95.
- Verhagen AM, Vaux DL (2002) Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis 7: 163-166.
- Chen DJ, Huerta S (2009) Smac mimetics as new cancer therapeutics. Anticancer Drugs 20: 646-658.
- Joaquin AM, Gollapudi S (2001) Functional decline in aging and disease: a role for apoptosis. J Am Geriatr Soc 49: 1234-1240.
- Bodyak N, Kang PM, Hiromura M, Sulijoadikusumo I, Horikoshi N, et al. (2002) Gene expression profiling of the aging mouse cardiac myocytes. Nucleic Acids Res 30: 3788-3794.
- Cao SX, Dhahbi JM, Mote PL, Spindler SR (2001) Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A 98: 10630-10635.
- Jiang CH, Tsien JZ, Schultz PG, Hu Y (2001) The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci U S A 98: 1930-1934.
- Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA (2002) Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A 99: 14988-14993.
- Lee CK, Klopp RG, Weindruch R, Prolla TA (1999) Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390-1393.
- Tollet-Egnell P, Flores-Morales A, Ståhlberg N, Malek RL, Lee N, et al. (2001) Gene expression profile of the aging process in rat liver: normalizing effects of growth hormone replacement. Mol Endocrinol 15: 308-318.
- Zou S, Meadows S, Sharp L, Jan LY, Jan YN (2000) Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc Natl Acad Sci U S A 97: 13726-13731.
- Zhang Y, Chong E, Herman B (2002) Age-associated increases in the activity of multiple caspases in Fisher 344 rat organs. Exp Gerontol 37: 777-789.
- Lynch AM, Lynch MA (2002) The age-related increase in IL-1 type I receptor in rat hippocampus is coupled with an increase in caspase-3 activation. Eur J Neurosci 15: 1779-1788.
- Martin DS, Lonergan PE, Boland B, Fogarty MP, Brady M, et al. (2002) Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem 277: 34239-34246.
- Kapasi AA, Singhal PC (1999) Aging splenocyte and thymocyte apoptosis is associated with enhanced expression of p53, bax, and caspase-3. Mol Cell Biol Res Commun 1: 78-81.
- Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11: 298-300.
- Harman D (1972) The biologic clock: the mitochondria? J Am Geriatr Soc 20: 145-147.
- Linnane AW, Marzuki S, Ozawa T, Tanaka M (1989) Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1: 642-645.
- Lee HC, Wei YH (2007) Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood) 232: 592-606.
- Müller-Höcker J (1989) Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol 134: 1167-1173.
- Müller-Höcker J (1990) Cytochrome c oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. J Neurol Sci 100: 14-21.
- Müller-Höcker J, Seibel P, Schneiderbanger K, Kadenbach B (1993) Different in situ hybridization patterns of mitochondrial DNA in cytochrome c oxidase-deficient extraocular muscle fibres in the elderly. Virchows Arch A Pathol Anat Histopathol 422: 7-15.
- Cooper JM, Mann VM, Schapira AH (1992) Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci 113: 91-98.
- Hsieh RH, Hou JH, Hsu HS, Wei YH (1994) Age-dependent respiratory function decline and DNA deletions in human muscle mitochondria. Biochem Mol Biol Int 32: 1009-1022.
- Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E (1999) Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev 111: 39-47.
- Trounce I, Byrne E, Marzuki S (1989) Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet 1: 637-639.
- Yen TC, Chen YS, King KL, Yeh SH, Wei YH (1989) Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun 165: 944-1003.
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, et al. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 102: 5618-5623.
- Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527-605.
- Wei Y, Scholes CP, King TE (1981) Ubisemiquinone radicals from the cytochrome b-c1 complex of the mitochondrial electron transport chain--demonstration of QP-S radical formation. Biochem Biophys Res Commun 99: 1411-1419.
- Sohal RS, Dubey A (1994) Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic Biol Med 16: 621-626.
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H (1994) Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev 74: 121-133.
- Lee HC, Yin PH, Chi CW, Wei YH (2002) Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci 9: 517-526.
- Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78: 547-581.
- Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A 90: 7915-7922.
- Beckman KB, Ames BN (1998) Mitochondrial aging: open questions. Ann N Y Acad Sci 854: 118-127.
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, et al. (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417-423.
