Research Article Open Access
Study on Slice-based Biosensor for Electrophysiological Propagation Measurement in Drugs Screening
| Qing-mei CHEN1,2, Rong LI1, Li-dan XIAO1, Qing-jun LIU1 and Ping WANG1* | |
| 1Biosensor National Special Lab, Key Lab for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, Zhejiang University, Hangzhou 310027, PR China | |
| 2Department of Biomedical Engineering, Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, PR China. | |
| Corresponding Author : | Dr. Ping WANG Biosensor National Special Lab Key Lab for Biomedical Engineering of Ministry of Education Department of Biomedical Engineering Zhejiang University Hangzhou 310027, PR China Tel: +86 571 87952832 Fax: +86 571 87951676 E-mail: cnpwang@zju.edu.cn |
| Received June 24, 2011; Accepted August 16, 2011; Published October 14, 2011 | |
| Citation: Chen QM, LI R, Xiao LD, LIU QJ, Wang P (2011) Study on Slice-based Biosensor for Electrophysiological Propagation Measurement in Drugs Screening. J Biochip Tissue chip S1:004. doi:10.4172/2153-0777.S1-004 | |
| Copyright: © 2011 Chen QM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
Abstract
In order to investigate the signal propagation of the cardiac tissue and the action of drugs, we developed a slice- based biosensor to monitor the electrophysiological activities of the cardiac tissues. At first, we analyzed the interface model of the slice-based biosensor and the electrolyte, and observed the characteristics of the slice-based biosensor with tissue over time. Then, using the slice-based biosensor we sampled the beating signals of the cardiac slices respectively in the presence of the normal Tyrode’s solution, Adrenaline Hydrochloride and Acetylcholine chloride. Further, we used statistic analysis and correlation analysis to review the propagation of beating signals and the action of drugs on propagation. Results showed that the slice-based biosensor is apt and stable enough to catch the character and tendency of signal propagation and Delay is the key to deduce the conduction tendency. Adrenaline Hydrochloride and Acetylcholine chloride respectively have excitatory and inhibiting action on Amplitude and Frequency of the beating signals, and appear dependent on dosage. Thus, slice-based biosensor offers a noninvasive and versatile method to study the cardiac beating and the electrophysiological propagation for drugs screening. It is significative to guide clinical remedy of the drugs’ acting targets in the future.
| Keywords |
| Slice-based biosensor; Electrophysiological propagation measurement; Drugs screening; Cardiac slice; Microelectrode array |
| Introduction |
| In recent years, the incidence and mortality of the heart diseases goes up visibly in lots of countries. Therefore, study about the cardiac biology, electrophysiology and pharmacology is being performed to seek for the pathogenesis and the effective treatments. Recently, biological therapy such as cell therapy, gene therapy and biological pacemaker has been advanced and is becoming hot [1-4]. However, to evaluate the effect of biological therapy, the cardiac electrophysiology is necessary to be considered. |
| In the experiments of cell culture, action potential of cardiac cells is often observed. Researchers have studied the characteristics of the action potential [5-8] and the effect of drugs on the potential [9,10]. Meiry et al. investigated gap junctions, conduction velocity, and propagation patterns of cultured neonatal rat ventricular myocytes, and analyzed the relationship among them [7]. Besides, Halbach et al. evaluated the relationship between the intracellular potentials and the field potentials [11]. These researches are helpful for the clinic diagnosis and medicine usage. However, the dissociated cardiomyocytes lose the anatomic and functional integrity of heart in vivo. So cardiomyocytes culture is not apt for conduction study. Accordingly, cardiac tissues and slices in vitro begin to become the present objects of electrophysiology study because they provide the possibility of preserving in vivo structure and normal electrophysiological. In 2005, Group of Pillekamp evaluated the viability of the embryonic heart slice from immunohistology and electrophysiology [12]. The results suggested that the heart slices from murine embryos can keep functionally intact. Accordingly, the electrophysiology of the ventricular slices in different stage has been investigated [13,14]. They successfully observed the propagation of the spontaneous signals and the induced signals by electrical stimulation, and discussed the conduction direction of signals using the map of the activation time. |
