Research Article Open Access
Study of Biochemical Risk Factors Involved in the Pathogenesis of Goiter in Adults in Sindh
Abdul Hafeez Kandhro1* and Fatehuddin Khand21Department of Biochemistry, Faculty of Medicine and Allied Medical Sciences, Isra University Hyderabad, Pakistan
2Supervisor & Chairman, Post Graduate Studies, Faculty of Medicine and Allied Medical Sciences, Isra University Hyderabad, Pakistan
- *Corresponding Author:
- Abdul Hafeez Kandhro
Clinical Lab Manager
Healthcare Molecular & Diagnostic Laboratory
Suit# 3,4 aziz avenue, opposite Jahan Plaza
Saddar, Hyderabad, Sindh, Pakistan, P.O# 71000
Tel: 0092-300-3061263
E-mail: hafeezjaan77@yahoo.com
Received Date: January 05, 2013; Accepted Date: April 23, 2013; Published Date: April 25, 2013
Citation: Kandhro AH, Khand F (2013) Study of Biochemical Risk Factors Involved in the Pathogenesis of Goiter in Adults in Sindh. J Clin Exp Pathol 3:138. doi: 10.4172/2161-0681.1000138
Copyright: © 2013 Kandhro AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Background: Deficiencies of iodine, Selenium, are the 2 most common micronutrient deficiencies in some areas of Pakistan, although control programs, when properly implemented, can be effective.
Objective: We investigate these deficiencies and their possible interaction in adult age in both gendersof Hyderabad & plane areas of Sindh. Design: Goiter, signs of Iodine deficiency, and biochemical markers of thyroid (Thyroid Hormones), Serum Selenium status were assessed in 100 younger aged 15–30 y.
Results: The goiter rate was 30.5%.TSH levels in adult goiter cases were significantly higher 11.40 ± 3.80 μIU/ml (p <0.002) than the control subjects 1.27 ± 0.42 μIU/ml (matched for age and gender and with no personal history of goiter). As compared to control subjects T3 levels were significantly higher 1.80 ± 1.02 ng/dl (p <0.001) in goiter cases. The T4 levels were comparable between goiter patients and control subjects. There were significantly lower 42.68 ± 11.07 μg/L (p <0.001) serum selenium levels in goiter cases as compared to control subjects 88.88 ± 10.39 μg/L. There were significantly lower 60.32 ± 20.47 μg/L (p <0.001) urine iodine levels in goiter cases as against the controls.
Conclusion: The finding of present study that T3 and T4 levels in goiter patients were within normal ranges indicates that the cause of enlarged thyroid gland in these patients is deficiency of iodine in the diet. The finding that iodine was excreted in significantly lower amounts in goiter patients than in the control subjects, also suggests mild iodine deficiency to be the cause of goiter in these patients.
Introduction
Thyroid gland swelling leads to Goiter. Thyroid gland tissue comprises follicular cells; these cell secrete iodine-containing hormones 3, 5, 3’, 5’ Tetraiodothyronine (T4) and Tri –iodothyronine (T3). In addition, this tissue contains Para follicular cells (also called C cells) which secrete calcitonin hormone. The major thyroid hormone synthesized by the thyroid gland is thyroxine (T4). Small amounts of tri-iodothyronine (T3) and reverse T3 (rT3) are also synthesized in the thyroid gland. T4 is converting to T3 by the removal of an iodine atom. This conversion occurs mainly in the liver and in certain tissues such as brain where T3 acts. The production of thyroid hormones is tightly regulated via the hypothalamic-pituitary-thyroid axis.
In adolescents, particularly women age ranges 11–18 years having commonly thyroid gland disorders (LaFranchi et al., 1994). Different iodine intakes in different geographical locations cause thyroid gland disorders [1].
In Pakistan, about 50% of the population is at risk of iodine deficiency disorders [2]. A national survey of iodine status in Pakistan conducted during 1993-1994 reported a median urinary iodine concentration (UI) of 16 μg/L and 90.4% of the population had a UI <100 μg/L.
