Research Article Open Access
Synthesis of Transition Metal 12 Hydroxy Oleate and their Effect on Photo and Biodegradation of Low Density Polyethylene Films
| Anniyyappa Umapathi Santhoskumar, Komaragounder Palanivelu*, ShailadraKumar Sharma and Sanjay Kumar Nayak | |
| Department of Plastics Technology, Central Institute of Plastics Engineering & Technology, Guindy, Chennai 600 032, India | |
| Corresponding Author : | Dr. Komaragounder Palanivelu Department of Plastics Engineering & Technology TVK Industrial Estate, Guindy Chennai-600 032, India Tel: +919362366651, +914422254708 Fax: +914422251707 E-mail: kpalanivelucipet@gmail.com |
| Received: September 11, 2010; Accepted: April 07, 2012; Published: April 09, 2012 | |
| Citation: Santhoskumar AU, Palanivelu K, Sharma S, Nayak SK (2012) Synthesis of Transition Metal 12 Hydroxy Oleate and their Effect on Photo and Biodegradation of Low Density Polyethylene Films. J Bioremed Biodegrad 3:142. doi:10.4172/2155-6199.1000142 | |
| Copyright: © 2012 Santhoskumar AU, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
A new additive Ferrous (Fe) and Nickel (Ni)12-hydroxy oleate was successfully synthesized and their performance on the photo degradability of polyethylene (LDPE) films were evaluated by observing the disparity in the structural characteristic, surface morphology, mechanical, and thermal properties upon degradation. LDPE films blended with different compositions (1, 3 & 5 wt %) of Fe and Ni 12-hydroxyl oleate were prepared by melt blending. Degraded films were subjected to Fourier Transformed Infrared spectroscopy, Scanning Electron Microscopy, Universal Testing Machine, Differential Scanning Calorimetry analysis to investigate its structural, morphological, mechanical and thermal properties respectively. The influence of low molecular weight organic compounds such as Fe 12-hydroxyl oleate and Ni 12-hydroxyl oleate additives on photo transformation of LDPE has been investigated. The results indicate that the additives play a vital role to accelerate and increase the efficiency of photodegradation in LDPE films but the hamper of photo cross linking and formation of the carbonyl group following of the Norrish type1 in this polymer. In this case Fe 12-hydroxyl oleate showed greater activity as photo initiator when compared to Ni 12-hydroxyl oleate and also the comparison of photo degraded films were subjected to biodegradation in the presence of the microbes such as Aspergillus niger and Pencillium funiculosum isolated from a dump. Fragments occur progressively in the biodegradation of the photodegradaded films. Moreover, the biodegradation test results reveals that the Fe 12-hydroxyl oleate shows 21% and Ni 12-hydroxyl oleate shows 19% of biodegradation on photodegraded LDPE films when observed at the end of 45 days.
| Keywords | ||||||||||||||||||
| Photodegradation; Ferrous 12-hydroxyl oleate; Nickel 12-hydroxyl oleate; Low density polyethylene; Biodegradation | ||||||||||||||||||
| Introduction | ||||||||||||||||||
| Polyolefin degradability offers a complimentary strategy to deal with this litter problem. One of the simplest ways of modifying the existing polymer is to accelerate the degradation process already taking place. Different approaches to develop photo and their biodegradable polyolefin’s have been adopted, including both copolymerization with ketone or CO groups, photo intiating metal complexes and addition of bioactive component [1]. | ||||||||||||||||||
