Editorial Open Access
Temperature Sensitivity of Respiratory Processes Vary Across Scales
Xuhui Zhou* and Lingyan Zhou
Research Institute for the Changing Global Environment, Coastal Ecosystems Research Station of Yangtze River Estuary, Ministry of Education Key Laboratory for Biodiversity Science and Ecological Engineering, The Institute of Biodiversity Science, Fudan University, 220 Handan Road, Shanghai 200433, China
- *Corresponding Author:
- Xuhui Zhou
Research Institute for the Changing Global Environment
Fudan University 220 Handan Road
Shanghai 200433, China
E-mail: zxuhui14@fudan.edu.cn
Received Date: March 20, 2012; Accepted Date: March 23, 2012; Published Date: March 27, 2012
Citation: Zhou X, Zhou L (2012) Temperature Sensitivity of Respiratory Processes Vary Across Scales. J Ecosys Ecograph 2:e109. doi:10.4172/2157-7625.1000e109
Copyright: © 2012 Zhou X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Predictions of terrestrial feedbacks to climate change from coupled climate -carbon (C) models differ remarkably in the magnitude and even the direction of soil and plant C responses [1,2]. These differences likely reflect the uncertainties inherent in how models parameterize temperature dependence of respiratory processes (i.e. temperature sensitivity), when the theoretical understanding of photosynthesis is well-established [3]. The sensitivity of respiratory processes to temperature is commonly described by Q10, which is a proportional change in respiration rate for a 10°C increase in temperature [4]. A majority of models usually assume a globally constant Q10 value of 2 to generate C dynamics based on biochemical and physiological studies, independent of temporal and spatial scales with changing environmental conditions and vegetation types [2,5]. However, modeling studies have found that even a small deviation of the Q10 values could significantly exacerbate or mitigate the buildup of atmospheric CO2 [5,6], with consequent feedbacks to climate change [1,2]. Temperature sensitivity of respiratory processes will largely determine the global pattern and magnitude of the predicted climate change. Therefore, understanding the sensitivity of respiratory processes to temperature is a critical step to quantify the climate-C cycle feedback in the future.
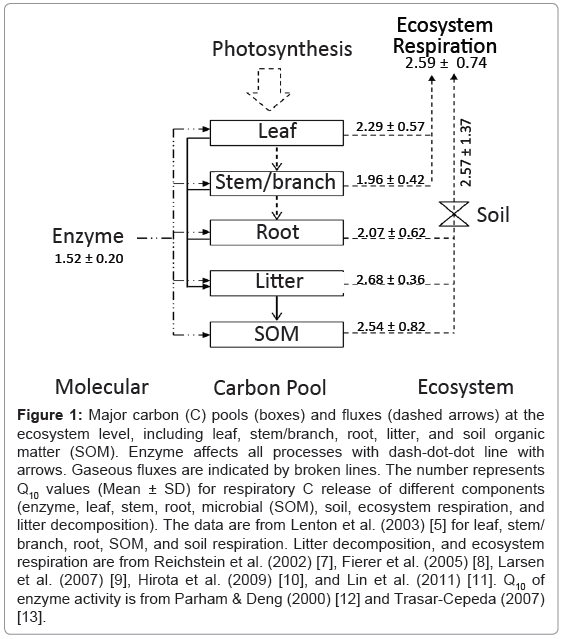
Carbon (C) enters the ecosystem via a single process, photosynthesis, but is returned back to the atmosphere via a variety of respiratory processes (Figure 1). At the biochemical level, a respiratory system involves numerous enzymes that drive glycolysis, the tricarboxylic acid (TCA) cycle and the electron transport train [4]. It is very difficult to measure the temperature sensitivity of each respiratory process individually. The Q10 values are often derived from its temperature variation at a specific period (e.g. weekly, seasonal or annual), dependent of the research need. The estimated Q10 values are thus the product of multiple processes in response to changes in temperature. In practice, respiratory processes include leaf, stem, root, microbial (or heterotrophic) respiration, litter decomposition, soil and ecosystem respiration (Figure 1).