| Presently, our laboratory has been developing the cell-based biosensors to study the electrophysiology [9,15-17]. As well as we are also developing tissue-based biosensors fit for investigating the signal conduction [18]. Here, we developed a slice-based biosensor to investigate the signal propagation of the cardiac tissues. In the previous studies, the delay of the activation time was the common parameter for propagation investigation. In the present study, we will use the slicebased biosensor to measure the beating signals of the cardiac slice, and assess the effect of several parameters as well as drugs on signal propagation. |
| Test principle |
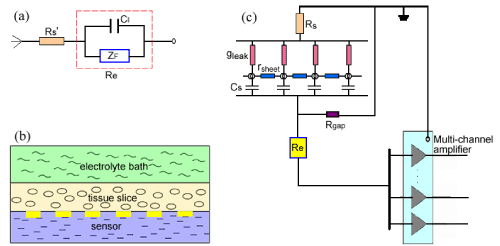
| Once slice-based biosensor is immerged in the electrolyte, chemical reactions occur immediately whereby electrons are transferred between the metal and the electrolyte, which is defined interface electrochemistry. The electrochemical principle of interface is usually described using Randles equivalent circuit model. Here we simply depicted the interface equivalent model of biosensor and the electrolyte as shown in (Figure 1a). Rs’ represents the electrolyte resistance, CI represents the interfacial capacitance induced by the electrodes, and ZF means faradic impedance. |
| When the tissue slices are placed on the biosensor, the interface of the tissue and the coupling degree of tissues/sensor must also be considered in the equivalent circuit model. In the present study, cardiac slice is taken as the sheet conductor model according to the discussed theory [19]. Here we will briefly discuss the interfacial change when the cardiac slice coupled to the biosensor. |
| As shown in (Figure 1b), surface of tissue slice is bathed in the electrolyte, and the substrate is contacted with the biosensor. Neglecting the current flow from slice to bath, we described the shunting effect of bath using an ohmic conductance per unit area gleak(=2/ρh), the slice itself using a sheet resistance rsheet(=ρ/h) and the interfacial capacitance of the tissue-substrate using a capacitance per unit area Cs (Figure 1c). As a result, the tissue slice is described as a sheet conductor in the x-y plane with a capacitive bottom and a leaky cover. Here ρ is the specific resistance of an isotropic and homogeneous volume and h is the effective thickness of the tissue slice. The larger h is, the smaller gleak and rsheet are and the smaller field potential can be acquired (Figure1c). If the slice thickness h is thin enough, we may also neglect the conduction along y orientation. To describe the adhesion of tissue to electrodes, we used Rgap to represent the sealing resistance of medium layer in the tissue-substrate gap. (Figure 1c) shows that the bigger Rgap is, the bigger the field potential is. |
| According to the circuit model of measurement using the slicebased biosensor (Figure 1c), when tissues placed on the biosensor the electrophysiological change of cells will induce the potential of electrodes to change. Thus, we can use the biosensor to measure the electrophysiological characteristics of tissues in real time and improve the measurement according to the circuit model and the experiment’s results. |
| In the equivalent circuit model of (Figure 1), faradic impedance ZF is caused by polarization, and the interfacial capacitance CI is mainly related with the material and the surface area of electrode. gleak and rsheet are basically due to the tissue itself, and Cs is induced by the interface change of tissue/electrolyte. Rgap maybe exhibits much importance during the electrophysiological measurement. It has reported that sealing resistance in the cell-substrate gap is highly related to the gap dimensions and is inversely proportional to the distance of cell-substrate [20]. Accordingly, for the slice-based biosensor Rgap will play the equivalent role during the measurement. |
| Materials and Methods |
| MEA fabrication and setup |
| In the present study, the slice-based biosensor is developed from microelectrode array (MEA). MEA fabrication adopts the process flow of standard microelectronic process. Briefly, evaporation or sputtering a thin layer of chromium (100 Å - 500 Å) followed with a thick gold layer (2000 Å - 5000 Å) on glass substrate (5” diameter, 500μm thickness). Then, coat the surface with an insulation layer such as polyimide or thick resist. In the end, open the holes where electrodes are located by reactive ion-etching. (Figure 2a) gives the view of the fabricated device. |
| Impedance measurement and chip surface modification |