| Transition metal element with ketone or CO containing some crude oil act as antioxidant and bioactive components are initiators of polymer degradation occurring in organic compounds [2-12]. They absorb a light up to above 290 nm, which cause their excitation or cleavage into free radicals. These ones may initiate polymer degradation and other transformations by abstraction of hydrogen atom from a macromolecule (PH) and formation of polymer alky radical (P°) [1,12-15]. | ||||||||||||||||||
| Transition metals like Co, Mn, Fe, Ni, Cu etc. containing the carbonyl group and purification of Ricinoleic acid especially in the form of Ferrous 12-hydroxyl oleate and Ni 12-hydroxyl oleate have been employed to initiate degradation in polyethylene films. The role of these metals/metallic compounds on the photodegradation of polyethylene has been extensively studied by several authors [15]. However, in the present work, we attempt a systematic study on the effect of Fe and Ni 12-hydroxyl oleate, especially due to the concentration and photooxidation of metals. This photoxidative degradation of metals leads to increases in low molecular weight fraction by chain scission in polyethylene, thereby facilitating biodegradation. It also leads to increase in the surface area through embrittlement. In addition, the formation of carbonyl group on the surface increases its hydrophilicity. Consequently, the possibility of further degradation induces a significant enhancement towards mineralization of polyolefin’s films [16]. | ||||||||||||||||||
| Experimental | ||||||||||||||||||
| Materials and methods | ||||||||||||||||||
| Ammonium iron (II) sulphate hexahydrate and Ammonium Nickel (II) sulphate hexahydrate, sodium hydroxide, Ricinoleic acid were used without further purification. General purpose film grade LDPE 24FS040 has been used to prepare films. Milli Q ultrapure water was used throughout the course of this work. | ||||||||||||||||||
| Synthesis of ferrous and nickel 12-hydroxyl oleate | ||||||||||||||||||
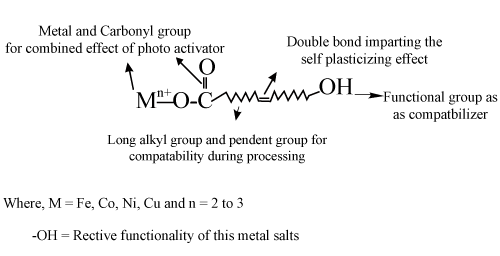
| 1 mole of NaOH is mixed with 1000 ml of ethanol in a volumetric flask to get 1 N NaOH solution. The required amount of NaOH solution is prepared in a volumetric flask. 720 ml of 1 N NaOH and 80 ml of fatty acid (Ricinoleic acid - Molecular formula C18H34O3) are taken in a round-bottomed flask and refluxed for one hour. Porcelain pieces are put inside the round-bottomed flask to avoid spitting out of solution. condenser is attached and continuous supply of water is provided during reflux. Cotton plug is kept on the neck of the condenser to avoid evaporation. 100 ml of 15 N HCl is mixed in 1400 ml of distilled water in beaker to get 1 N HCl. 1 mole of NaOH was dissolved in 1000 ml of distilled water to get one mole aqueous NaOH solution. 60 g of the collected mixture was dissolved in 200 ml of 1mole aqueous NaOH solution and as a result a single phase solution of sodium salt is got. 1N Ferrous or Nickel ammonium sulphate is dissolved in required amount of water to get one mole of Fe or Ni salt solution. One mole of Fe and Ni salt solution was mixed with water several times till a pure water layer is separated. Then the remaining mixture is washed with a small amount of ethanol and the resulting mixture is called the Fe or Ni 12-hydroxyl oleate as shows in Figure 1 basis structure of synthesized additive. | ||||||||||||||||||
| Blending and film preparation of LDPE | ||||||||||||||||||
| The Fe 12-hydroxyl oleate or Ni 12-hydroxyl oleate was melt blended with LDPE at three different formulations 1, 3 & 5% respectively in (Haake, Rheomex OS, PTW16, Thermo scientific, Germany) Modular Torque Rheometer. In this instrument condition are as given below. | ||||||||||||||||||