In 2005, Pakistan’s Ministry of Health started the National Plan for action with technical and financial assistance from the Micronutrient Initiative (MI). One of its high prime priorities was to increase access to and use of adequately iodized salt in Pakistan [3]. In 2008, the Nutritional Wing of the Ministry of Health in Pakistan launched a project in 65 districts of Pakistan to enhance production of iodized salt in the country. As a result, iodized salt production increased from 17% to 70% in these districts [4].
Study of the referred patients at NIMRA, Jamshoro for screening of thyroid disease, reported that females were five times more prone to develop goiter than the males and the peak age for presentation of goiter in both sexes was 15–25 year [5]. Although pregnancy can be suggested as part of the explanation of the gender difference in the prevalence of goiter, peak occurrence of goiter in both the genders in between 15-25 years age suggests involvement of other risk factors as well.
Iodine
Iodine is an important component of the hormones produced by the thyroid gland. Iodine deficient soils areas are most frequent in inland regions, mountainous areas and areas of frequent flooding, but can also occur in coastal regions [6]. In addition, this arises from the distant past through glaciation, compounded by the leaching effects of snow, water and heavy rainfall, which removes iodine from the soil. Boiling, baking, and canning of foods containing iodized salt cause only small losses (≤ 10%) of iodine [7].
Pathophysiology of iodine deficiency
When low intake of iodine abnormally, adequate secretion of thyroid hormones may still be achieved by marked modifications of thyroid activity. (Such as stimulation of the trapping mechanism of iodide by the thyroid as well as stimulation of the subsequent steps of the intrathyroidal metabolism of iodine leading to preferential synthesis and secretion of T3). These adaptive processes are triggered and maintained by increased secretion of thyroid stimulating hormone (TSH). There is a clear inverse relation between iodine supply and thyroidal uptake of iodide of radioiodide. The increase uptake may be accompanied by and may result from an increase in the serum levels of TSH. However, in extreme iodine deficiency (Endemic Goiter) there were elevated TSH level. In mild iodine deficiency, small fraction of subjects having elevated TSH, usually the youngsters [8].
Iodine deficiency in endemic goiter is well established, but a variety of naturally occurring compounds have been identified that might be goitrogenic in human beings [9,10]. Goitrogens cause goiter only if iodine supply is limited and/or goitrogenic substances is of long duration. These compounds belong to the following chemical groups:
• Sulfurated organics (thiocyanate, isothiocyanate, goitrin & disulphides)
• Flavonoids (polyphenols)
• Polyhydroxyphenols and phenol derivatives
• Pyridines, phthalate esters and metabolites,
• Polychlorinated (PCB) and polybrominated (PBB) biphenyls Organochlorines (like DDT)
• Polycyclic aromatic hydrocarbons (PAH)
• Inorganic iodine (in excess)
• Lithium.
Selenium
Selenium (Se) discovered by Berzelius in 1817, is now recognized as an essential trace element [11]. Like iodine, selenium is present in highest concentration in thyroid gland. It is essential for normal thyroid function and thyroid hormone homeostasis [12], since selenium is capable of exerting multiple actions on endocrine system by modifying the expression of at least 30 selenoproteins, many of which have clearly defined functions. Well-characterized selenoenzymes are the families of glutathione peroxidase, thioredoxin reductase, and iodothyronine deiodinase [13].
Selenium deficiency can effects on thyroid hormone metabolism and possibly also on the thyroid gland itself [11,14,15]. In this situation the function of type I deiodinase (a selenoprotein) is impaired. In peripheral tissues, Type I deiodinase plays a major role in T4 deiodination.
Selenium deficiency also leads to a reduction in the selenium containing enzyme glutathione peroxidase. It detoxifies H2O2, which is mostly present in the thyroid gland as a substrate for the thyroperoxidase that catalyzes iodide oxidation and its subsequent binding to thyroglobulin at tyrosyl residues, and the oxidative coupling of iodotyrosines into iodothyronines. Thyroid cell death can occur due to reduced detoxification of H2O2 [16]. Elevated H2O2 levels in thyrocytes may be more toxic under situations of increased TSH stimulation such as is present in areas with severe iodine deficiency. China indicated in extensive epidemiological data that all selenium- deficient areas were IDD-endemic areas. However, IDD can be very severe in many selenium-rich areas [16].
