Research Article Open Access
Subjective Effects of Thiopental in Young Adults with and without a Family History of Alcoholism
Ismene L Petrakis1,2*, Karin Kerfoot1,2,3, Brian Pittman1, Elizabeth Ralevski1,2, Albert Perrino1,2, Julia Koretski1,2, Jenelle Newcomb1,2, Diana Limoncelli1,2 and Gregory Acampora1,21NIAAA Center for the Translational Neuroscience of Alcoholism and Department of Psychiatry, Yale University School of Medicine, Connecticut, USA
2Department of Veterans Affairs, Alcohol Research Center, VA Connecticut Healthcare System, Connecticut, USA
3Department of Psychiatry, University of Western Ontario, Canada
- *Corresponding Author:
- Ismene Petrakis
West Haven Veterans Administration Medical Center #116-A
950 Campbell Avenue
West Haven, Connecticut 06516, USA
Tel: (203) 932-5711 x2244
Fax: (203) 937-3886
E-mail: ismene.petrakis@yale.edu
Received December 20, 2011; Accepted May 11, 2012; Published May 14, 2012
Citation: Petrakis IL, Kerfoot K, Pittman B, Ralevski E, Perrino A (2012) Subjective Effects of Thiopental in Young Adults with and without a Family History of Alcoholism. J Addict Res Ther S7:002. doi:10.4172/2155-6105.S7-002
Copyright: © 2012 Petrakis IL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: The development of alcohol use disorders is genetically influenced, and may be mediated through differences in the subjective response to alcohol. There is some evidence to suggest that response differences to alcohol could be conveyed by heritable differences in GABAA receptors. The purpose of this study was to investigate whether individuals with a family history positive (FHP) for alcohol dependence would experience alterations in response to the GABAA receptor agonist thiopental, in comparison to family history negative (FHN) subjects. Methods: 73 subjects (24 FHP and 49 FHN) between the ages of 21 and 30 years were administered subanesthetic doses of the GABAA receptor agonist thiopental and placebo on two separate test days. Various alcoholrelated measures were administered, including those examining subjective effects, coordination, and cognition. Results: Sub-anesthetic doses of thiopental produced alcohol-like subjective effects, as well as alcohol-like impaired coordination and cognition in healthy subjects. While there were no significant main effects in subjective, coordination, or cognitive effects between FHP and FHN individuals, analysis of peak effects suggested FHP had blunted sedative, but not stimulant effects compared to FHN. Conclusion: Thiopental produced alcohol-like effects and perceived similarities to alcohol in healthy individuals. Subtle differences in sedative effects are consistent with reports of blunted FHP response to the negative but not stimulant effects of alcohol. Future studies are needed to better understand how this insight informs our understanding of the heritable risk for alcoholism and the treatment of alcohol use disorders.
Keywords
Thiopental; Alcohol; Family history; Vulnerability; GABA
Introduction
Alcohol use disorders are well recognized to be common, debilitating, and genetically influenced. A family history of alcoholism has been repeatedly identified as a risk factor for the development of alcohol use disorders in numerous studies [1-4]. Several lines of investigation have evaluated potential mechanisms to explain this risk, and have included the study of co-morbid psychiatric disorders, alcohol metabolism, and personality factors [5]. Research has also suggested that response to alcohol, including the balance of positive and negative effects experienced by individuals (or “reward valence” of alcohol), is an important area for consideration (see review [6]). Several studies have supported the intergenerational transmission of response to alcohol as a mechanism for the genetic risk of alcoholism (e.g. [7]).
There are several ways subjective effects may influence risk for alcoholism see review [6]. Several studies, pioneered by Dr. Mark Schuckit and his colleagues [8] have shown that healthy individuals with a family history positive (FHP) for alcoholism are more likely to experience a low level of response (LR) to the negative effects of alcohol in a laboratory setting: these include sedation and cerebellar dysfunction, compared to those individuals negative for a family history of alcoholism (FHN). FHP individuals with a LR were shown to be at increased risk for the subsequent development of an alcohol use disorder [9]. Other studies have suggested that FHP individuals demonstrate both increased and decreased sensitivity to alcohol [10]; specifically that FHP individuals show increased sensitivity to the rewarding, excitatory effects of alcohol and decreased sensitivity to the negative effects (e.g. sedation, depression, anxiety) (e.g. [11,12]). In fact, some have suggested that the stimulant effects are more important as they predict future alcohol consumption [13]. It should be noted, however, that several studies have failed to find clear support for either (e.g. [14]), have reported null findings (e.g. [15]), and have identified potential confounding factors such as drinking history (e.g. [16]).