Figure 1: Major carbon (C) pools (boxes) and fluxes (dashed arrows) at the ecosystem level, including leaf, stem/branch, root, litter, and soil organic matter (SOM). Enzyme affects all processes with dash-dot-dot line with arrows. Gaseous fluxes are indicated by broken lines. The number represents Q10 values (Mean ± SD) for respiratory C release of different components (enzyme, leaf, stem, root, microbial (SOM), soil, ecosystem respiration, and litter decomposition). The data are from Lenton et al. (2003) [5] for leaf, stem/ branch, root, SOM, and soil respiration. Litter decomposition, and ecosystem respiration are from Reichstein et al. (2002) [7], Fierer et al. (2005) [8], Larsen et al. (2007) [9], Hirota et al. (2009) [10], and Lin et al. (2011) [11]. Q10 of enzyme activity is from Parham & Deng (2000) [12] and Trasar-Cepeda (2007) [13].
Based on data compiled nearly 30 years ago, the global median values of Q10 for leaf, stem/branch, root, microbial, soil, ecosystem respiration and litter decomposition are 2.29 ± 0.57 (SD, standard deviation), 1.96 ± 0.42, 2.07 ± 0.62, 2.54 ± 0.82, 2.57 ± 1.37, 2.59 ± 0.74 and 2.68 ± 0.36, respectively (Figure 1) [5,7-11]. Temperature sensitivity of enzyme activity is 1.52 ± 0.20, which is relatively low compared to other respiratory processes (Figure 1) [12,13]. However, past research have shown that the Q10 values of respiratory processes vary widely from little more than 1 (low sensitive) to more than 10 (even 30, high sensitive) across temporal and spatial scales [8,14-16]. For example, Fierer et al. [8] found that the range in Q10 values of microbial respiration was from 2.2 to 4.6 in the continental USA. Within one year in a beech forest, Zealand, the Q10 values of soil respiration were from 2.0 to 32.7 for 4-7 days windows [14]. But considerable uncertainties remain associated with the temperature sensitivity of respiratory processes across scales and its controlling processes.
What are temporal and spatial patterns of Q10 values for different respiratory processes? Few studies have examined the temporal and spatial patterns of Q10 values for microbial and ecosystem respiration to date. For example, Zhou et al. [16] found that the Q10 values for microbial respiration are spatially heterogeneous and largely vary with environmental factors, which tend to be high in the high-latitudinal regions (e.g. tundra) and low in arid ecosystems (e.g. deserts). Although Mahecha et al. [17] found that short-term temperature sensitivity of ecosystem respiration does not differ among biomes across 60 FLUXNET sites, apparent and/or long-term temperature sensitivity may be highly variable among sites. Large variation in the Q10 values also occurred for soil respiration on the regional and global scales [15,18,19] . Moreover, roots and leaves can also differ in their Q10 values as can other respiratory processes [20]. Therefore, more studies should concentrate on the spatial and temporal variations in Q10 values of respiration to modify the ecosystem Rs model. Such variable Q10 values need to be fully explored in global C modeling.
What are the controlling factors for temporal and spatial patterns of temperature sensitivity of respiratory processes? The local patterns of temperature, light and water availability have both short- and long term effects on individual organisms and then potentially influence temperature sensitivity of respiratory processes. For example, many studies have shown that the temperature sensitivity of soil respiration negatively correlated with temperature and positively correlated with soil moisture [14,15,18,19]. For a fine scale of enzyme, the Q10 values of extracellular enzymes changed seasonally, although temperature sensitivity of intracellular enzymes has a relative uniform inner environment [21]. However, it is largely unclear what controls temperature sensitivity of respiratory processes on regional and global scales as well as the interannual and longer-scale variability of the Q10 values.
Respiratory acclimation to temperature is very common in the long term, which in effect reduces the temperature sensitivity [20,22-24]. In the short-term, changes in temperature sensitivity of respiratory processes may result from physical or chemical reaction to substrate, enzyme and environmental changes. The variation in the degree of acclimation may result from the interactive effects of temperature and other abiotic factors (e.g. irradiance, drought and nutrient availability) and passive responses to change in respiratory substrate availability [20]. Much remains unclear what the underlying mechanisms are responsible for respiratory acclimation to temperature with decreased Q10 values and what determines the degree of acclimation? In addition, the sensitivity of respiratory processes to temperature has often been modeled based on thermodynamic principles by using simple, first-order exponential equations with temperature (e.g. Arrhenius or Van’t Hoff’s equation). This simple temperature- dependency poorly reflects the complex nature of the numerous respiratory processes, which is currently being questioned [25]. Therefore, new insights are needed to improve the estimated methods for the sensitivity of respiratory processes to temperature, especially using integrated methods with both experiments and models.