Materials and Methods
Study subjects
A total of 100 (50 goiter patients and 50 normal control subjects) between the ages 15–30 years, and of either gender were recruited from the OPD patients and patients admitted in Medicine wards of Isra University Hospital and OPD of Nuclear Institute of Medicine & Radiotherapy (NIMRA), Jamshoro.
The distribution of goiter patients studied was as follows:
Fifty Goiter patients (25 males, 25 females)
Fifty Control subjects (24 males, 26 females)
Study design
This was prospective, case-control study.
Setting
The experimental work was carried out in the department of Biochemistry, Faculty of Medicine and Allied Medical Sciences, Isra University, Hyderabad in collaboration with Isra University Hospital, Sindh University High tech Central Research Laboratory, Jamshoro and Nuclear Institute of Medicine and Radiotherapy, Jamshoro.
For this study patients admitted with raised or decreased TSH levels and lower or higher thyroid hormone levels were recruited from Medicine wards and OPD of Isra University Hospital and OPD of Nuclear Institute of Medicine and Radiotherapy, Jamshoro.
Inclusion criteria for goiter patients:
a) Age : Adults (15-30) years
b) Sex : Both genders (Male / Female)
c) H/O : Goiter
d) TSH : Increased / Decreased e) T3, e
e) T4 : Increased / Decreased
f) T3, t4 : Normal (In Euthyroid patients) or (Treated patients)
Inclusion criteria for non-goiter subjects
a) Age : Adults (15-30) years
b) Sex : Both genders (Male / Female)
c) H/O : Non-goiter
d) TSH :Normal
e) T3, T4 : Normal
Exclusion criteria for goiter patients
a) Hepatic disorders
b) Cardiac dysfunction
C) Endocrine dysfunctions other than thyroid gland
Exclusion criteria for controls:
a) Hepatic disorders
b) Cardiac dysfunction
c) Endocrine dysfunctions
Samples
Blood and random urine samples were be collected from every individual under study.
Measurement parameters
1. T3, T4 & TSH levels in serum
2. Selenium concentration in serum
3. Iodine level in urine samples
Methods
T3, T4 & TSH levels in serum were analyzed by Electro –-Chemilumeniscence technology on Cobas e 411 Analyzer (Roche, UK). Iodine content of urine samples were measured by using a Hitachi –220 Spectrometer by the method of Sandell-Kolthoff. Selenium levels in serum samples were measured using Hitachi–220 Spectrometer.
Statistical analysis
Standard methods were used for calculation of mean, Standard Deviation (SD), standard error of the mean (SEM), and Coefficient of Variation (CV). Statistical comparisons were done using student’s paired t-test. Results were considered statistically significant at P < 0.05.
Measurement of iodine
I Principle: Sample was digested with ammonium persulfate. Iodide (I-) is the catalyst in the reduction of ceric ammonium sulfate (yellow) to cerous form (colorless), and is detected by rate of color disappearance (Sandell-Kolthoff reaction). The time taken for the color disappearance was inversely proportional to the amount if iodide catalyzing it.

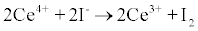
II Standards: Standards were prepared through serial dilution of the stock potassium iodate (500 μg / L) solution yielding final concentrations of 10, 40, 80, 120, 200 μg / L, while deionized water was used as 0 μg / L standard.
III Calculation of results: Constructed a standard curve on graph paper by plotting the iodine concentration of each standard against its absorbance at 420 nm on the ordinate.
Measurement of selenium
Oxidation by acid permanganates converts all selenium compounds to selenate. Selenate is reduced to selenite in warm 4N HCL. Temperature, time and acid concentrations are specified to obtain quantitative reduction without loss of selenium. The optimum pH for the formation of piazselenol is approximately pH 1.5. Above pH 2, the rate of formation of the colored compound is critically dependant on the pH.
I. Standards: Standard selenium solution:
Diluted an appropriate volume of stock selenium solution which distilled water upto 100 ml = 100 ug Selenium.
