Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Study of Bacterium Fixation Stability on Gamma Radiation Synthesized Carriers to Improve Degradation
| Assia Djefal-Kerrar1*, Khalida Ouallouche Abdoun1, Ryma Chouikrat2, Yakout Guendouz El-Kahina2, Assia Nacer Khodja1 and Mohamed Mahlous1 | |
| 1Centre de Recherche Nucléaire d'Alger (CRNA), 2, Bd Frantz Fanon, Alger Algérie | |
| 2Faculté des Sciences Biologiques, Université des Sciences et Technologie Houari Boumediene, Alger Algérie | |
| Corresponding Author : | Dr. Assia Djefal-Kerrar Centre de Recherche Nucléaire d'Alger (CRNA) 2, Bd Frantz Fanon, Alger Algérie Tel: 213-21434444 Fax: 213- 21434280 E-mail: kerasdjef@yahoo.fr |
| Received April 05, 2011; Accepted April 07, 2011; Published April 09, 2011 | |
| Citation: Djefal-Kerrar A, Ouallouche-Abdoun K, Chouikrat R, El-Kahina YG, Nacer-Khodja A, et al. (2011) Study of Bacterium Fixation Stability on Gamma Radiation Synthesized Carriers to Improve Degradation. J Bioremed Biodegrad 2:117. doi:10.4172/2155-6199.1000117 | |
| Copyright: © 2011 Djefal-Kerrar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Hydrogels that are formed by gamma radiation, as a threedimensional network of macromolecules, are considered as stable insoluble matrices. They exhibit very interesting mechanical properties and are thus suitable for a wide range of applications.
| Keywords |
| Biodegradation; Anthracene; Naphthalene; Poly (vinylpyrrolidone), Cell immobilization, Adhesion |
| Introduction |
| Hydrogels that are formed by gamma radiation, as a threedimensional network of macromolecules, are considered as stable insoluble matrices. They exhibit very interesting mechanical properties and are thus suitable for a wide range of applications [1,2]. Furthermore, for the sake of increasing their performance, their surfaces can be modified in a controlled manner through grafting of side chains containing functional groups such as acrylic acid and acrylamide. Polyvinylpyrrolidone (PVP) is an example of polymers that are used for the synthesis of such hydrogels, which find applications in medicine, environment and biotechnology [3]. For those purposes, enzymes, antibodies, fungi and bacteria can be fixed on these crosslinked matrices providing thus an effective system of pollutants' treatment when dealing with pollution remediation issues [4,5]. |
| Polycyclic aromatic hydrocarbons (PAHs) are considered as environmental pollutants due to their recalcitrance, their potentially deleterious effects as well as their toxicity [6]. Among PAHs, both naphthalene and anthracene are classified by the US Environmental Protection Agency (EPA) as major recalcitrant toxic pollutants [6]. |