| Speed Range : 0 to 550 min-1 | ||||||||||||||||||
| Temperature Range : 0 to 440°C | ||||||||||||||||||
| Pressure Display Range : 0 to 700 Bar | ||||||||||||||||||
| Torque Measuring Range : 0 to 400 Nm | ||||||||||||||||||
| LDPE was blended with the synthesized Fe and Ni 12-hydroxyl oleate additive in varying percentages (1%, 3% and 5%), by using Torque Rheometer, Blending was carried out at a temperature range of 130-190°C and at a screw speed of 75 rpm. Subsequently, the pellets are dried in a dehumidifier at 70°C for two hours to remove moisture. The film was prepared by using film die for all the three percentages of additives. The wall thickness of the film was kept as 50 microns by controlling the speed of the nip rollers and output rate. | ||||||||||||||||||
| Photo degradation | ||||||||||||||||||
| All blended samples were subjected to photodegradation studies using QUV UV Weather-o-meter. Films of 25 mm width were used to evaluate the degradation phenomenon. Samples were exposed to two different test cycles of UV irradiation and condensation as shown in table 1. Subsequently the samples were tested and characterized for 1,2,3,4,5 and 6th day. For 24 hours for eight hours UV cycle was carried out and for next four hours condensation cycle was carried. For the remaining 12 h, the cycle was repeated in the same order. Photodegradation affect the physical and optical properties of a plastic relative to the initial specified properties. | ||||||||||||||||||
| Polyethylene was blended with the synthesized Fe or Ni 12-hydroxyl oleate additive, the photodegradation mechanism both metal 12-hydroxyl oleate in presence of double bond acting plasticizing effect to virgin polymer so the glass transition temperature reduced, OH functional group as compatibilizer, long alkyl group and pendent group for compatibility during processing, metal and carbonyl group for combined effect of photo activator in virgin polymer. In this case additive contains hydroxyl group, carbonyl group and double bond acting various functionalized compatabilizing agent, photoactivator and plasticizing effect respectively. This may know as Multifunctional Active Additive (MFA) as shown in Figure 1. | ||||||||||||||||||
| Transition metal 12-hydroxyl oleate reacted within virgin polymer during UV weathering condition M2+ to M3+ when exposed to UV radiation. This could be due to the fact that in the photolysis process of Metal 12-hydroxyl oleate, the M2+ ions Oxidizes to M3+ more readily and these ions causes photodegradation of the polymer chains to form carbonyl groups following of the Norrish type 1 reaction. | ||||||||||||||||||
| Fourier transform infrared spectrophotometer (FTIR) | ||||||||||||||||||
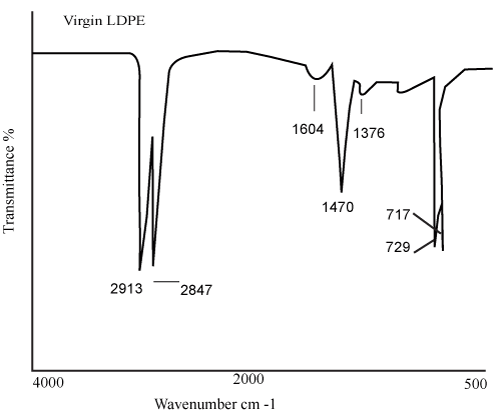
| The structural changes in LDPE films due to the presence of Fe and Ni 12-hydroxyl oleate effect of UV exposure were studied by Nicolet 6000 (USA) Fourier Transform Infrared Spectrometer (FTIR) with the wave number range of 400-4000 cm-1. | ||||||||||||||||||
| Mechanical properties | ||||||||||||||||||
| Tensile properties (ASTM D 882): Tensile properties of virgin LDPE with Fe and Ni 12-hydroxyl oleate blended sample before and after UV exposure, with dimensions 150 x 25 x 0.060 mm were subjected to tensile tests as per ASTM D 882, using Universal Testing Machine (UTM), Lloyd Instrument Ltd, UK. A cross head speed of 500 mm/min and gauge length of 50 mm in both machine and transverse directions. | ||||||||||||||||||
| Thermal properties | ||||||||||||||||||