II. Standard Preparation: Prepared standards containing 0.0, 10.0, 25.0 and 50.0 μg Selenium in 50 ml Erlenmeyer flasks. Diluted to approximately 25 ml, add 10 drops methyl orange indicator solution, 2 ml 0.1 N HCL, 5 ml CaCl2 solution, 3 drops 0.1 N KMnO4, and few glass beads to prevent bumping. Boiled vigorously for approximately 5 minutes.
III. Triiodothyronin (T3)
a) Technology: The Electrochemiluminescence immunoassay “ECLIA”
b) Instrument: Cobas e 411 Immunoassay analyzer.
c) Test principle
1st incubation: 30 μL of sample and a T3-specific antibody labeled with a ruthenium complex; bound T3 is released from the binding proteins in the sample by ANS.
2nd incubation: After addition of streptavidin-coated microparticles and biotinylated T3, the still-free binding sites of the labeled antibody become occupied, with formation of an antibody-hapten complex. The entire complex becomes bound to the solid phase via interaction of biotin and streptavidin.
The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of the electrode. Unbound substances were then removed with ProCell. Application of a voltage to the electrode then induced chemiluminescent emission which was measured by a photomultiplier.
Results were determined via a calibration curve, which is instrument- specifically generated by 2-point calibration, and a master curve provided via the reagent barcode.
d) Calibration
Every Elecsys T3 reagent set has a barcoded label containing the specific Information for calibration of the particular reagent lot.
III Tetraiodothyronin (T4)
a) Technology: The Electrochemiluminescence immunoassay “ECLIA”
b) Use: In vitro quantitative determination of Tetraiodothyronin (T4) in human serum and plasma
c) Instrument: Cobas e 411 Immunoassay analyzer
d) Test principle
1st incubation: 15 μL of sample and a T4-specific antibody labeled with a ruthenium complex; bound T4 is released from binding proteins in the sample by ANS.
2ndincubation: After addition of streptavidin-coated microparticles and biotinylated T4, the still-free binding sites of the labeled antibody become occupied, with formation of an antibody-hapten complex. The entire complex becomes bound to the solid phase via interaction of biotin and streptavidin.
The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of the electrode. Unbound substances were then removed with ProCell. Application of a voltage to the electrode then induced chemiluminescent emission which was measured by a photomultiplier.
Results were determined via a calibration curve which is instrument specifically generated by 2-point calibration and a master curve provided via the reagent barcode.
e) Calibration
Every Elecsys T4 reagent set has a barcoded label containing the specific information for calibration of the particular reagent lot.
IV. Thyroid Stimulating Hormone (TSH)
a) Technology: The Electrochemiluminescence immunoassay “ECLIA”
b) Use: In vitro quantitative determination of Thyroid Stimulating Hormone (TSH) in human serum and plasma
c) Instrument: Cobas e 411 Immunoassay analyzer
d) Test principle
1st incubation: 50 μL of sample, a biotinylated monoclonal TSH specific antibody and a monoclonal TSH-specific antibody labeled with a ruthenium complex react to form a sandwich complex.
2nd incubation: After addition of streptavidin-coated microparticles, the complex becomes bound to the solid phase via interaction of biotin and streptavidin.
The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of the electrode. Unbound substances were then removed with ProCell. Application of a voltage to the electrode then induced chemiluminescent emission, which was measured by a photomultiplier.
Results were determined via a calibration curve, which is instrument specifically generated by 2-point calibration, and a master curve provided via the reagent barcode.
e) Calibration
Every Elecsys TSH reagent set has a barcoded label containing the specific information for calibration of the particular reagent lot.