| Before electrophysiological experiments, we evaluated the properties of the sensing electrodes. The measurements were performed by exciting each electrode with sinusoidal waves (50mV in amplitude) and by sweeping the frequency from 1Hz to 100 kHz. |
| To improve the characteristics of microelectrodes, we tried modifying the electrodes by electroplating platinum black. Here electroplating was made on electrochemical workstation (Potentiostat/ Galvanostat Model 273A, EG&G Princeton Applied Research, USA) and the electroplating solution was prepared according to the previous method [21]. The plating voltage was set at -100mV, and the plating time was controlled at about 40s for each electrode. Finally we can obtain the device as shown in (Figure 2b). |
| To evaluate the characteristic of electrodes after being electroplated platinum black (PB-plating) and observe the influence of the tissue slice on platinum black, we also measured the impedance under the different stages. |
| Cardiac slice preparation |
| Experiments were carried out in accordance with the guidelines of the local welfare committee and the use of animals in the study was approved by the local animal welfare and ethical committee. Mice (ICR, regardless of sex, about 4 weeks old) were killed after anesthetized using 20% urethane (7ml/kg). The whole heart was quickly isolated and immersed in the oxygenated ice-cold (2-4o) Ca2+-free Tyrode’s solution containing (mmol/L) 126 NaCl, 5 KCl, 1.25 NaH2PO4, 2 MgCl2, 10 glucose, 5 hepes; pH7.4 adjusted with NaOH. Then the heart should be quickly taken out from the ice-cold Ca2+-free Tyrode’s solution and cut into slices along the short axis using the vibratome (Vibratome 1000 Plus, Vibratome, USA). In the present experiments, the slice thickness was controlled within 400μm. |
| According to the sketch of slicing (Figure 3a), we got the cardiac slices as shown in (Figure 3b). During the experiments, slices with good configuration were stored in the standard ice-cold Tyrode’s solution (adding 2mmol/L CaCl2 to the Ca2+-free Tyrode’s solution) continuously bubbled with pure oxygen, then were incubated in DMEM without serum at room temperature for at least 30min. |
| System testing |
| At the beginning of measurement, the flow velocity of the solution and the flux of oxygen need to be adjusted. Usually the velocities of the solution and the oxygen were respectively 2.5ml/min and 0.5-1L/min. Then the multi-channel amplifier (MEDl6, Multi-channel systems, GmbH, Germany), MEA chip and the computer were connected. In the end, we detected the status, stability and noise level of the system. |
| To eliminate the power-line interference, all measurements were made in the shielding box. Data acquisition was carried out by the analyzing software (MC_Rack) accessory with the system. The software can realize 16-channel synchronous inspection in real-time and elementary data-processing. During the present experiments, we simultaneously acquired the data from 16-channels with a sampling frequency of 20 kHz. |
| Signal recording and processing |
| Placing the cardiac slices onto MEA, we monitored their electrophysiological activities and investigate the propagation of the beating signals. Furthermore we observed the action of Adrenaline Hydrochloride and Acetylcholine chloride on the cardiac slices as well as to know about the detecting capability of this slice-based biosensor. The final concentrations of drugs included 0.1μmol/L and 1μmol/L by dissolved in the normal Tyrode’s solution. |
| Firstly, we sampled the raw data of the control group (in the normal Tyrode’s solution) which was recorded at least 1h. The drug groups were performed after recording 20min in the normal Tyrode’s solution. Drug’s action was controlled within 10min, then transferred to irrigate with the normal Tyrode’s solution to remove the drug’s action. The drug groups were recorded at least 20min once in the presence of the drugs. |
| All data are represented by the mean ± SEM. Student’s t-test (Paired Samples Test) was used to calculate if there was a significant difference between the control and the drug groups. |
| Results |
| MEA characteristics |
| (Figure 2a) Is the overall view of MEA. The electrode diameter is 30μm and the space between electrodes (center to center) is 150μm. According to chapter 3.2, we got the impedance spectroscopy under the different conditions. |
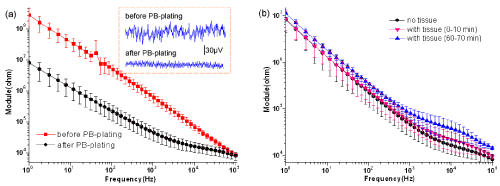