| Many biological agents such as bacteria are efficient in degrading those pollutants even when those agents are immobilized on inert matrices [7-10]. Fixed cells can grow, invade, and focus on large surfaces; this improves their activity [11]. |
| In the present work, the study of the adhesion of R. erythropolis B4 strain on three crosslinked matrices was implemented. The characterization of these materials and the determination of their immobilization rates have been performed. In addition, the reuse of this system was studied over two treatment cycles in order to assess the process performance. |
| Materials and Methods |
| Chemicals |
| Anthracene and naphthalene were obtained from Sigma-Aldrich. Poly (Vinylpyrrolidone) K90 with an average molecular weight of 360 000 Da was purchased from Fluka-Chemica. All used chemicals were of analytical grade. |
| Microorganisms |
| R. erythropolis B4 strain was obtained from the Microbiology Laboratory Collection, of the Science and Technology University of Algiers. It was maintained on nutrient agar (Difco) slants at 4°C. |
| Culture conditions |
| Tryptic Soy Broth (TSB) was used for the reactivation of the bacterial strain. A vitamin- supplemented mineral salt medium (MSM) containing respectively (per litre of distilled water) Na2HPO4 (4.5g), NH4NO3 (1g), KH2PO4 (0.68g), MgSO4 7H2O (0.1g), FeSO4 7H2O (0.001g), trace elements and vitamins [12] were used both for the free and the immobilized cell studies. |
| Naphthalene and anthracene at respective concentrations of 0.1 and 0.15%, 0.7 and 0.1% were dissolved in a minimum amount of ethanol for naphthalene and NN'dimethylformamide for anthracene before being added to the media as the only source of carbon. |
| Hydrogel matrices preparation |
| Polymeric hydrogels were crosslinked, grafted and sterilized in a single step by Gamma radiation induced crosslinking and by sterilisation according to the method reported previously [13]. The poly (vinylpyrrolidone) at a concentration of 7% w/v was dissolved in distilled water at 70°C overnight. Three matrices were prepared namely: poly (vinylpyrrolidone) PVP, PVP with different concentrations of acrylamide (AAm) (1-2, 8-4 and 7%) and PVP with different concentrations of acrylic acid (AAc) (1-4 and 7%). The different solutions were poured into plastic syringes, sealed with Parafilm and were then gamma irradiated to a dose of 25 kGy and a dose rate of 10.66 Gy / min. |
| Crosslinked gels were cut into discs of about 0.5 cm thickness and 1.2 cm diameter and maintained in sterile conditions until their utilisation. |
| Matrices characterization |
| Gel fraction: The gel fraction (Gel %) is the ratio of the dry gel weight (Wdg) to the initial weight of the polymer (Wip) according to the formula: |
| % Gel = (Wdg/Wip) X 100 (1) |