Given that multiple neurotransmitter targets in the brain independently contribute to the effects of alcohol, altered alcohol sensitivity in vulnerable individuals may be mediated by more than one mechanism. Alcohol’s subjective effects are thought to be related to its actions as a potent gamma-amino-butyric acid (GABA) A receptor agonist and an n-methyl-D-aspartate (NMDA) glutamate receptor antagonist [17]. Attempts to understand the neurobiology of alcohol-induced effects have included studies that probe specific components of alcohol’s action in the brain using relatively selective pharmacologic agents. This “pharmacologic dissection” of vulnerability to alcoholism has included studies examining the effects of NMDA receptor antagonism by agents such as ketamine (e.g. [18,19]) and drugs that facilitate GABA type A (GABAA) receptor function, such as benzodiazepines and barbiturates (e.g. [20-23]).
The facilitation of GABAA receptor function by alcohol may be among the most potent consequences of alcohol intoxication (see review [24]). Alcohol produces sedative-hypnotic effects resembling other drugs that facilitate GABAA receptor function, particularly the benzodiazepines and barbiturates [25]. To indirectly test the hypothesis that alterations in response to alcohol might be conveyed by heritable differences in GABAA receptors, several groups have investigated response to benzodiazepines and barbiturates in healthy subjects with and without a family history of alcohol dependence (see review [24]). Though results have been mixed, FHP individuals have shown decreased negative responses (such as sedative, amnestic, and ataxic effects) and increased pleasurable responses to benzodiazepines, relative to FHN individuals [20]. Response to barbiturates has also been shown to differentiate between FHP and FHN subjects [22,23].
Thiopental is an ultra short-acting barbiturate with a history of extensive use as an induction anesthetic [26]. Its clinical uses include the induction and maintenance of sleep, therapy for brain hypoxicischemic diseases, and management of status epilepticus refractory to more conventional therapy. The metabolism of thiopental is consistent with first-order kinetics, with a half-life of 5-22 hours. Thiopental binds to the choloride ion channel of the GABAA receptor predominantly and acts by opening the chloride ion channel. However, it does affect other ion channels, such as the nicotinic acetylcholine receptor and the serotonin 3R receptor [27]. Thiopental’s clinical pharmacology makes it an ideal pharmacologic “probe” of the GABA system.
The aim of this study was to investigate whether individuals with a family history positive for alcohol dependence would experience an alteration in the reward valence (balance of positive and negative effects) of the GABAA receptor agonist thiopental, in comparison to family history negative age-matched subjects. We hypothesized that individuals with a family history positive for alcoholism would experience enhanced stimulatory effects and/or fewer sedative and negative effects in response to an intravenous infusion of thiopental when compared to the family history negative control subjects.
Methods
These methods were also described in detail in a previous publication (Dickerson et al.) [28].
Subjects
Healthy subjects (n = 73) were recruited by advertisement and compensated for their participation. Inclusion criteria included: 1) males and females, 2) between the ages of 21 and 30 years, 3) medically and neurologically healthy on the basis of history, physical examination, electrocardiogram and screening laboratories, and 4) no lifetime Axis I psychiatric or substance use disorders. Exclusion criteria included: 1) individuals with a history of counseling or psychotherapy, except family therapy centered around another family member, 2) unwillingness to remain alcohol-free for three days prior to each test day, 3) positive urine toxicology on test days for drugs, including marijuana, cocaine, benzodiazepines, amphetamines and opioids, 4) for women, positive pregnancy test at screening or intention to engage in unprotected sex during the study, 5) alcohol naïve, and 6) adoptees with no contact with family members. Family history positive (FHP) subjects were required to have a biological father and another first- or second-degree biological relative with histories of alcoholism by the Family History Assessment Module (FHAM) developed by Collaborative Studies on Genetics of Alcoholism (COGA). Biological mothers had to be without a history of alcoholism, in order to ensure that any effects were not the result of direct toxicity of alcohol during pregnancy. Family history negative (FHN) subjects were required to have no family history of alcoholism in any first- or second-degree relatives. Institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine approved this study.