| (Figure 4a) Gives the impedance spectroscopy of electrodes before and after being plated platinum black (PB-plating) without tissue. The red curve (representing the naked Au electrodes) is visibly higher than the black curve (representing the electrodes with platinum black) especially at low frequency. Furthermore, after PB-plating the thermal noise of electrodes reduced (see the dashed frame of (Figure 4a). Before PB-plating, the noise value fluctuated around 60μV. However, after PB-plating the noise value was almost within 25μV. From the actual baselines, we also find that the baseline is more stable after PB-plating than before PB-plating. Results of (Figure 4a) suggest that PB-plating can effectively lower the impedance especially at low frequency, as well as calm the baseline. |
| To see whether the interface with tissue will change over time, we reviewed it by the impedance values. In (Figure 4b), the black curve (without tissue slice) has smaller impedance than the other two curves (with tissue slice) basically at high frequency. However, at low frequency, the impedance with tissue has not visible difference from that without tissue. From (Figure 4b), we also find that the impedance with slice will increase over time, but the change is very small. This result argues that the tissue slice brings additional impedance components and plays important role in the impedance of high frequency. Whereas at low frequency, the impedance with tissue especially at the beginning has not visible difference from that without tissue, which indicates that the impedance induced by the electrode dominates at low frequency. The result shows that adhesion of tissue affects little on platinum black within 1h. That is to say, we can realize long-term recording on the electrodes with platinum black. |
| Propagating signal recording synchronously with slice-based biosensor |
| Using the multi-channel amplifier and acquisition system, we simultaneously obtained the multi-site signals from the cardiac slices. (Figure 5a) gives the 16-channel signals in the normal Tyrode’s solution with MEA acquisition. Usually the maximal amplitude can reach several hundred micro volts, and the beating period is from 800ms to 2500ms. In the present experiments, not all slices can give birth to beating signals. Generally, about 50% slices per experiment can exhibit spontaneous contractions and appear the beating. Here, we reviewed four parameters: Amplitude, Frequency, Duration and Delay. Amplitude is defined with the absolute value of dispersion of FPpre and FPmin (Figure 5b), Frequency means the beating times within 1s, and Duration is the time between FPmin and the last FPmax. According to the former definition [1], the time of the maximal negative deflection of the field potential is taken as the time of activation, which is defined Delay in the present study. |
| Using principal component and factor loading analysis, we investigated the contribution of all parameters to the propagation. The factor loading reflects the correlation degree of the new variables and the original parameters. Results show that the 4 parameters have strong correlation to the two principal components, and Amplitude as well as Frequency belongs to a new factor, Duration and Delay to another factor. By comparison, we find that the contribution of Amplitude, Frequency and Delay to the signal propagation is relatively large, but that of Duration is smaller. Further, we reviewed the correlation about Amplitude, Frequency and Delay to Distance. Here Distance is defined by the interval of the centers between any two electrodes, and the start is the electrode site with minimal Delay. We found Delay and Distance have strong relativity. Since Delay is taken as the time of activation, we can deduce the direction of the signal propagation according to the map of local time activation which was automatically plotted with Matlab software. |
| Besides, we can calculate the conduction velocity on the basis of the Delay. Here, the conduction Velocity is the mean velocity of 16 electrodes, which is gotten by calculating Δs/Δt. Thereinto Δs, and Δt are respectively the conduction distance and time of signals. In the present experiments, the conduction Velocity is about 13.05 cm/s. |
| Drug action on beating signal and propagation |
| Experiments were also made to observe the drug’s effect on the beating signals. The data were acquired for 3 min after using the drugs. Results showed that Adrenaline Hydrochloride can increase the amplitude and frequency of the beating signals, but Acetylcholine chloride can depress the amplitude and frequency. (Table 1) gives the statistic of Amplitude, Frequency, Duration and Velocity. From (Table 1), we find that in the presence of the high dose of drugs, Amplitude, Frequency and Duration change obviously. |
| Discussion |
| Effect of electroplating platinum black on microelectrodes |