Results
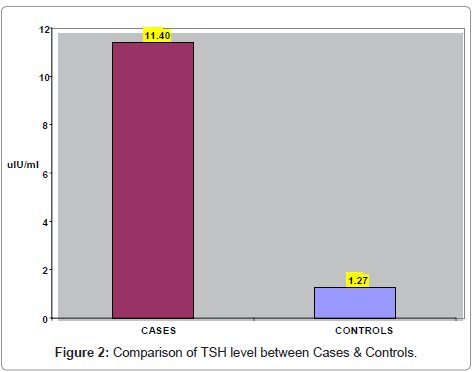
Gender-wise distribution of goiter patients and control subjects is shown in (Table 1). There were 49 males (25 goiter cases and 24 control subjects) and 51 females (25 goiter cases and 26 control subjects). (Figure 1) presents gender- wise distribution of goiter patients and control subjects. In (Table 2) comparison of TSH levels between cases & control subjects is shown. The mean TSH was 11.4 ± 3.8 uIu/ml in goiter cases 1.27 ± 0.4 in control subjects.TSH levels in goiter cases were significantly higher (p <0.002) than the control subjects. (Figure 2) represents the comparison of TSH levels between cases & control subjects.
| GROUP | MALES | FEMALES | TOTAL |
|---|---|---|---|
| Goiter Cases | 25 | 25 | 50 |
| Non-Goiter subjects | 24 | 26 | 50 |
| TOTAL | 49 | 51 | 100 |
Table 1: Gender-wise distribution of goiter cases and controls.
| Parameter | Cases (n=50) | Controls (n=50) | P-Value |
|---|---|---|---|
| TSH (uIU/ml)Mean + SD | 11.40 + 3.8 | 1.27 + 0.42 | 0.002 |
Table 2: Comparison of TSH levels between cases & control subjects.
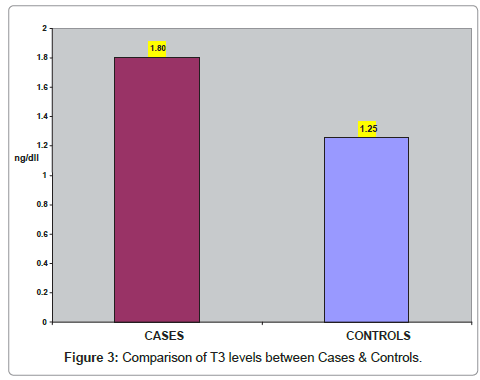
In (Table 3) comparison of T3 levels between cases & control subjects is shown. The mean T3 was 1.80 ± 1.02 ng/dl in goiter cases and 1.25 ± 0.16 in control subjects. As compared to controls T3 levels were significantly higher (p<0.001) in cases. (Figure 3) represents the comparison of T3 levels between cases & control subjects.
| Parameter | Cases (n=50) | Controls (n=50) | P-Value |
|---|---|---|---|
| T3 (ng/dl) Mean + SD | 1.80 + 1.02 | 1.25 + 0.16 | <0.001 |
Table 3: Comparison of T3 level between Cases & Control.
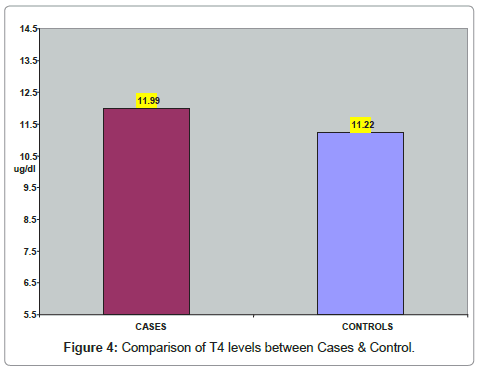
In (Table 4) comparison of T4 levels between cases & control subjects is shown. The mean T4 was 11.99 ± 5.35 ug/dl in goiter cases and 11.22 ± 1.48 in controls. The T4 levels were comparable between the subjects of the two groups. (Figure 4) represents the comparison of T4 levels between cases and control subjects.
| Parameter | Cases(n=50) | Controls(n=50) | P-Value |
|---|---|---|---|
| T4 (ug/dl)Mean + SD | 11.99 + 5.35 | 11.22 + 1.48 | 0.321 |
Table 4: Comparison of T4 levels between Cases & Controls.