- Wei YH (1992) Mitochondrial DNA alterations as ageing-associated molecular events. Mutat Res 275: 145-155.
- Kroemer G, Dallaporta B, Resche-Rigon M (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 60: 619-642.
- Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47: 143-183.
- Armstrong JS (2006) The role of the mitochondrial permeability transition in cell death. Mitochondrion 6: 225-234.
- Mather M, Rottenberg H (2000) Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun 273: 603-608.
- Nitahara JA, Cheng W, Liu Y, Li B, Leri A, et al. (1998) Intracellular calcium, DNase activity and myocyte apoptosis in aging Fischer 344 rats. J Mol Cell Cardiol 30: 519-535.
- Phaneuf S, Leeuwenburgh C (2002) Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282: R423-430.
- Li M, Ona VO, Guégan C, Chen M, Jackson-Lewis V, et al. (2000) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288: 335-339.
- Sun W, Funakoshi H, Nakamura T (2002) Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J Neurosci 22: 6537-6548.
- Vukosavic S, Stefanis L, Jackson-Lewis V, Guégan C, Romero N, et al. (2000) Delaying caspase activation by Bcl-2: A clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 20: 9119-9125.
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, et al. (1997) Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA 278: 1363-1371.
- Ribe EM, Serrano-Saiz E, Akpan N, Troy CM (2008) Mechanisms of neuronal death in disease: defining the models and the players. Biochem J 415: 165-182.
- Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, et al. (1999) Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell 97: 395-406.
- Ivins KJ, Thornton PL, Rohn TT, Cotman CW (1999) Neuronal apoptosis induced by beta-amyloid is mediated by caspase-8. Neurobiol Dis 6: 440-449.
- Jordán J, Galindo MF, Miller RJ (1997) Role of calpain- and interleukin-1 beta converting enzyme-like proteases in the beta-amyloid-induced death of rat hippocampal neurons in culture. J Neurochem 68: 1612-1621.
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, et al. (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98-103.
- Sheng JG, Jones RA, Zhou XQ, McGinness JM, Van Eldik LJ, et al. (2001) Interleukin-1 promotion of MAPK-p38 overexpression in experimental animals and in Alzheimer's disease: potential significance for tau protein phosphorylation. Neurochem Int 39: 341-348.
- Raina AK, Hochman A, Zhu X, Rottkamp CA, Nunomura A, et al. (2001) Abortive apoptosis in Alzheimer's disease. Acta Neuropathol 101: 305-310.
- Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH (2001) Activation of caspase-8 in the Alzheimer's disease brain. Neurobiol Dis 8: 1006-1016.
- Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, et al. (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease. Evidence for apoptotic cell death. Am J Pathol 155: 1459-1466.
- Cotman CW, Anderson AJ (1995) A potential role for apoptosis in neurodegeneration and Alzheimer's disease. Mol Neurobiol 10: 19-45.
- LeBlanc A, Liu H, Goodyer C, Bergeron C, Hammond J (1999) Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer's disease. J Biol Chem 274: 23426-23436.
- Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, et al. (2000) A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat Med 6: 397-404.
- Pompl PN, Yemul S, Xiang Z, Ho L, Haroutunian V, et al. (2003) Caspase gene expression in the brain as a function of the clinical progression of Alzheimer disease. Arch Neurol 60: 369-376.
- Pellegrini L, Passer BJ, Tabaton M, Ganjei JK, D'Adamio L (1999) Alternative, non-secretase processing of Alzheimer's beta-amyloid precursor protein during apoptosis by caspase-6 and -8. J Biol Chem 274: 21011-21016.
- Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, et al. (2000) Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci 20: 1386-1392.
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, et al. (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A 100: 10032-10037.
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, et al. (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114: 121-130.
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, et al. (2002) Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci 22: 4015-4024.
- Klevenyi P, Andreassen O, Ferrante RJ, Schleicher JR Jr, Friedlander RM, et al. (1999) Transgenic mice expressing a dominant negative mutant interleukin-1beta converting enzyme show resistance to MPTP neurotoxicity. Neuroreport 10: 635-638.
- Lu G, Kwong WH, Li Q, Wang X, Feng Z, et al. (2005) bcl2, bax, and nestin in the brains of patients with neurodegeneration and those of normal aging. J Mol Neurosci 27: 167-174.