Procedures
After signing informed consent, subjects underwent baseline screening, including structured interview, physical examination, and laboratory testing, including urine toxicology. Prior to administration of any study medication, subjects were warned that thiopental has addictive potential and that its effects could resemble the effects of alcohol. Individuals were encouraged not to participate if they were concerned about an increased risk for subsequent development of a substance use disorder. Subjects were then scheduled to receive thiopental and placebo on two separate test days at least three days apart in a randomized order under double-blind conditions.
Prior to each test session, participants fasted overnight and remained in a fasting state during the test session. They presented to the Biological Studies Unit of the VA Connecticut Healthcare System, West Haven campus, at approximately 8:30 AM. Prior to testing, subjects underwent urine drug screening for toxicology and breathalyzer screening. Provided that these tests were negative, an intravenous line was placed. Subjects received a 60-minute infusion of either thiopental at a 1.5 mg/kg loading dose and infusion rate of 40 mcg/kg/min or a saline solution. The dose of thiopental was chosen to achieve a desired level of sedation (relaxed with eyes open) at which point subjects were able to complete required paperwork. In agreement with hospital policies on conscious sedation study medication was administered by a licensed anesthesiologist (AP).
Subjective intoxication ratings were obtained at baseline and 15, 45, 80, 110, 170, and 230 minutes after the start of the infusion using the Number of Drinks Scale (NDS), Biphasic Alcohol Effects Scale (BAES), and Visual Analogue Scale (VAS). Subjects also reported on similarity to drugs of abuse as measured by the Visual Analogue Scales of Similarity to Drugs of Abuse (VASSDA). All subjects had used alcohol in the past and were asked to assess alcohol-like effects. As a comparison, subjects who were familiar with marijuana (n=48) were also asked to rate similarity to marijuana. The VASSDA consists of VASs (0 = not at all, 7 = extremely) measuring the perceived similarity to alcohol and other drugs, and has been used in previous challenge studies conducted by this group. Subjects were asked to report on the number of drinks they felt like they had consumed using the NDS, which has also been used in several previous challenge studies conducted by this group. The BAES measures both the stimulating and sedating effects associated with alcohol intoxication. The stimulating effects of alcohol intoxication include feeling energized, excited, stimulated, talkative, ‘up’ and vigorous. The sedating effects associated with alcohol intoxication include feeling ‘down,’ heavy headed, inactive, sedated, sluggish, having slow thoughts, and difficulty in concentrating.
Mood ratings were assessed using Visual Analogue Scales of Mood States (VASMS). These scales are VASs marked proportionately to the perceived intensity of the subjective experience (0 = not at all, 7 = extremely) for mood states, including high, depressed and anxious. These mood rating scales have shown convergent validity with other measures of mood states in previous studies.
Dissociative states were assessed using the Clinician Administered Dissociative States Scale (CADSS) at baseline and 15, 80, 110 minutes post-infusion. The CADSS measures perceptual alterations and consists of 19 self-report items and 8 clinician-rated items (0 = not at all, 4 = extremely). Total scores were calculated for both the objective and subjective scales. This scale has been used previously by our group and has been a sensitive measure of a differential response to ketamine in FHP vs. FHN individuals [19].
Changes in hand-to-eye coordination were assessed using the Grooved Pegboard Test (Lafayette Instrument Company) administered at baseline and 15 minutes post-infusion. This is a manipulative dexterity test, consisting of a board with randomly positioned slots into which the subject inserts pegs under timed conditions.
