Research Article Open Access
Structural and Functional Characterization of a Soil Microbial Community which is Able to Convert Waste Cooking Oil to Fatty-acid-Derived Fuels
| Xuanyu Tao1, Li Yan1, Zhengsheng Yu1, Mengxin Zhao2, Xiaowei Zhang1, Pu Liu1 and Xiangkai Li1* | |
| 1MOE Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou, Gansu, 730000, China | |
| 2State Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing, 100084, China | |
| Corresponding Author : | Xiangkai Li Key Laboratory of Cell Activities and Stress Adaptations School of Life Sciences Lanzhou University Gansu, 730000, PR China Tel: 86-931-8912561 Fax: 86-931-8912560 E-mail: xkli@lzu.edu.cn |
| Received May 24, 2013; Accepted August 23, 2013; Published August 25, 2013 | |
| Citation: Tao X, Yan L, Yu Z, Zhao M, Zhang X, et al. (2013) Structural and Functional Characterization of a Soil Microbial Community which is Able to Convert Waste Cooking Oil to Fatty-acid-Derived Fuels. J Bioremed Biodeg 4:201. doi:10.4172/2155-6199.1000201 | |
| Copyright: © 2013 Tao X, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Waste Cooking Oil (WCO) is classified as waste material and harmful to the environment and human health. Searching a cost-effective and eco-friendly approach to recycle WCO is urgent in China. In this study, three soil samples were collected and their WCO degradation ability was investigated. Gas Chromatography-Mass Spectroscopy (GC-MS) analysis revealed that a soil sample collected near a restaurant disposal site was able to convert WCO to fatty-acid-derived fuels after 90 days’ anaerobic fermentation. The calorific value of the fermentation products increased by 17.2%. 16S meta sequencing data showed that this microbial community has a unique structure. Proteobacteria was the most abundant microbial phylum representing 60.4298% of the whole community. The percentage was almost three times higher than that in the other two microbial communities which cannot degrade WCO. Magnetospirillum, a genus of Proteobacteria, was much more abundant than the other genera in this phylum, accounting for 11.2% of the total population. The unusual community composition might correlate with its ability of WCO degradation and Proteobacteria phylum and Magnetospirillum genus may play key roles in the decomposition of WCO. To our knowledge, this is the first finding that a microbial community is able to convert WCO to fatty-acid-derived fuels, which might provide an alternative approach of reprocessing WCO.
| Keywords |
| Waste cooking oil; Degradation; Soil microbial community; Fatty-acid-derived fuels |
| Introduction |
| The consumption of edible oils is twenty one million tons each year in China, producing six million tons of Waste Cooking Oil (WCO) [1]. Most of the WCO is not rationally recycled or utilized [2]. Some of them is directly dumped into environment which causes atmospheric pollution and water contamination [3,4]. Some factories illegally resell WCO as edible oils after simple treatments. As WCO contains aflatoxins which is known as carcinogen and other toxic substances, such as heavy metal and peroxide compounds, WCO is toxic and hazardous to human health. Long-term consumption of WCO can cause hepatic and renal diseases [5-7]. Therefore, seeking efficient approaches to process WCO for avoiding damaging human health and environment contamination is urgent in China [7]. |
| Nowadays, many countries in the world are facing energy crisis [8]. A recent survey indicates that well-accessible sources will be exhausted in 50-70 years [9]. Fatty-Acid-Derived Fuels (FADF) are one of the promising crude oil substitutes, composed of fatty acids and synthesized by chemical catalysis. Utilization of FADF can reduce the carbon dioxide emission compared with fossil energy. Additionally, FADF are biodegradable, non-toxic, and essentially free of sulfur and aromatic components [10,11]. FADF are the major products in the biofuel market, which have already been utilized as the first generation biofuels to substitute gasoline or diesel [12]. |