| Generally, the signal-to-noise ratio (SNR) of extracellular recording with MEA is very small, so it is necessary to lower the impedance as well as the noise. According to the equivalent circuit model in (Figure 1a), changing the electrode material or increasing the electrodes effective area may improve the impedance characteristic. In the present study, we modified the surface of MEA by electroplating platinum black to enhance the surface roughness and effective area of electrodes. Result of (Figure 4a) shows that electroplating platinum black reduces the electrode impedance as well as the noise. It is speculated that the increment of the effective surface area of the electrodes makes the interfacial capacitance (CI shown in (Figure 1) enhanced and the impedance decreased. The results suggest that surface modification by electroplating platinum black is an effective method to improve the electrode characteristics. |
| Evaluation of the cardiac slice-based biosensor |
| When placing the cardiac slice on MEA, we found the electrode impedance increased especially at high frequency (Figure 4b). This result argues that the tissue slice brings additional impedance components and plays important role in the impedance of high frequency. (Figure 4b) also tells us that the impedance with slice increased faintly over time. The result indicates that adhesion of tissue affects little on platinum black within 1h. Thereby, we can realize long-term recording on the electrodes with platinum black. Present results indicate that the cardiac slice-based biosensor is feasible, and is useful for conduction study and drugs’ evaluation. Moreover, the technique operates simply and can get high ratio of signal-to-noise. |
| However, the present experiment reveals that not every slice’s beating can be detected. This issue maybe arises from various causes. At first, the cardiac slice may be damaged to be in a bad status during slicing. Secondly, the dead cells on the slice surface came into being an insulating layer, which made tissue slice and electrodes badly coupling. Thus Rgap became small and the signal amplitude was too small to be visible. |
| To lessen the number and pain of animals and enhance the efficiency of experiments, we will take action to increase the longevity of the slices as well as the slice-based biosensor. Firstly, we will improve the manipulation and condition of slicing and measurement. Besides, we can design the novel electrode arrays to solve the coupling problem and enhance SNR. Now, the three dimensional microelectrode arrays have been advanced [22,23], which can make good contact between the electrodes and the active cells in the slice. Therefore, we can design three dimensional microelectrode arrays to develop the slice-based biosensor for more effective measurement than now. |
| Significance of the cardiac slice-based biosensor |
| We monitor the electrophysiological activities of the cardiac slices respectively in the presence of the normal Tyrode’s solution, Adrenaline Hydrochloride and Acetylcholine chloride. Results suggest that Amplitude, Frequency and Delay can reflect the property of signal propagation and Delay is helpful for deducing the conduction tendency. In the present experiments, the neighboring cells in a tissue slice represent nicer synchronization, which is accordant to the excitationcontraction coupling mechanism. So it is befitting for investigating the signal conduction of heart. |
| Table 1 display the action of Adrenaline Hydrochloride and Acetylcholine chloride on beating and propagation of the cardiac slices. Results indicate that action of high dose is more obvious than the low dose. Additionally, we find that Adrenaline Hydrochloride not only increases the amplitude and the beating frequency, sometimes can also excite some channels without signals under the normal Tyrode’s solution. That is to say Adrenaline Hydrochloride can excite some sites and even propagate to active other sites nearby. These results indicate that the slice-based biosensor can implement cardiac drugs screening and evaluate the dependence of signals on the drugs. It is helpful to guide clinical remedy and to search drug’s acting targets in the future. |
| Acknowledgements |
| This work was supported by the National Natural Science Foundation of China (Grant No. 60725102, No. 30970765), the National Basic Research Program of China (Grant No. 2009CB320303). |
| References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13473
- [From(publication date):
specialissue-2011 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 8959
- PDF downloads : 4514