Assessment for changes in cognitive function included use of the Hopkins Verbal Learning Test (HVLT). The HVLT is a word list learning test of verbal memory and has the advantage of six different versions that permit multiple episodes of testing [29]. The procedures associated with the test allow some degree of distinction between immediate recall, delayed recall, and recognition. The procedures associated with the test allow some degree of distinction between immediate recall, delayed recall (after 30 minutes), and recognition. This test was administered at 15 minutes after the infusion. The Verbal Fluency Task (VFT) was also used and this task required subjects to generate as many words as possible, beginning with a specified letter during a 1-minute interval. Equivalent versions of this task were administered on the 3 test days at 15 minutes after the infusion by using letters equated for frequency in English. Both the HVLT and VFT were administered once at +15 minutes post-infusion on each test day.
The Mini Mental Status Examination (MMSE) was used as a safety measure, with the stipulation that any subject with a post-infusion score of <27 would stay overnight, prior to consideration for discharge.
Data Analysis
Data were checked for normality prior to analysis using Kolmogorov-Smirnov test statistics and normal probability plots. The outcomes for the HVLT, VFT, and pegboard tasks were sufficiently normal. Linear mixed models with medication (placebo, thiopental) as a within-subjects factor and group (family history: FHP vs. FHN) as a between-subjects factor were used to analyze the HVLT and VFT. The pegboard task was analyzed using the same model described above with time (baseline, +15min) included as an additional withinsubjects factor. All other outcome measures were heavily skewed and/ or exhibited floor effects. These outcomes were analyzed using the nonparametric approach for repeated measures data by Brunner [30] where the data were first ranked, and then fitted using a mixed effects model with an unstructured variance-covariance matrix and p-values adjusted for ANOVA-type statistics (ATS). These models included both medication (placebo, thiopental) and time (study time points) as within-subjects explanatory factors and group (FHP vs. FHN) as a between-subjects factor. These models allowed for testing of all main and interactive effects of group, medication and time. For behavioral measures, additional analyses used peak change from baseline as the dependent measure. Only significant effects for the additional analyses are reported. All reported p-values are Bonferroni adjusted, applied within but not across domains. Data were analyzed using SAS, version 9.1 (SAS Institute Inc., Cary, NC).
Results
Subject characteristics
| Variable | Totals (n=73) | FHN (n= 49) |
FHP (n = 24) |
|---|---|---|---|
| Gender* Female Male |
n, % 35 (47.95) 38 (52.05) |
n, % 25 (51.02) 24 (48.98) |
n, % 10 (41.67) 14 (58.33) |
| Ethnicity* Caucasian African American Other |
n, % 58 (79.45) 8 (10.96) 7 (9.59) |
n, % 36 (73.47) 7 (14.29) 6 (12.24) |
n, % 22 (91.67) 1 (4.17) 1 (4.17) |
| Mean (SE) | Mean (SE) | Mean (SE) | |
| Age* | 24.2 (0.31) | 24.0 (0.36) | 24.6 (0.63) |
| Years of education* | 15.8 (0.25) | 15.8�? (0.32) | 15.9�? (0.37) |
| # of Drinking Days past 30 days* | 5.0 (0.47) | 5.1 (0.63) | 4.9 (0.66) |
| # of Drinks during past 30 days* | 18.0 (2.38) | 17.6 (3.04) | 18.8 (3.82) |
| Age began to drink regularly* | 17.1 (0.24) | 17.2 (0.32) | 17.1 (0.33) |
* No significant differences between groups .
Table 1: Study Participants.
As shown in Table 1, seventy-three individuals participated in this study, 24 (33%) were FHP, and 49 (67%) were FHN. Sixty-two (86%) completed both test days, while seven and four subjects completed only the placebo or thiopental test day, respectively. One subject did not want to continue after feeling “uncomfortable” with the medication on test day 1 (thiopental); 1 subject stopped the active medication infusion; another subject completed 1 test day and was discontinued. Five subjects completed 1 test day but withdrew from the study because of scheduling conflicts. Three subjects were withdrawn from the study after they completed test day 1 because we were not able to obtain the active medication (thiopental) to complete their test days. All available subject data were included in the data analysis.