| Differential scanning calorimeter (DSC) analysis: Melting behavior of transition metal 12-hydroxyl oleate blended samples is being studied by employing Perkin Elmer (USA) differential scanning calorimeter. Sample 5 mg weights were scanned from 45 to 200°C at the heating rate of 5°C/min to detect the melting characteristics of the sample before and after exposure to UV radiation. The percentage of crystallinity of 12-hydroxyl oleate blended LDPE films was calculated as follows. | ||||||||||||||||||
| % of Crystallinity = (ΔHm-ΔHc)/ ΔHc° | ||||||||||||||||||
| Where ΔHm - Enthalpy of melting (J/g) | ||||||||||||||||||
| ΔHc - Enthalpy of Crystallization (J/g) | ||||||||||||||||||
| ΔHc° - Enthalpy of 100% crystalline polymer (277.3 J/g) | ||||||||||||||||||
| Thermo gravimetric analysis: Thermal degradation of LDPE Fe 12-hydroxyl oleate and LDPE Ni 12-hydroxyl oleate blended samples of before and after UV Exposure were analyzed by Perkin Elmer (USA), at the heating rate of 10°C/min from 50 to700°C. | ||||||||||||||||||
| Optical properties | ||||||||||||||||||
| Optical properties such as luminous transmittance and haze were studied for the Fe 12-hydroxyl oleate and Ni 12-hydroxyl oleate blended samples (LDPE) before UV exposure and after UV to find the effect of additive on the optical characteristics of the film. For measuring haze and luminous transmittance, The BYK Gardner Spectrophotometer was employed (ASTM D 1003). | ||||||||||||||||||
| Scanning electron morphology | ||||||||||||||||||
| The scanning electron microscopic analysis of fractured surface of LDPE-Fe 12-hydroxyl oleate, LDPE-Ni 12-hydroxyl oleate film was carried out using CARL ZESIS Model; EVO MA 15 scanning electron microscope. The surface of the samples were coated with conductive heavy metal such as gold. | ||||||||||||||||||
| Elemental analysis | ||||||||||||||||||
| The carbon content of the each test sample determined by elemental analysis by using Carlo Erbal model 1106 elemental analysis. | ||||||||||||||||||
| Titration method of CO2 determined test | ||||||||||||||||||
| The degradation of chemical compounds in the titration CO2 evolution was based on the same principle as the conventional CO2 evolution test. Test vessels were cylindrical bottles with a 2-liter volume containing the same mixture of inorganic (Barium hydroxide) medium, organic test substance, and mixed inoculums in a liquid volume of 1.5 liter. Incubation, aeration with CO2-free air, and agitation of the test mixture, blank, and control vessels were also comparable. The exhaust gas from the vessels was passed through a glass chamber (volume, 1500 ml) that was immersed in the test mixture and filled with 50 ml of a 0.25 N aqueous Ba(OH)2 solution titrated with HCl. The remain Barium Hydroxide was calculated after the end point. By subtracting the blank values from titrant value biodegradation was calculated and expressed as a percentage of the CO2. There was a linear correlation between the amounts of carbon dioxide liberated. This correlation was determined for each volume and concentration of absorption solution used. | ||||||||||||||||||
| Oxygen undergoes complete oxidative biodegradation of the test compound. The concentration of inoculums was 30 mg of dry matter of Solid compost, and the concentration of the test compound was 100 mg of substance liter-1, or 50 to 100 mg liter-1 of theoretical oxygen demand (ThOD). The test duration was minimum 45 days, and the tests were performed at an incubation temperature of 58 ± 2°C [17]. | ||||||||||||||||||
| ASTMD-5338: Test procedure | ||||||||||||||||||
| The amount of CO2 produces pass through Ba(OH)2 solution and is precipitated as BaCO3 | ||||||||||||||||||