| The conventional process to generate FADF is through transesterification [13,14]. Homogeneous basic and acid catalysts are the most commonly used catalysts in industries [15-17]. However, both acid and basic catalysts need neutralization and separation from the reaction mixture, which will cause environmental problems as waste water is generated during the process [18]. Previous data have shown that for every100 liter FADF produced, 20 liter waste water will be generated [19]. Moreover, to produce one ton of FADF, 50kW of electricity is needed, of which 60-70% is consumed in glycerin refining [20]. These are the obvious disadvantages of conventional chemical processing. Biotechnological method to degrade WCO could be an alternative to conventional chemical process. Not only might this method reduce the cost of catalysis process, but also it is ecofriendly [9,21]. Additionally, WCO is toxic to normal bacteria, such as acetogens and methanogens, and prevents anaerobic metabolism of many bacteria [22-24]. Thus, it is difficult to construct an engineered strain which has the abilities of tolerating and degrading WCO. |
| In this study, a microbial community from a restaurant dumping site soil demonstrated ability to convert WCO to FADF. The calorific value of degradation products has a significant increase. Additionally, 16S meta analysis indicated that phylum Proteobacteria was three times more abundant than the controls. And genus Magnetospirillum of Proteobacteria, accounting for 11.2 % of the total population, was more abundant than other genera. It suggested that the unique community structure might play an important role in the process of converting WCO. To our knowledge, this is first report of utilization of microbial community to convert WCO to fatty-acid-derived fuels. |
| Materials and Methods |
| Soil sample collection |
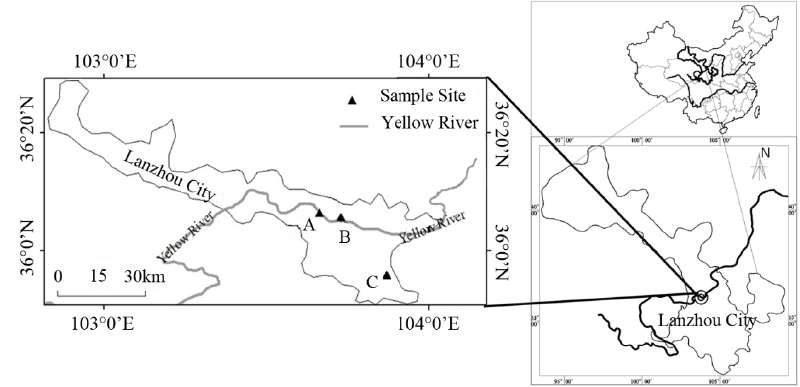
| Soil samples were collected from three sites designated A, B, and C in the city of Lanzhou (Figure 1). Site A soil sample was collected from 7 cm below surface at 17°C in an area where industrial effluent was drained into the Yellow River. Soil sample from site B was collected from 7 cm below surface at 17°C near the bank of the Yellow River. Soil sample C was collected near a waste oil draining site from 7 cm below surface at 14°C. All samples were transported to laboratory on ice and stored at 4°C until analyzed. |
| Measurement of soil physicochemical parameters |
| Samples were measured by standard gravimetric techniques to determine the water content [25]. Soils were then air dried and sieved through 150μm filters to measure Soil pH, organic carbon, available nitrogen, and phosphorus following standard methods [26]. Total concentration of metals (K, Ca, Mg, Cd, Cu, Cr, Pb and Mn) in soil samples were determined using Varian AA240 following digestion with a mixture of HNO3/HClO4 [27,28]. |
| Genomic DNA extraction |
| Genomic DNA was extracted from each soil sample using ‘Soil DNA Kit’ (OMEGA BIO-TEK, USA) according to the manufacturer’s instructions. DNA quality was estimated by using a NanoDrop ND- 2000 spectrophotometer (Thermo) to measure the absorbance ratios of A260/A280 and A260/A230. Then the purified DNA was dried by CentriVap DNA Concentrator (Labconco) and stored at -20°C for further analysis. |
| 16S meta sequencing |
| Pyrosequencing of V4-V5 hypervariable regions of 16SrRNA was performed by 454 FLX System (Roche) in the Beijing Genomics Institute. 16S meta sequencing results were analyzed by QIIME software. Software mothur was used to perform all samples’ reads OTU clustering analysis, and the similarity of 97% sequences was clustered into one OTU [29,30]. |
| WCO degradation |
| WCO was purchased from a restaurant in city of Lanzhou. WCO was mixed with each of the three soil samples (1:1,v/wt) in the fermentation cylinder at 28°C for 90 days. WCO was fermented with sterilized soil for 90 days as controls. |