The average age was 24.2 ± 2.7. Thirty-eight (52%) were male and subjects were predominantly Caucasian (n=58, 79%). All subjects had at least a high school education (12 years) with an average of 15.8 ± 2.1 years of education. The average age at which subjects began drinking was 17.1 ± 2.0 years. The average number of drinking days within the past 30 days before infusion was 5.0 ± 4.0 (range: 0-17) and the average number of standard drinks within the past 30 days before infusion was 18.0 ± 20.3 (range: 0-90). There were no differences in baseline characteristics between FHP and FHN groups.
Subjective intoxication
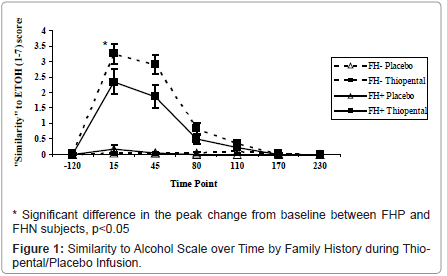
Perceived Similarity to Alcohol: Thiopental demonstrated similarities to alcohol [medication by time: ATS=95.9, num df=3.7, p<0.0001], but did not differ by family history status [group by medication by time: ATS=0.67, num df=3.68, p=0.60] in the primary analysis. In the analysis of peak change from baseline FHP subjects reported a lower similarity to alcohol compared to FHN subjects during thiopental administration (ATS=4.91, num df=1, p=.027) (Figure 1). In order to understand whether this was a non-specific “high” or specific to alcohol, similarity to alcohol was compared to perceived similarity to marijuana. The perceived similarity to alcohol during thiopental administration was more robust than the observed similarity to marijuana; nonetheless, thiopental was rated as more marijuana-like than placebo [ATS=26.8, num df=1.8, p<0.0001]. There was no significant group difference based on family history status [ATS=0.25, num df=1.8, p=0.76].
Number of Drinks Scale (NDS): Thiopental was rated as the equivalent of 3.3 standard drinks (sd=2.3) during the infusion on the basis of the NDS [medication by time: ATS=99.5, num df=3.0, p<0.0001]. There were no differences in the perceived number of drinks between FHP and FHN groups [ATS=0.59, num df=3.0, p=0.62].
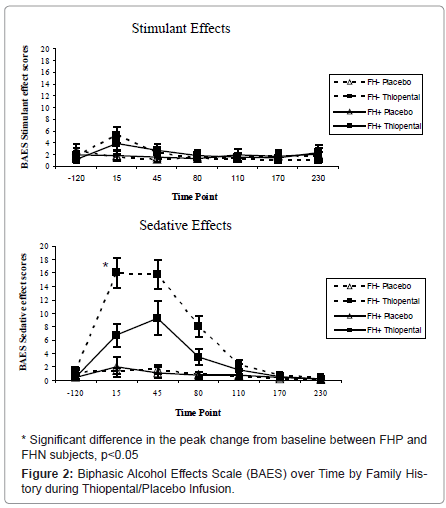
Biphasic Alcohol Effects Scale: As shown in Figure 2, thiopental produced significant effects associated with both the stimulant [medication by time: ATS=8.4, num df=4.3, p<0.0001] and sedative [ATS=38.9, num df=4.0, p<0.0001] effects of alcohol intoxication effects over time among both groups of subjects. No group differences were observed for stimulant effects; peak sedative effects were blunted in FHP subjects during thiopental administration relative to the FHN subjects (ATS=4.92, num df=1, p=.027).
Self-reported high and drowsiness: Based on the visual analog scale (VAS), subjects felt more “buzzed” [medication by time: ATS=40.1, num df=2.7, p<0.0001], “high” [ATS=56.6, num df=2.7 p<0.0001], and “drowsy” [ATS=16.4, num df=4.2, p<0.0001] during thiopental administration. There were no statistically significant differences between family history groups (all p>0.12).
Intensity of Mood States: On the basis of visual analog scale (VAS), no significant differences were observed between thiopental and placebo for measures of high, depressed and anxious.