| • Determination of CO2 by titrating the remaining Ba(OH)2 with 0.05N HCL | ||||||||||||||||||
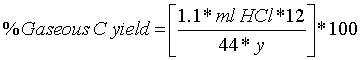 |
||||||||||||||||||
| Y = mg carbon charged to flask | ||||||||||||||||||
| Biodegradation Calculation | ||||||||||||||||||
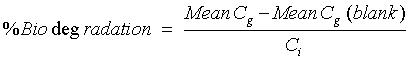 |
||||||||||||||||||
| Where ; | ||||||||||||||||||
| Cg = Amount of gaseous carbon produced g, and | ||||||||||||||||||
| Ci = Amount of carbon in test compound in the sample, g. | ||||||||||||||||||
| Results and Discussion | ||||||||||||||||||
| Characterization of ferrous and nickel 12-hydroxyl oleate | ||||||||||||||||||
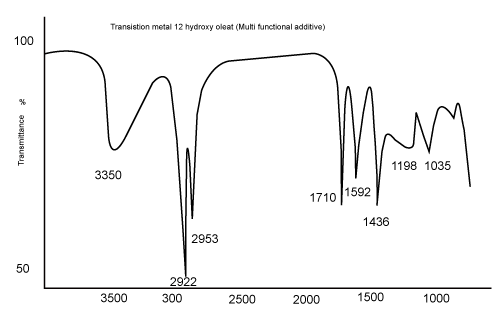
| The FTIR spectra of all the Fe and Ni 12-hydroxyl oleate exhibited absorbance at 1592 cm-1 due to asymmetric vibration stretching of the carboxylic group coordinated to the metal ion. The UV-Vis spectra of the oleate in ethanol show absorption maximum at 290- 360 nm as Fe and 290-305 nm as Ni 12-hydroxyl oleate. | ||||||||||||||||||
| Photo-oxidation of LDPE films containing ferrous and nickel 12-hydroxyl oleate | ||||||||||||||||||
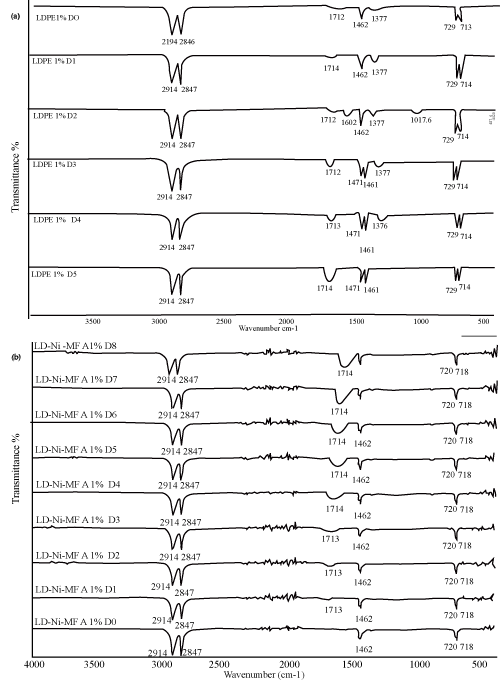
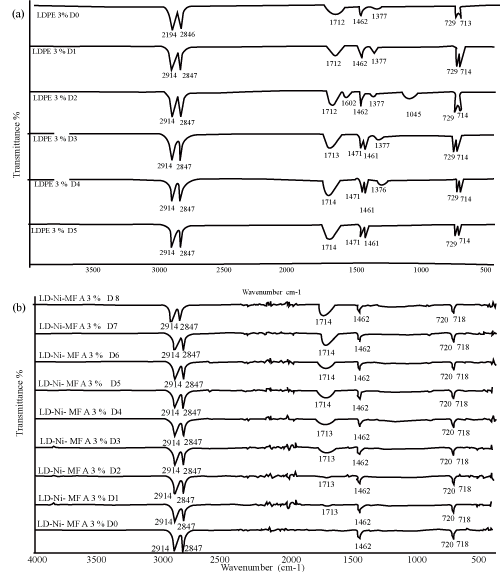
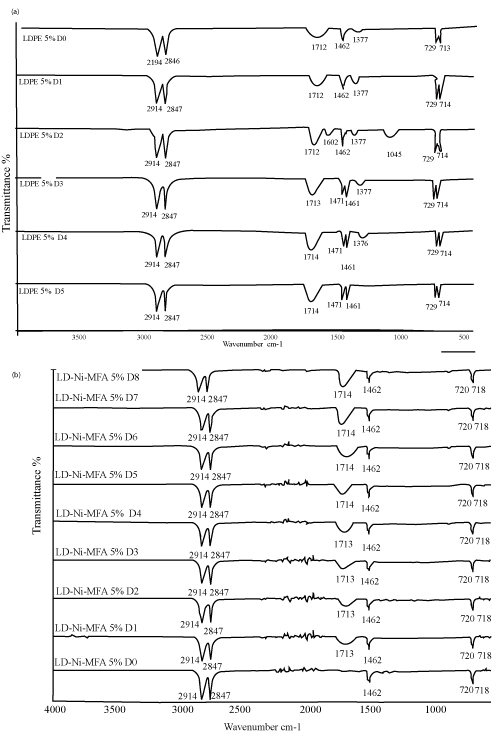
| With an aim to investigate the chemical changes taking place in the polyethylene films due to photo-oxidation, the evolution of the FTIR Spectra was studied with respect to exposure time, similar changes in the FTIR spectra were observed in all the samples, the extent of change depends on the concentration and nature of oleate. Some new features that show increased intensity with UV exposure. As observed by other workers, a new (structured) band appears at -1712 cm-1 which can be attributed to the generation of carbonyl groups primarily on the surface of the polymer. | ||||||||||||||||||
| The increases in the absorbance of these bands were however more pronounced for samples containing Fe and Ni 12-hydroxyl oleate than neat LDPE as far as the band shape is concerned, a progressive broadening of the carbonyl band was observed. The carbonyl band is a result of overlapping of absorption bands due to several functional groups like ketones, carboxylic acids, aldehydes, esters and peroxylcarbolylic acids, etc. the broadening [18,19]. | ||||||||||||||||||
| The characteristic peak absorptions of virgin LDPE film and transition metal 12-hydroxyl oleate are given in Table 2 and 3 respectively. The FTIR spectra of transition metal 12-hydroxyl oleate was shown in Figure 2 and LDPE in Figure 3. | ||||||||||||||||||
| As shown in Figure 4, 5 and 6 (a,b) a peak at around 1712 cm-1 corresponding to carbonyl group of transition metal 12-hydroxyl oleate was observed for LDPE films with 1%, 2% and 3% additive. It can be seen from the figure that the absorption intensity increases with increasing the concentration of the additive from 1% to 3%. On exposure of the films to the accelerated UV radiation further increases the intensity which is due to the formation of new carbonyl groups on photodegradation involving the chain scission following the Norrish type 1 reaction. | ||||||||||||||||||
| Mechanical properties evaluation | ||||||||||||||||||
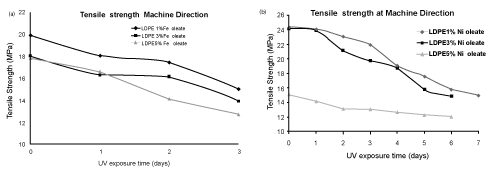
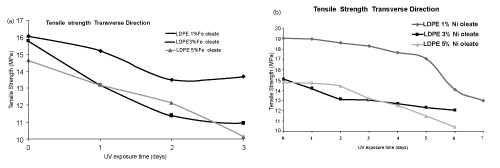
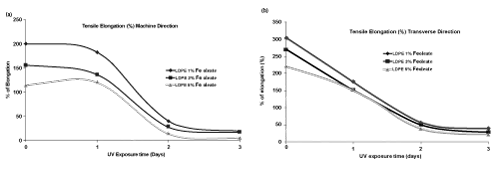
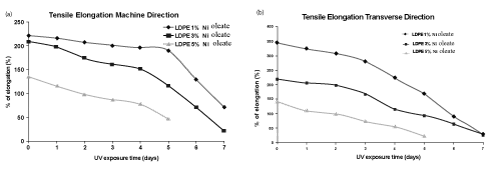
| The tensile strength data of LDPE film with iron (Fe) and nickel (Ni) 12-hydroxyl oleate before and after UV exposure are presented in the Tables 4 and 5. | ||||||||||||||||||