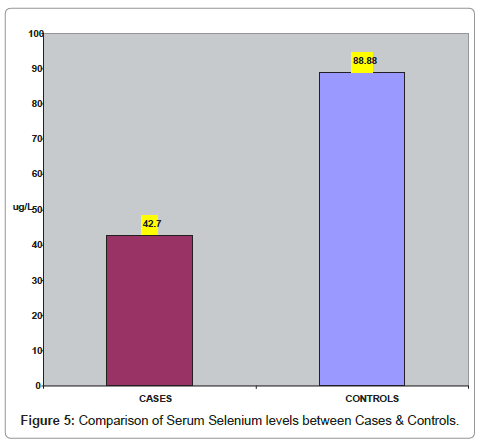
In (Table 5) comparison of serum selenium levels between cases & control subjects is shown. The mean selenium was 42.68 ± 11.07 ug/L in goiter cases 88.88 ± 10.39 in control subjects. There was significantly lower (p <0.001) selenium levels in goiter cases than the control subjects. (Figure 5) represents the comparison of serum selenium levels between cases & control subjects.
| Parameter | Cases(n=50) | Controls(n=50) | P-Value |
|---|---|---|---|
| Selenium (ug/L)Mean + SD | 42.68 + 11.07 | 88.88 + 10.39 | <0.001 |
Table 5: Comparison of Serum Selenium levels between Cases & Controls.
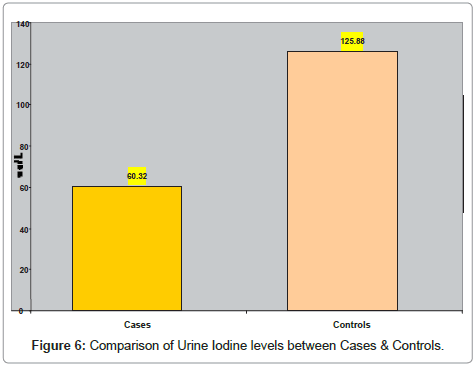
In (Table 6) comparison of urine iodine levels between cases & control subjects is shown. The mean urine iodine was 60.32 ± 20.47 ug/L in goiter cases and 25.8 ± 9.57 in control subjects. There was significantly lower (p <0.001) urine iodine level in goiter cases as against the control subjects. (Figure 6) represents the comparison of urine iodine levels between cases and control subjects.
| Parameter | Cases (n=50) | Controls(n=50) | P-Value |
|---|---|---|---|
| Urine Iodine (ug/L)Mean + SD | 60.32 + 20.47 | 125.8 + 9.57 | <0.001 |
Table 6: Comparison of Urine Iodine levels between Cases & Controls.
Discussion
The finding of present study that statistically T3 and T4 levels in goiter patients were within normal ranges with (mild raise TSH level & enlarged thyroid gland) indicates that the cause of enlarged thyroid gland in these patients is deficiency iodine in the diet, In mild iodine deficiency, small fraction of subjects having elevated TSH, usually the youngsters [8]. This is because abnormally enlarged thyroid gland can result either from under – or over production of thyroid hormones or from a deficiency of iodine in the diet [17].
The finding that iodine was excreted in significantly lower amounts in goiter patients than in the control subjects. Also suggests mild iodine deficiency to be the cause of goiter in these patients. According to WHO, UNICEF and International Council for the Control of Iodine Deficiency Disorder criteria, urinary iodine excretion in the range of 50 99 ug/L is indicative of mild iodine deficiency [18].
Like iodine, selenium is present in highest concentration in thyroid gland. It is essential for normal thyroid function and thyroid hormone homeostasis [12]. The finding that serum selenium levels were significantly higher in control subjects as against goiter patients suggests that selenium plays protective role via antioxidant defense system. Selenium is capable of exerting multiple actions on endocrine system by modifying the expression of at least 30 selenoproteins, Well-characterized selenoenzymes are the families of glutathione peroxidase, thioredoxin reductase, and iodothyronine deiodinase [13].
This is because selenium deficiency leads to a reduction of glutathione peroxidase an enzyme that detoxifies H2O2, which is abundantly produced in the thyroid gland as a substrate for the thyroperoxidase, [14,16]. Thyroperoxidase catalyzes iodide oxidation and its subsequent binding to thyroglobulin at tyrosyl residues and also the oxidative coupling of iodotyrosines into iodothyronines [11,14,15].