- Yao M, Nguyen TV, Pike CJ (2005) Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J Neurosci 25: 1149-1158.
- Biswas SC, Shi Y, Vonsattel JP, Leung CL, Troy CM, et al. (2007) Bim is elevated in Alzheimer's disease neurons and is required for beta-amyloid-induced neuronal apoptosis. J Neurosci 27: 893-900.
- Cha JH, Kosinski CM, Kerner JA, Alsdorf SA, Mangiarini L, et al. (1998) Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human huntington disease gene. Proc Natl Acad Sci U S A 95: 6480-6485.
- Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, et al. (1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399: 263-267.
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, et al. (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6: 797-801.
- Kiechle T, Dedeoglu A, Kubilus J, Kowall NW, Beal MF, et al. (2002) Cytochrome C and caspase-9 expression in Huntington's disease. Neuromolecular Med 1: 183-195.
- Sánchez I, Xu CJ, Juo P, Kakizaka A, Blenis J, et al. (1999) Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron 22: 623-633.
- Goldberg YP, Nicholson DW, Rasper DM, Kalchman MA, Koide HB, et al. (1996) Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet 13: 442-449.
- Wellington CL, Ellerby LM, Hackam AS, Margolis RL, Trifiro MA, et al. (1998) Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J Biol Chem 273: 9158-9167.
- Taub DD, Longo DL (2005) Insights into thymic aging and regeneration. Immunol Rev 205: 72-93.
- Aggarwal S, Gollapudi S, Gupta S (1999) Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J Immunol 162: 2154-2161.
- Aggarwal S, Gupta S (1998) Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol 160: 1627-1637.
- Higami Y, Shimokawa I (2000) Apoptosis in the aging process. Cell Tissue Res 301: 125-132.
- Zhang JH, Zhang Y, Herman B (2003) Caspases, apoptosis and aging. Ageing Res Rev 2: 357-366.
- Lacelle C, Xu S, Wang E (2002) Identification of high caspase-3 mRNA expression as a unique signature profile for extremely old individuals. Mech Ageing Dev 123: 1133-1144.
- Aggarwal S, Gupta S (1999) Increased activity of caspase 3 and caspase 8 in anti-Fas-induced apoptosis in lymphocytes from ageing humans. Clin Exp Immunol 117: 285-290.
- Gupta S (2000) Molecular steps of cell suicide: an insight into immune senescence. J Clin Immunol 20: 229-239.
- Castro JE, Listman JA, Jacobson BA, Wang Y, Lopez PA, et al. (1996) Fas modulation of apoptosis during negative selection of thymocytes. Immunity 5: 617-627.
- Berzins SP, Uldrich AP, Sutherland JS, Gill J, Miller JF, et al. (2002) Thymic regeneration: teaching an old immune system new tricks. Trends Mol Med 8: 469-476.
- Bar-Dayan Y, Afek A, Bar-Dayan Y, Goldberg I, Kopolovic J (1999) Proliferation, apoptosis and thymic involution. Tissue Cell 31: 391-396.
- Andrew D, Aspinall R (2001) Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol 166: 1524-1530.
- Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, et al. (1996) Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol 271: H1215-1228.
- Bernecker OY, Huq F, Heist EK, Podesser BK, Hajjar RJ (2003) Apoptosis in heart failure and the senescent heart. Cardiovasc Toxicol 3: 183-190.
- Condorelli G, Morisco C, Stassi G, Notte A, Farina F, et al. (1999) Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation 99: 3071-3078.
- Lee WL, Chen JW, Ting CT, Ishiwata T, Lin SJ, et al. (1999) Insulin-like growth factor I improves cardiovascular function and suppresses apoptosis of cardiomyocytes in dilated cardiomyopathy. Endocrinology 140: 4831-4840.
- Li Z, Bing OH, Long X, Robinson KG, Lakatta EG (1997) Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am J Physiol 272: H2313-2319.
- Yue TL, Ma XL, Wang X, Romanic AM, Liu GL, et al. (1998) Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res 82: 166-174.
- Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, et al. (1996) Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 74: 86-107.
- Liu L, Azhar G, Gao W, Zhang X, Wei JY (1998) Bcl-2 and Bax expression in adult rat hearts after coronary occlusion: age-associated differences. Am J Physiol 275: R315-322.
- Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, et al. (2006) Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res 99: 553-560.
- Azhar G, Gao W, Liu L, Wei JY (1999) Ischemia-reperfusion in the adult mouse heart influence of age. Exp Gerontol 34: 699-714.
- Liu JJ, Peng L, Bradley CJ, Zulli A, Shen J, et al. (2000) Increased apoptosis in the heart of genetic hypertension, associated with increased fibroblasts. Cardiovasc Res 45: 729-735.
- Liu P, Xu B, Cavalieri TA, Hock CE (2002) Age-related difference in myocardial function and inflammation in a rat model of myocardial ischemia-reperfusion. Cardiovasc Res 56: 443-453.
- Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6: 508-519.
- Kuiper J, van Puijvelde GH, van Wanrooij EJ, van Es T, Habets K, et al. (2007) Immunomodulation of the inflammatory response in atherosclerosis. Curr Opin Lipidol 18: 521-526.
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, et al. (2009) Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119: 480-486.
- Baba T, Ishizu A, Iwasaki S, Suzuki A, Tomaru U, et al. (2006) CD4+/CD8+ macrophages infiltrating at inflammatory sites: a population of monocytes/macrophages with a cytotoxic phenotype. Blood 107: 2004-2012.
- Chamberlain CM, Granville DJ (2007) The role of Granzyme B in atheromatous diseases. Can J Physiol Pharmacol 85: 89-95.
- Adams CS, Horton WE Jr (1998) Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec 250: 418-425.
- Spaeny-Dekking EH, Hanna WL, Wolbink AM, Wever PC, Kummer JA, et al. (1998) Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J Immunol 160: 3610-3616.
- Tak PP, Spaeny-Dekking L, Kraan MC, Breedveld FC, Froelich CJ, et al. (1999) The levels of soluble granzyme A and B are elevated in plasma and synovial fluid of patients with rheumatoid arthritis (RA). Clin Exp Immunol 116: 366-370.
- Marzetti E, Leeuwenburgh C (2006) Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41: 1234-1238.
- Higami Y, Shimokawa I, Okimoto T, Tomita M, Yuo T, et al. (1996) Susceptibility of hepatocytes to cell death induced by single administration of cycloheximide in young and old F344 rats. Effect of dietary restriction. Mutat Res 357: 225-230.
- Higami Y, Shimokawa I, Okimoto T, Tomita M, Yuo T, et al. (1997) Effect of aging and dietary restriction on hepatocyte proliferation and death in male F344 rats. Cell Tissue Res 288: 69-77.
- Higami Y, Shimokawa I, Tomita M, Okimoto T, Koji T, et al. (1997) Aging accelerates but life-long dietary restriction suppresses apoptosis-related Fas expression on hepatocytes. Am J Pathol 151: 659-663.
- Muskhelishvili L, Hart RW, Turturro A, James SJ (1995) Age-related changes in the intrinsic rate of apoptosis in livers of diet-restricted and ad libitum-fed B6C3F1 mice. Am J Pathol 147: 20-24.
- Hanke J (2000) Apoptosis and occurrence of Bcl-2, Bak, Bax, Fas and FasL in the developing and adult rat endocrine pancreas. Anat Embryol (Berl) 202: 303-312.
- Wu J, Zhang L, Wang X (2000) Maturation and apoptosis of human oocytes in vitro are age-related. Fertil Steril 74: 1137-1141.
- Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW (2009) Beta cell apoptosis in diabetes. Apoptosis 14: 1389-1404.
- Xiao ZQ, Moragoda L, Jaszewski R, Hatfield JA, Fligiel SE, et al. (2001) Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mech Ageing Dev 122: 1849-1864.
- Bogazzi F, Russo D, Locci MT, Chifenti B, Ultimieri F, et al. (2005) Apoptosis is reduced in the colonic mucosa of patients with acromegaly. Clin Endocrinol (Oxf) 63: 683-688.
Citation: Cooper DM (2012) The Balance between Life and Death: Defining a Role for Apoptosis in Aging. J Clin Exp Pathol S4:001. DOI: 10.4172/2161-0681.S4-001
Copyright: © 2012 Cooper DM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 18449
- [From(publication date): 0-2012 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 13640
- PDF downloads: 4809