| The tensile strength of LDPE virgin sample decreases with the incorporation of Fe 12-hydroxyl oleate as well as Ni 12-hydroxyl oleate in the concentration of 1%, 3% and 5%. There was a considerable decrease in the tensile strength and elongation at break on exposure of the films with additive to UV radiation. Both iron based as well as nickel based additives show considerable decrease in the tensile strength and elongation at break as shown in Figure 7-10 (a,b). The deterioration in mechanical properties of films was observed in both the machine direction and transverse direction, but the rate of deterioration was high in transverse direction. Also, increasing the concentration of additive from 1% to 5% increases the degradation. | ||||||||||||||||||
| In the case of LDPE films with Fe 12-hydroxyl oleate additive show decrease in tensile strength and elongation and ultimately become brittle when exposed to UV radiation for 96 hrs (four days). Whereas the films with Ni 12-hydroxyl oleate additive show decrease in tensile properties and become brittle only after sixth day (144 hours) when exposed to UV radiation. This could be due to the fact that in the photolysis process of Ferrous 12-hydroxyl oleate, the Fe2+ ions oxidizes to Fe3+ more readily and these ions causes photodegradation of the polymer chains to form carbonyl groups following the Norrish type 1 reactions. In other words, in absorption of energy in the form of light, the Fe and Ni 12-hydroxyl oleate undergo decarboxylation leading to the formation of the free radicals. These generate radicals on the main chain of the polymer matrix leading to chain scission which finally | ||||||||||||||||||
| affects the mechanical properties. This phenomenon is relatively slower in case of LDPE films not containing additive, where UV irradiation leads to cross linking and hence higher tensile strength [20]. | ||||||||||||||||||
| Thermal properties | ||||||||||||||||||
| Differential scanning calorimeter (DSC) analysis: The differential scanning calorimetric data pertaining to the melting point and degree of crystallinity of Fe and Ni 12-hydroxyl oleate blended LDPE film before and after exposure to accelerated UV is presented in Table 6 and 7. | ||||||||||||||||||
| The virgin LDPE shows its melting point at 111.45°C. On the incorporation of Fe 12-hydroxyl oleate and Ni 12- hydroxyl oleate, the melting point is found to change slightly due to the presence of additive in LDPE matrix. In case of the LDPE, 5% Fe 12-hydroxyl oleate samples exposed to UV for five days and a marginal decrease in the melting point from 111.29 to 109.21¢ªC was observed whereas in the case of Ni-12-hydroxyl oleate samples exposed to UV a marginal decrease in the melting point from 111.35 to 109.23¢ªC was observed only after eight days. This could be due to the faster photodegradation of LDPE films in the presence of Fe 12-hydroxyl oleate additive. In this case LDPE blend Fe and Ni 12-hydroxyl oleate additive there is no much difference in the melting point compare to virgin LDPE and the same period of time (five days in the presence of UV exposure) due to oleate additive which contains a double bond and carbonyl group, some inter and intra molecular interaction in the polymer effected by UV exposure. Corresponding ¥ÄH peak get broadening indicates the formation of low molecular weight species due to photodegradation. The percentage of crystallinity decreases by increasing the additive concentration. It was observed that the degree of crystallinity of LDPE films with 5% Fe 12-hydroxyl oleate decreases from 100 to 63 when the samples were exposed to UV for 5 days. These results are in agreement with the mechanical properties data thus revealing higher degradation rate at higher additive percentage. | ||||||||||||||||||