The sensitivity of respiratory processes to temperature is one of the major uncertainties in predicting climate-C cycle feedback. In this essay, we simply review temporal and spatial patterns of temperature sensitivity of multiple respiratory processes and identify four important questions to be answered: What are spatial patterns of the Q10 values, what are the controlling factors, what are the underlying mechanisms responsible for respiratory acclimation to temperature and how to improve the estimated methods of the Q10 values? These questions will guide our research to improve the understanding on projecting climate change in the future.
References
- Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408: 184-187.
- Friedlingstein P, Cox P, Betts R (2006) Climate-carbon cycle feedback analysis: Results from the (CMIP)-M-4 model intercomparison. Journal of Climate 19: 3337-3353.
- Trumbore S (2006) Carbon respired by terrestrial ecosystems - recent progress and challenges. Glob Change Biol 12: 141-153.
- Luo Y, Zhou X (2006) Soil Respiration and the Environment, San Diego, CA, Academic Press/ Elsevier.
- Lenton TM, Huntingford C (2003) Global terrestrial carbon storage and uncertainties in its temperature sensitivity examined with a simple model. Glob Change Biol 9: 1333-1352.
- Cramer W, Bondeau A, Woodward FI, Prentice Ic, Richard AB, et al. (2001) Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Glob Change Biol 7: 357-373.
- Reichstein M, Tenhunen JD, Roupsard O, Ourcival JM, Rambal S, et al. (2002) Ecosystem respiration in two Mediterranean evergreen Holm Oak forests: drought effects and decomposition dynamics. Funct Ecol 16: 27-39
- Fierer N, Craine JM, Mclauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86: 320-326.
- Larsen KS, Ibrom A, Beier C, Jonasson S, Michelsen A (2007) Ecosystem respiration depends strongly on photosynthesis in a temperate heath. Biogeochemistry 85: 201-213.
- Hirota1 M, Zhang P, Gu S, Du M , Shimono A, et al. (2009) Altitudinal variation of ecosystem CO(2) fluxes in an alpine grassland from 3600 to 4200 m. J Plant Ecology-Uk 2: 197-205
- Lin X, Zhang Z, Wang S, Hud Y, Xu G, et al. (2011) Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agricultural and Forest Meteorology 151: 792-802.
- Parham JA, Deng SP (2000) Detection, quantification and characterization of beta-glucosaminidase activity in soil. Soil Biol Biochem 32: 1183-1190.
- Trasar-Cepeda C, Gil-Sotres F, Leiros MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biol Biochem 39: 311-319.
- Janssens IA, Pilegaard K (2003) Large seasonal changes in Q10 of soil respiration in a beech forest. Glob Change Biol 9: 911-918.
- Chen H, Tian HQ (2005) Does a general temperature-dependent Q(10) model of soil respiration exist at biome and global scale? J Integr Plant Biol 47: 1288-1302.
- Zhou T, Shi PJ, Hui DF, Luo YQ (2009) Global pattern of temperature sensitivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback. J Geophysical Research-Biogeosciences 114.
- Mahecha MD, Reichstein M, Carvalhais N, Lasslop G, Lange H, et al. (2010) Global Convergence in the Temperature Sensitivity of Respiration at Ecosystem Level. Science 329: 838-840.
- Kirschbaum MUF (1995) The Temperature-Dependence of Soil Organic-Matter Decomposition, and the Effect of Global Warming on Soil Organic-C Storage. Soil Biol Biochem 27: 753-760.
- Wang X, Piao S, Ciais P, Janssens IA, Reichstein M, et al. (2010) Are ecological gradients in seasonal Q(10) of soil respiration explained by climate or by vegetation seasonality? Soil Biol Biochem 42: 1728-1734.
- Atkin OK, Bruhn D, Hurry VM, Tjoelker MG (2005) The hot and the cold: unravelling the variable response of plant respiration to temperature. Functional Plant Biology 32: 87-105.
- Wallenstein MD, Allison SD, Ernakovich J, Steinweg JM, Sinsabaugh RL (2010) Controls on the Temperature Sensitivity of Soil Enzymes: A Key Driver of In Situ Enzyme Activity Rates. In: Soil Enzymology. (eds Shukla G, Varma A). Berlin Heidelberg, Springer-Verlagg 245-258.
- Luo YQ, Wan SQ, Hui DF, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413: 622-625.
- Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Sci 8: 343-351.
- Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, et al. (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecology Letters 11: 1316-1327.
- Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440: 165-173.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 14643
- [From(publication date):
April-2012 - Nov 25, 2025] - Breakdown by view type
- HTML page views : 9863
- PDF downloads : 4780