| Swelling measurements: Swelling kinetics was investigated through measurements of the hydrogels' water absorption as a function of time. Hydrogel samples were cut into cylindrical pieces and placed in deionised water at room temperature. The mass of the swollen gel was measured at different time intervals until swelling equilibrium was reached. The water uptake was calculated according to the formula: |
| Wat = (Wst – Wo) / Wo X 100 (2) |
| Where: |
| Wat is the weight of water absorbed by the hydrogel at time t, Wst is the weight of the swollen hydrogel at time t and Wo is the initial weight of the hydrogel sample. |
| Grafting rate: Grafted hydrogel samples were immersed into acetone during 1 minute in order to precipitate acrylic acid and acrylamide monomers which were not grafted. Samples were then dried until constant weight is reached. Grafting rate Gr (%) was calculated by means of the following formula: |
| Gr = Wd / Wi X 100 (3) |
| where Wd is the weight of dry gel and Wi is the initial weight of gel sample. |
| FTIR spectroscopic analysis: Samples for Fourrier Transformée Infra Red (FTIR) spectroscopic characterization were prepared by grinding 1 mg of dry blends with 100 mg of KBr and compressing the mixtures to form pellets. The FTIR spectra of samples were measured with a Nicolet 380 spectrometer in the wavelength range of 4000 to 400 cm-1. |
| Immobilization of bacteria |
| 2 ml of a suspension cell containing about 108 CFU (colony forming units)/ml were mixed with hydrogel discs into 50 ml of sterile vitaminsupplemented MSM. The mixture was incubated at 4°C under gentle shaking. After 24 h, the discs were removed and washed with distilled water to remove non adherent cells. |
| Determination of the immobilization rate |
| Free and non-adherent cells of R.erythropolis were enumerated by means of the plate count method on plate count agar medium (PCA). The plates on duplicates were inoculated with 0.1 ml of several dilutions. The average colony forming unit was determined after 48 h of incubation period at 30°C. The number of immobilized cells and the immobilization rate are then determined. |
| Scanning electron microscopy |
| The fixation of the cells on radiation crosslinked poly(vinylpyrrolidone) hydrogel matrix was observed making use of an environmental scanning electron microscope, i.e. Philips ESEM XL 30 FEG. The matrices containing cells were fixed with 2.5 % (v/v) glutaraldehyde in a saline solution for 1 hour, then rinsed with water and then sequentially dehydrated by immersing them for 10 min in increasing concentration of ethanol solutions (10%, 50%, 80%, 100%). Subsequently, the specimens were freeze-dried and then coated with gold. |
| Degradation of PAHs by free and immobilized cells |