| Thermo gravimetric analysis (TGA): The thermo gravimetric analysis of LDPE with Fe 12-hydroxyl oleate and Ni 12-hydroxyl oleate additive is summarized in Table 8 and 9. The results show that the initial decomposition temperature of LDPE after blending with Fe 12-hydroxyl oleate as well as Ni 12-hydroxyl oleate decreases significantly. The increase in percentage of additive further decreases the initial decomposition temperature. In fact about 120¢ªC decrease in initial decomposition temperature was observed with 5% additive concentration. This is because of the initiation of degradation due to the presence of metal ions. The degradation due to UV exposure involves the chain scission and formation of carbonyl groups following the Norrish type 1 reaction. However, there is a significant increase in ultimate decomposition temperature with increasing the concentration of Fe 12-hydroxyl oleate and Ni 12-hydroxyl oleate in LDPE films. It was observed that about 30°C increase in ultimate decomposition temperature for LDPE films with 5% additive. This could be due to 5% additive contained the inorganic elements residual such as Fe and Ni. So the ultimate decomposition temperature increases on addition of Fe 12-hydroxyl oleate and Ni 12-hydroxyl oleate. | ||||||||||||||||||
| Optical properties | ||||||||||||||||||
| The results of optical properties of Ferrous 12-hydroxyl oleate and Nickel 12-hydroxyl oleate blended LDPE before and after exposure to UV radiation are given in Table 10 and 11. | ||||||||||||||||||
| It is evident that with the increase in additive concentration there was a decrease in transmittance level, which is due to the carbonyl formation increases (absorption increases by light) in the process of photo oxidative degradation of LDPE film. LDPE film containing the percentage of oleate additive increases with increase in cloudy appearance of an otherwise transparent films caused by light scattered from within the films or from its surface. As the UV exposure time increases the percentage of additive increases the oxidative degradation. So haze is caused increases by surface imperfection (degradation) density changes (decreases) or inclusion that produces light scattering. In case of the films containing 5% additive possess low transmittance and high haze. | ||||||||||||||||||
| Morphology | ||||||||||||||||||
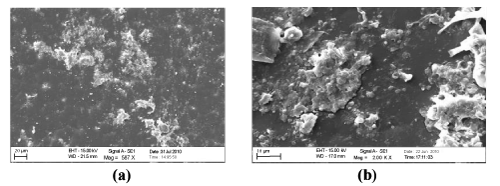
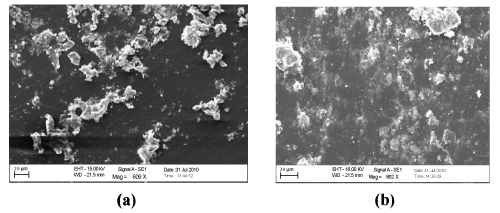
| Scanning electron micrograph (SEM): A comparative compilation of scanning electron micrographs of two samples (LDPE-Fe 12 hydroxyl oleate and LDPE-Ni 12 hydroxyl oleate) at magnification of 500 to 2000 was done. Figure 11 as is apparent from the surface of non degraded LDPE is smooth, without fractured and free from defects. Figure 12 (a,b) The SEM micrographs of LDPE Fe 12-hydroxyl oleate and LDPE Ni 12-hydroxyl blended films with 5% of additives show the uniform dispersion of additive in the polymer matrix. Figure 13- 15 (a) shows the SEM of LDPE after 96 hours of UV degradation. It was observed that the surface developed some fractured and grooves due to UV exposure. However the extent of damage was much more pronounced in the samples containing Fe 12-hydroxyl oleate. | ||||||||||||||||||
| Figure 13- 15 (b) shows the electron micrographs of Ni 12-hydroxyl oleate before and after UV exposure. The damage is more evident in this case and sample after 144 hours showed extensive grooves and pits as a result of UV irradiation. A 1000 fold magnification SEM photograph of Ni 12-hydroxyl oleate after 144 hours of UV exposure is displayed in figure 12 (b), where the deepening of the pit is more evident. | ||||||||||||||||||