Clinician Administered Dissociative States Scale (CADSS): Increased effects due to thiopental administration were observed for both the patient- [medication by time: ATS=40.8, num df=2.4, p<0.0001] and clinician-rated [ATS=19.5, num df=2.5, p<0.0001] components of the CADSS scale. These effects did not differ by family history status (all p>0.58).
Pegboard: Thiopental impaired hand-to-eye coordination in both the dominant [medication by time: F(1,191)=35.5, p<0.0001] and non-dominant hand [F(1,191)=36.6, p<0.0001]. These effects were not distinguished by family history status (all p>0.27).
Cognitive effects: Thiopental impaired both total [F(1,71)=17.5, p<0.0001] and delayed recall [F(1,71)=20.8, p<0.0001] during the HVLT, but there were no differences between FHP and FHN subjects (group by medication: all p>0.42 On the basis of the VFT there were no significant differences in learning observed between thiopental and placebo.
Discussion
Results from this study show that intravenous administration of the GABAA agonist thiopental produce alcohol-like subjective effects, as well as alcohol-like impaired coordination and cognition. While there were no significant differences in effects between FHP and FHN individuals in the primary analysis, there is some indication of FHP vs. FHN differences in some, but not all of the subjective effects of thiopental. Specifically, the FHP is less sensitive to thiopental’s sedative effects and reported a lower similarity of thiopental to alcohol compared to FHN. The results from this study could be considered consistent with reports that find differences in the sedative or negative, but not stimulant subjective effects of alcohol between FHP and FHN
The subjective effects of thiopental included a subjective similarity to alcohol, a rating that thiopental was the equivalent of 3.3 standard drinks, euphoria and sedation. Previous animal studies have shown that GABA-ergic medications including the barbiturates and benzodiazepines have alcohol-like discriminative effects [31-33]. GABA-ergic medications which are cross-tolerant with alcohol, predominately the benzodiazepines but also barbiturates, which have been abandoned because of a less advantageous safety profile are the mainstay of treatment for alcohol withdrawal [34]. The development of other agents which influence GABA receptors, including baclofen [35], gamma hydroxybutyric acid (GHB) [36] and steroid anesthetic agents, such as ganaxolone, may hold promise as a therapeutic tool for alcoholism [24]. A promising new line of investigation includes the use of benzodiazepine inverse agonists, such as iomazenil [37] as a potential antidote for intoxication or as potential treatments for alcohol dependence.
Interestingly, the results from this study differ from previous studies showing enhanced FHP response to both barbiturate administration [23] and to benzodiazepine administration compared to FHN individuals [22,38]. In this study, the only differences between FHP and FHN are a decreased sensitivity to the sedative effects of thiopental. The discrepancy in results mirrors the inconsistent findings in subjective alcohol response between FHP and FHN. Some studies find differences only in the sedative or negative response to alcohol [8], while other studies have suggested that FHP individuals demonstrate both increased and decreased sensitivity to alcohol [10]. Several explanations for the discrepancy are possible. There has been much written about the potential confounding effect of level of alcohol consumption in studies comparing subjective effects of alcohol, and by extrapolation other pharmacologic agents, in FHP vs. FHN [13]; in this study subjects were carefully screened and there were no group differences between baseline levels of alcohol consumption. Another important issue is difference in effective dose across studies. Our group has previously shown that this dose of thiopental produces the same level of sedation as the N-methyl-D-aspartate (NMDA) antagonist ketamine infusion at a 0.23 mg/kg loading dose and infusion rate of 58 mcg/kg/min, yet at this dose despite similarities in sedation, ketamine’s effects were more alcohol-like than thiopental effects [28]. The optimal alcohol like effects of thiopental may be unable to be reached because of its sedating properties.
Our group has previously shown that FHP individuals were less sensitive to the NMDA antagonist ketamine than FHN individuals [19] but there was no difference in euphoria between groups. Both of these studies support the hypothesis that the differences in FHP vs. FHN are mediated by the more negative effects of alcohol, but whether those effects are mediated by glutamate via the NMDA receptor, or GABA, is unclear. Some recent evidence is emerging that individuals with polymorphisms in the GABAA gene may have different subjective responses to alcohol compared to individuals without the polymorphism [39,40]. It would be interesting to see if polymorphisms in genes that affect GABA function, synthesis or receptors are associated with differences in response to thiopental. Unfortunately, genetic data are not available from this sample to follow up on this hypothesis.