| The PAHs degradation capacity of free and immobilized bacteria cells was experienced in MSM containing naphthalene or anthracene as the sole carbon source and energy. The PAHs concentrations used were 0.7 and 1 g/l for anthracene and, 1 and 1.5 g/l for naphthalene. 2 ml of free cells suspension and 30 hydrogel discs containing fixed cells were used separately to inoculate the culture media. Incubation was performed at 30°C under continuous shaking during 10 days. Periodically, samples were aseptically collected from the culture flask for High Performance Liquid Chromatography (HPLC) analysis. |
| Reusability of the immobilized cells |
| To evaluate the reuse potential of radiation synthesized matrices immobilized cells, PVP based hydrogel discs containing fixed cells were removed from the first culture broth, were washed with a physiological saline solution and then transferred to a fresh culture medium. Two repeated degradation tests were carried out at the same operating conditions as in the first cycle. |
| Analyses |
| For both free and fixed cells experiments, remaining naphthalene and anthracene were determined by means of HPLC using a UV detector at a wavelength of 254 nm with a Waters 2487 system. Suitable aliquots of samples were clarified by centrifugation and afterwards liquid-liquid extracted by means of equal volume of cyclohexane for 2 h while shaked at 150 rpm. 20 µl samples were analysed using a spheri-PAK-C18 column and an elution flow rate of 1 ml/min (water/ acetonitrile 25/75 v/v). |
| Results and Discussion |
| Hydrogels characterization |
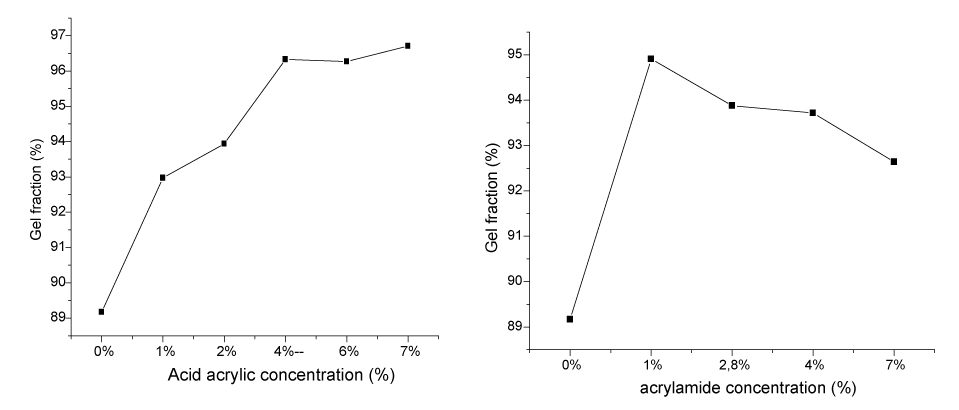
| Gel fraction: Gel fractions of PVP/AAc and PVP/AAm hydrogels plotted as a function of concentrations are illustrated in Figure 1. |
| For PVP/AAc hydrogel, gel fraction increases with increasing concentrations until 4 %. Beyond that percentage, the gel fraction remains stable. This means that acrylic acid, plays the role of crosslinker and leads to the formation of bridges between macromolecular chains until a given high crosslinking degree is reached. For PVP/ AAm hydrogel, the gel fraction increases until 1 % of acrylamide then decreases beyond this concentration. This can be explained by the polymerization of acrylamide in such a way that we obtain a PVP co-polyacrylamide complex. |
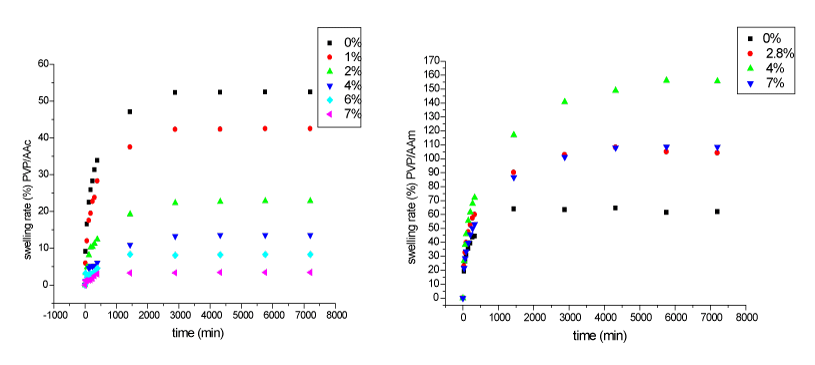
| Swelling kinetics: The swelling behavior of PVP/AAc and PVP/ AAm versus acrylic acid and acrylamide concentrations is represented in Figure 2. |
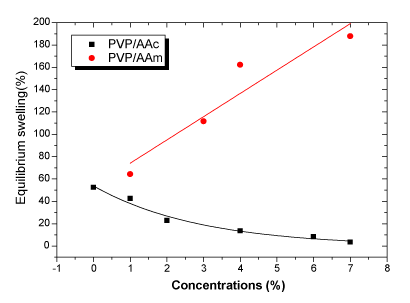
| The equilibrium swelling rates versus concentrations relationship is represented in Figure 3. The water uptake is found to decrease for acrylic acid grafted matrix. This behavior can be explained by the existence of an important crosslinking density which is in accordance with an increase in the gel fraction from 89.17 % to 96.71 %. |