Increased generation of H2O2 caused by the high TSH associated with iodine deficiency, together with a loss of thyroidal selenoperoxidase activity due to concurrent selenium deficiency, produces marked thyroid atrophy [16]. Selenium deficiency can effects on thyroid hormone metabolism and possibly also on the thyroid gland itself [11,14,15].
Conclusion
From the present study it is concluded that mild iodine deficiency and selenium deficiency in the diet playing major role in the pathogenesis of goiter in plain areas of Sindh.
More research work is required to:
1) Investigate the dietary habits of goiter and non-goiter subjects.
2) Measure the levels of selenium in drinking water samples.
3) Evaluate the causes for differences in serum selenium levels between goiter and non-goiter subjects.
4) Find out the levels of selenoenzymes in goiter patients and control subjects.
References
- Knudsen N, Bülow I, Jørgensen T, Laurberg P, Ovesen L, et al. (2000) Comparative study of thyroid function and types of thyroid dysfunction in two areas in Denmark with slightly different iodine status. Eur J Endocrinol 143: 485-491.
- UNICEF (2001). Iodine Deficiency Disorders (IDD). Medical Herald (special supplement) 18: 4-11.
- World Health Organization. Iodine status worldwide WHO Global Database on Iodine Deficiency. Department of Nutrition for Health and Development. Geneva: World Health Organization 2004
- Micro Nutrient Initiative. Pakistan.
- Khand, FD, Awan MR, Choudhary MM and Memon R (2002) Prevelance of goiter in Hyderabad and adjoining areas. 3rd International and 13th National Chemistry Conference held in Karachi. Abstract P. 47.
- Assey VD, Greiner T, Mzee RK, Abuu H, Mgoba C, et al. (2006) Iodine deficiency persists in the Zanzibar Islands of Tanzania. Food Nutr Bull 27: 292-299.
- Akhter P, ur-Rehman K, Orfi SD, Ahmad N (2004) Assessment of iodine levels in the Pakistani diet. Nutrition 20: 783-787.
- Spitzweg C, Morris JC (2002) The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol (Oxf) 57: 559-574.
- Gaitan, E (1980) Goitrogens in the etiology of endemic goiter. In Endemic goiter and endemic cretinism. Iodine nutrition in health and disease. New York: John Wiley publ, USA.
- Delange F (2000) Iodine deficiency. In The thyroid. A fundamental and clinical text. Philadelphia: Lippincott publ.
- Vanderpas JB, Contempré B, Duale NL, Goossens W, Bebe N, et al. (1990) Iodine and selenium deficiency associated with cretinism in northern Zaire. Am J Clin Nutr 52: 1087-1093.
- Dickson RC, Tomlinson RH (1967) Selenium in blood and human tissues. Clin Chim Acta 16: 311-321.
- Beckett GJ, Arthur JR (2005) Selenium and endocrine systems. J Endocrinol 184: 455-465.
- Contempré B, de Escobar GM, Denef JF, Dumont JE, Many MC (2004) Thiocyanate induces cell necrosis and fibrosis in selenium- and iodine-deficient rat thyroids: a potential experimental model for myxedematous endemic cretinism in central Africa. Endocrinology 145: 994-1002.
- Thomson CD, McLachlan SK, Grant AM, Paterson E, Lillico AJ (2005) The effect of selenium on thyroid status in a population with marginal selenium and iodine status. Br J Nutr 94: 962-968.
- Contempre B, Denef JF, Dumont JE, Many MC (1993) Selenium deficiency aggravates the necrotizing effects of a high iodide dose in iodine deficient rats. Endocrinology 132: 1866-1868.
- Delange F (1994) The disorders induced by iodine deficiency. Thyroid 4: 107-128.
- WHO, UNICEF, and ICCIDD (1994) Indicators for assessing Iodine Deficiency Disorders and their control through salt iodization. Geneva:WHO publ. WHO/NUT.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14440
- [From(publication date):
June-2013 - Nov 15, 2025] - Breakdown by view type
- HTML page views : 9745
- PDF downloads : 4695