The strengths of this study include that the study complements previous studies that utilized oral GABAergic agents as probes including secobarbital [22,23] and diazepam [22,23] in understanding differences in subjective effects between FHP and FHN. The advantages of thiopental include its rapid onset and short duration of action and its tolerability. For the sake of feasibility, a single dose of thiopental was studied; however studies utilizing thiopental at different doses for comparison may assist in providing further information regarding the potential utility of this agent as a reliable probe in studying the role of GABAA receptor activity in alcoholism. There were several methodologic limitations of this study. For safety and ethical reasons, subjects were informed that thiopental could potentially be alcohollike. Since expectancy can influence subjective responses, this may confound the results. Pharmacologically, thiopental does not act upon the same subclass of GABAA receptors as alcohol [41], limiting generalizability of the results of thiopental administration to alcohol administration. Finally, although pharmacologically attractive, thiopental must be administered intravenously following conscious sedation policies and is not as easy to administer as other barbiturates or benzodiazepines that can be given orally. This may limit the ability for others to replicate or follow up on these results.
In summary, this study characterizes the effects of the GABAergic receptor agonist thiopental and compares response in a group of FHP vs. FHN individuals. Thiopental produced alcohol-like effects in addition to perceived similarities to alcohol; some group differences in subjective response, particularly to the sedative effects, between FHP and FHN were observed. GABAA receptors have been established to have a role in human alcohol intoxication, but alterations in receptor function as a mechanism of the heritable risk to develop alcoholism is not yet established. Future studies, perhaps including genotyping, will be needed to better understand their role in the pathophysiology and treatment of alcohol use disorders.
Acknowledgements
The authors acknowledge the important contributions of Angelina Genovese, R.N.C., M.B.A., Elizabeth O’Donnell, R.N., and Michelle Lynn SanPedro, R.N., Willie Ford of the Neurobiological Studies Unit of the VA Connecticut Healthcare System, West Haven Campus, West Haven, CT. In addition, the authors acknowledge support for this work from the Department of Veterans Affairs (VA Merit Review Grant, Alcohol Research Center, VISN 1 Mental Illness Research Education and Clinical Center) and National Institute on Alcohol Abuse and Alcoholism (2P50-AA012870-07).
References
- Cloninger CR, Bohman M, Sigvardsson S (1981) Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry 38: 861-868.
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G, et al. (1973) Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch Gen Psychiatry 28: 238-243.
- Cotton NS (1979) The familial incidence of alcoholism: a review. J Stud Alcohol 40: 89-116.
- Dawson DA, Harford TC, Grant BF (1992) Family history as a predictor of alcohol dependence. Alcohol Clin Exp Res 16: 572-575.
- Hines LM, Ray L, Hutchison K, Tabakoff B (2005) Alcoholism: the dissection for endophenotypes. Dialogues Clin Neurosci 7: 153-163.
- Morean ME, Corbin WR (2010) Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res 34: 385-395.
- Schuckit MA (2009) An overview of genetic influences in alcoholism. J Subst Abuse Treat 36: S5-14.
- Schuckit MA (1994) Low level of response to alcohol as a predictor of future alcoholism. Am J of Psychiatry 151: 184-189.
- Schuckit MA (1985) Genetics and the risk for alcoholism. JAMA 254: 2614-2617.
- Newlin DB, Thomson JB (1990) Thomson, Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull 108: 383-402.
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O'Connor S (2002) Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res 26: 1299-1306.
- King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002) Biphasic Alcohol Response Differs in Heavy Versus Light Drinkers. Alcohol Clin Exp Res 26: 827-835.
- King AC, de Wit H, McNamara PJ, Cao D (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68: 389-99.
- Evans SM, FR Levin (2003) Response to alcohol in females with a paternal history of alcoholism. Psychopharmacology (Berl) 169: 10-20.