| For acrylamide, water uptake increases with increase of concentration as a result of the co- polymerization of acrylamide which gives a low crosslinking density. |
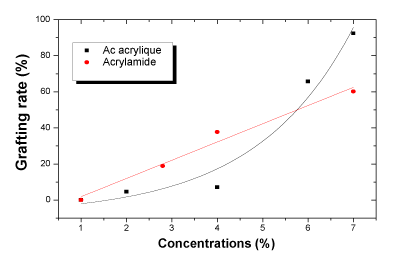
| Grafting rate: The degree of PVP/AAc and PVP/AAm grafting is given in Figure 4. The degree of grafting increases with polymers' increasing concentrations. It is however more important for acrylic acid (92.44%) than for acrylamide (60.14%). This may be explained by the co-polymerization of acrylamide which reduces the formation of the crosslinking network with PVP. |
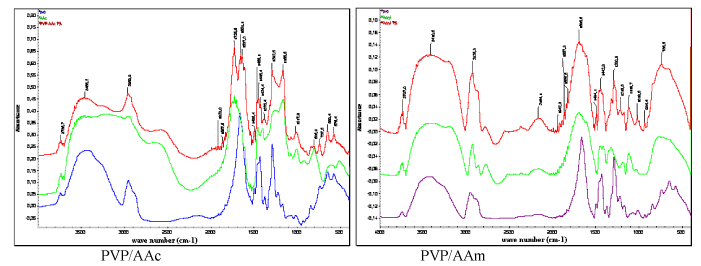
| FTIR analysis: PVP/AAc and PVP/AAm FTIR spectra are illustrated in Figure 5. Acrylic acid shows the typical band of the carbonyl function at 1725.6 cm-1 while it is absent on the spectrum of PVP. The acrylamide amino function absorption band clearly appears at 3410.8 cm-1. On another hand, the absorption band of C=O which is present in AAm, PVP and PVP/AAm spectra becomes visible at 1695.6 cm-1. |
| Immobilization rates |
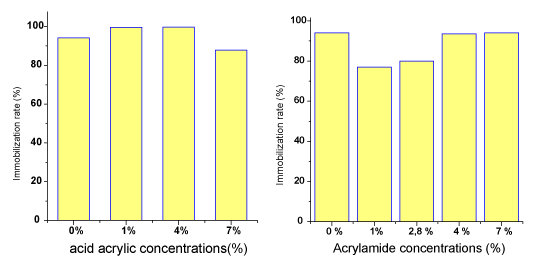
| The immobilization rates of Rhodococcus erythropolis on crosslinked PVP/AAc and PVP/AAm are illustrated in Figure 6. |
| The immobilization rates are high in all cases. They exceed 90 % for PVP alone (94 %) then for PVP grafted with acrylic acid (99 %) or PVP grafted with acrylamide (93%). The nature of the matrices does not seem to play a major role in the bacteria cell adhesion, which is practically the same in all cases. Moreover, the basic PVP carrier can be the most appropriate. Such high immobilization rates on the different matrices reflect the adhesion ability of Rhododcoccus erythropolis thanks to the extracellular structures such as exopolysaccharides fibers [14]. |
| Fixed cells observed on ESEM microphotographs are illustrated in Figure 7. One can observe the presence of a fiber network forming a film and covering the bacteria cells, which are attached onto the surface supports. These fibers seem to be responsible of forming a stable monolayer of cells [15], where some cells were even encapsulated [9]. The presence of these extracellular structures has been induced by the attachment of cells onto the inert surfaces [16]. |
| Moreover, there is a close relationship between the adhesion phenomenon and a wide variety of parameters such as porosity, water content of the polymer matrix, nature of the surface (electrical charge, flexibility, roughness, hydrophobicity) as reported in [1]. |
| Influence of immobilization on biodegradation |
| The strain R. erythropolis grows well in minimal medium containing naphthalene or anthracene as sole source of carbon and energy. Its ability to degrade these PAHs has been well demonstrated in previous study [7]. |