| Elemental analysis | ||||||||||||||||||
| In Table 12 (a,b) C, H and N elemental analysis was reported. It can shows only the Carbon, Hydrogen and Nitrogen analysis. Carbon values of cellulose depends on carbon, where as hydrogen analysis is independent of the oxygen in molecular structure. By elemental analysis it shows carbon content as 84% than pure Fe and Ni 12-hydroxyl oleate. This Oleate containing the percentage of Fe as 8.87% and as Ni 6.37%. | ||||||||||||||||||
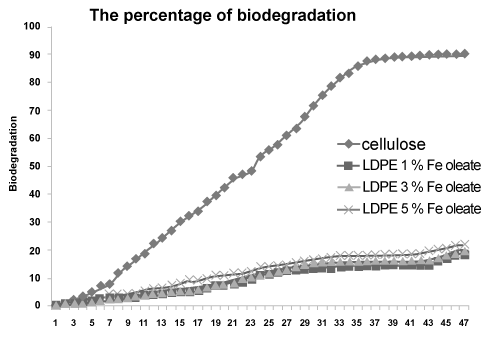
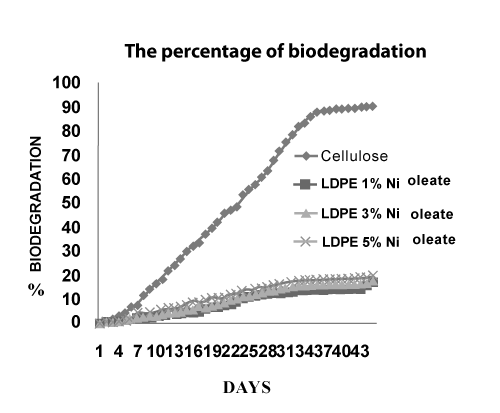
| Biodegradation of the LDPE with Fe and Ni 12-hydroxyl oleate | ||||||||||||||||||
| Figure 16 and Figure 17 shows conditions of reaction mixtures: Organ of compost; livestock excrement, municipal and Vegetable waste used, the method used for the determination of the biodegradability of the polyolefin’s was based on the International Standard (ASTMD 5338- 98) that measures the evolved CO2 amount from both the blank vessel without a sample and the sample vessel including a 10g LDPE with Fe and Ni 12-hydroxyl oleate samples. According to the experimental 2.9 ASTMD 5338 test procedure, Fragments occur progressively in the biodegradation of the photodegradaded films. Moreover, the biodegradation test results reveals that the Fe 12- hydroxyl oleate shows 21% and Ni 12-hydroxyl oleate shows 19% of biodegradation on photodegradaded LDPE films when observed at the end of 45 days. | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Conclusion | ||||||||||||||||||
| The rate of photo degradation on LDPE film is very high at higher concentration of Fe and Ni 12-hydroxyl oleate. LDPE films containing 5% Fe 12-hydroxyl oleate has shown highest degradation in mechanical, thermal properties and reduction in degree of crystallinity when compared to 5% Ni 12-hydroxyl oleate on UV exposure. The FTIR analysis shows that a peak at around 1712 cm-1 corresponding to carbonyl group of transition metal 12-hydroxyl oleate was observed for LDPE films with 1%, 3% and 5% additive. The SEM micrographs indicate the fractured surface and brittle mode on films after UV exposure. The present study indicates that the Fe and Ni 12-hydroxyl oleate promote the photodegradation of LDPE film and in the following order. Fe 12-hydroxyl oleate > Ni 12-hydroxyl oleate. It can be concluded that besides oleate content, the metal also plays a vital role in photodegradation of polyethylene films and also comparison of the photodegraded films were subjected to biodegradation. Fragments occur progressively in the biodegradation of the photodegradaded films. Moreover, the biodegradation test results reveals that the Fe 12-hydroxyl oleate shows 21% and Ni 12-hydroxyl oleate shows 19% of biodegradation on photodegradaded LDPE films when observed at the end of 45 days. | ||||||||||||||||||
| Acknowledgements | ||||||||||||||||||
| Funding of this work by the Department of Science and Technology, grant number. DST/TSG/WP/2006/58 is gratefully acknowledged. | ||||||||||||||||||
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
| Table 6 | Table 7 | Table 8 | Table 9 |
| Table 10 | Table 11 | Table 12a | Table 12b |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
 |
 |
 |
 |
| Figure 11 | Figure 12 | Figure 13 | Figure 14 |
 |
 |
 |
| Figure 15 | Figure 16 | Figure 17 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15828
- [From(publication date):
April-2012 - Nov 21, 2025] - Breakdown by view type
- HTML page views : 11015
- PDF downloads : 4813