- de Wit H, McCracken SG (1990) Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res 14: 63-70
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, et al. (2002) Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. J Stud Alcohol 63: 734-744.
- Krystal JH, Tabakoff B (2002) Ethanol abuse, dependence, and withdrawal: Neurobiology and clinical implications. Neuropsychopharmacology: a fifth generation of progress 1425-1443.
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, et al. (1998) Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry 55: 354-360.
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, et al. (2004) Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry 161: 1776-1782.
- Cowley DS, Roy-Byrne PP, Radant A, Hommer DW, Greenblatt DJ, et al. (1994) Eye movement effects of diazepam in sons of alcoholic fathers and male control subjects. Alcoholism, Clinical & Experimental Research 18: 324-332.
- Cowley DS, Roy-Byrne PP, Greenblatt DJ, Kramer GL, Petty F (1996) Effect of diazepam on plasma gamma-aminobutyric acid in sons of alcoholic fathers. Alcohol Clin Exp Res 20: 343-347.
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE (1990) Alcohol and secobarbital effects as a function of familial alcoholism: acute psychophysiological effects. Alcohol Clin Exp Res 14: 704-712.
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE (1991) Alcohol and secobarbital effects as a function of familial alcoholism: extended intoxication and increased withdrawal effects. Alcohol Clin Exp Res 15: 94-101.
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, et al. (2006) Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry 63: 957-968.
- Grant KA, Lovinger DM (1995) Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci 3: 155-164.
- Russo H, Bressolle F (1998) Bressolle, Pharmacodynamics and pharmacokinetics of thiopental. Clin Pharmacokinet 35: 95-134.
- Flood P, Krasowski MD (2000) Intravenous anesthetics differentially modulate ligand-gated ion channels. Anesthesiology 92: 1418-1425.
- Dickerson D, Pittman B, Ralevski E, Perrino A, Limoncelli D, et al. (2008) Ethanol-like effects of thiopental and ketamine in healthy humans. J Psychopharmacol 24: 203-212.
- Brandt J (1991) The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clinical Neuropsychologist 5: 125-142.
- Brunner E, Domhof S, Langer F (2002) Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley & Sons, New York.
- McMahon LR, France CP (2005) Combined discriminative stimulus effects of midazolam with other positive GABAA modulators and GABAA receptor agonists in rhesus monkeys. Psychopharmacology (Berl) 178: 400-409.
- Porcu P, Grant KA (2004) Discriminative stimulus effects of ethanol in rats using a three-choice ethanol-midazolam-water discrimination. Behav Pharmacol 15: 555-567.
- Besheer J, Hodge CW (2005) Hodge, Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology 30: 747-757.
- Mayo-Smith MF, Beecher LH, Fischer TL, Gorelick DA, Guillaume JL, et al. (2004) Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 164: 1405-1412.
- Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA (2010) Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res 34: 1849-1857.
- Leone MA, Vigna-Taglianti F, Avanzi G, Brambilla R, Faggiano F (2010) Gamma-hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses. Cochrane Database Syst Rev CD006266.
- http://clinicaltrials.gov/
- Cowley DS, Roy-Byrne PP, Godon C, Greenblatt DJ, Ries R, et al. (1992) Response to diazepam in sons of alcoholics. Alcohol Clin Exp Res 16: 1057-1063.
- Petrakis I, et al. (2011) Role of GABA-Related Genes in Mediating Subjective Response to IV Ethanol in Healthy Subjects, in Research Society on Alcoholism (RSA), 34th Annual RSA Scientific Meeting, Alcoholism Clinical & Experimental Research: Atlanta, GA.
- Uhart M, et al. (2011) Association of Variants in GABA(A) Receptor Subunit Gene Cluster on Chromosome 5 with Subjective Effects of Alcohol, in Research Society on Alcoholism (RSA), 34th Annual RSA Scientific Meeting, Alcoholism Clinical & Experimental Research, Atlanta, GA.
- Krystal JH, D'Souza DC, Gallinat J, Driesen N, Abi-Dargham A, et al. (2006) The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotox Res 10: 235-252.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14073
- [From(publication date):
specialissue-2013 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 9639
- PDF downloads : 4434