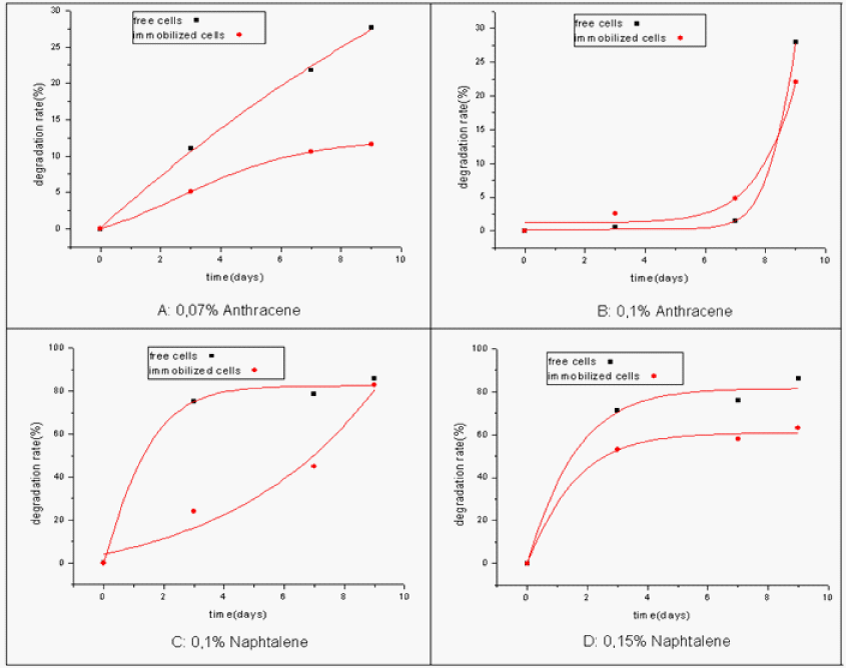
| The rate of degradation by immobilized cells was found less important than that by free ones (Figure 8). However, the fixed cells remain active and 83% of naphthalene degradation was obtained in the fixed system whereas 86% were noted for the free one. We noticed the same thing both for anthracene and naphthalene at all the concentrations. |
| This little decrease in biodegradation by immobilized cells can be explained by diffusional limitations of carbon sources [17] that are imposed by the porosity of the gel matrix but also by the impact of the biomass accumulation [18]. |
| Reuse experiments |
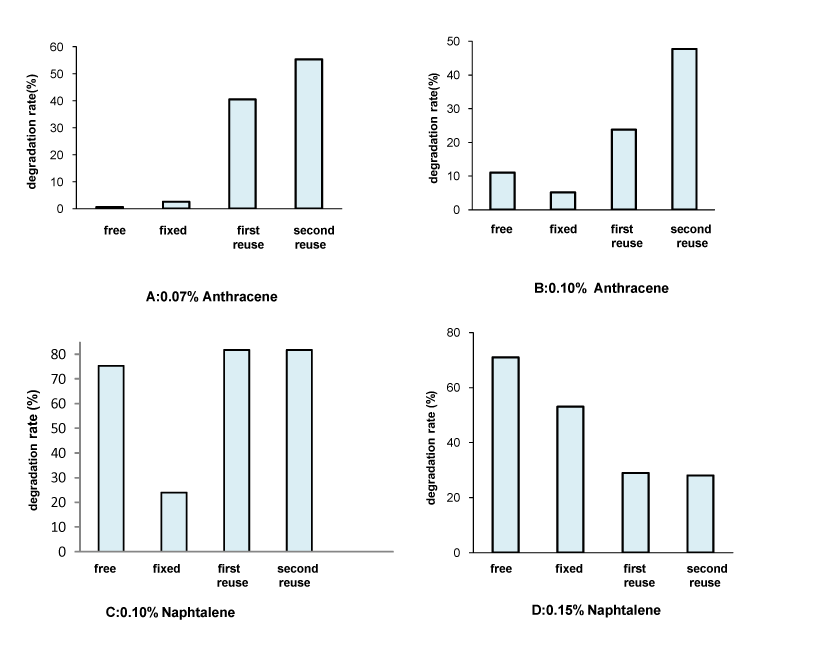
| It was observed that bacteria cells fixed on radiation crosslinked PVP hydrogel remained pretty well active. The matrices do not affect the microbial activity [7] .The results of the reused immobilized cells as compared to those with the free and the immobilized ones for PAHs degradation are given in Figure 9. |
| One can notice that the reused immobilized cells have a better capability to degrade PAHs. The above-mentioned process is highly increased by the repeated use. Degradation rates obtained after only two days of incubation with reused cells were higher than those obtained after 9 days of first use. This was most likely due to the prolonged acclimatization of the cells towards the contaminants which rather improves the degradation efficiency of the culture [19]. Moreover, the efficiency of hydrocarbons' oxidation depends on the hydrocarbons-adsorbing capability for the carrier [20]. It has thus been suggested that there is a direct relationship between cell surface hydrophobicity and the initial irreversible adhesion onto surfaces [14]. These lead to an increased availability of the substrates for the cells and a better interaction between the substrates and the immobilized cells [21]. The efficiency of immobilized cells repeated use has been reported by several authors [21,22], who demonstrated that recycling experiments gradually lead to increased degradation activity. |
| Conclusion |
| In the present work, we have shown that grafting monomers on PVP could increase the area that is colonized by bacteria. On the other hand, it did however not significantly improve immobilization rates. The observed rates are fairly comparable in the three different supports. Furthermore, recycling runs lead to gradually increased degradation activity. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 |
Post your comment
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13232
- [From(publication date):
June-2011 - Jul 18, 2024] - Breakdown by view type
- HTML page views : 8898
- PDF downloads : 4334