| Gas Chromatography-Mass spectroscopy (GC-MS) analysis |
| Each oil sample which was from WCO degradation tests was mixed with hexane and 0.5 mol/l KOH-CH3OH solution in a volume ratio of 1:4:2. The mixture was incubated in 70°C water bath for 10 min and 10 ml distilled water was added. The mixture was then fully mixed by ultrasonification and centrifugation, and prepared for GC-MS that dried with sodium sulfate [31,32]. GC-MS was performed as described before. Qualitative results of used cooking oil were obtained from the GC-MS library [33]. |
| Analysis of calorific value |
| After fermentation for 90 days, the supernatant from the fermentation cylinder was transferred and sealed in medicinal capsules. After centrifugation the capsules were put in combustionspoons, and 15 to 25 cm of Nichrome wire was attached to the electrical terminals of a standard oxygen bomb calorimeter, and 45 ml distilled water was added to the calorimeter. Nichrome wire was then ignited, and the change of temperature was recorded by temperature sensor. Finally, calorific value of each sample was calculated [34]. |
| Data analysis |
| 16S meta sequencing data were further analyzed by different statistical methods: ( i ) microbial diversity indices were analyzed by PAlaeontological Statistics (version 2.00, http://folk.uio.no/ohammer/past/) [35]; (ii) Detrended correspondence analysis (DCA), hierarchical clustering of communities and canonical correspondence analysis (CCA) were performed using R program (version 2.15.2, http://cran.rproject.org/) [36]. |
| Results |
| Different physicochemical parameters of three soil samples |
| Soil physicochemical parameters and total heavy metal content were summarized in Table 1. The soil texture was different from each other. Total concentrations of heavy metals in site A and B were higher than those in site C, especially the content of cadmium, chromium and copper in sample A, as site A was closed to industrial heavy metal discharge sites. The organic carbon content in sample C was almost six times higher than that in sample A and B, which may be derived from the cooking oil sorbed in the soil of site C. |
| Change of WCO composition and increase of calorific value after fermentation |
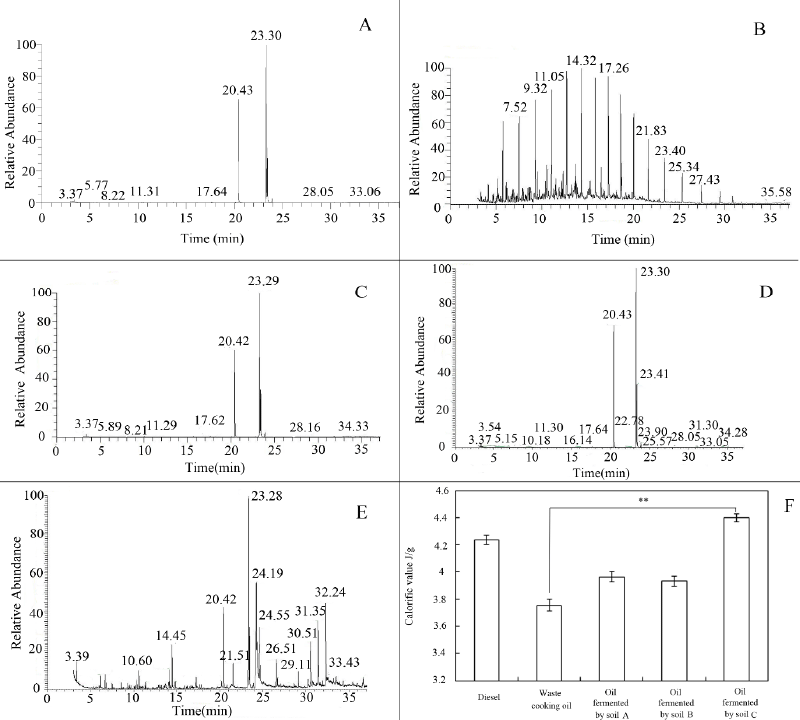
| To evaluate the composition of waste cooking oil, GC-MS was performed.As demonstrated in Figure 2A and 2B, the WCO before microbial degradation was mainly composed of hexadecanoic acid and octadecadienoic acid, whose composition percentages were 26.69% and 52.29% respectively (Supplementary table 2A), and the main components of diesel were alkanes (Supplementary table 2B). After incubation with soil microbes for 90 days, the components of waste oil with soil sample A and B were almost unchanged, and the composition percentages of hexadecanoic acid and octadecadienoic acid were still around 25% and 50% (Figure 2C and 2D and Supplementary Table 2C and 2D). However, the components of oil degraded by soil C were very different from those of the primitive waste oil. The degradation products contained many new alkanes existing in diesel, such as undecane and dodecane (Figure 2E and supplementary Table 2E), and the percentages of hexadecanoic acid and octadecadienoic acid decreased to 4.27% and 34.58% respectively and the composition of WCO treated with sterilized soil samples of site A, B and C remained unchanged (data not shown). Altogether, it demonstrated that the changes of WCO composition are mainly due to the combined activities of microbial communities in soil sample C rather than chemical interaction. |
| After 90 days of incubation, the calorific value of oil fermented by soil C showed a significant increase by 17.2% compared with primitive WCO (Figure 2F). This result, combined with the composition analysis of fermentation products, suggested that the microbial community in soil sample C were able to degrade WCO into biodiesel. |
| Microbial community structural differences in three soil samples |
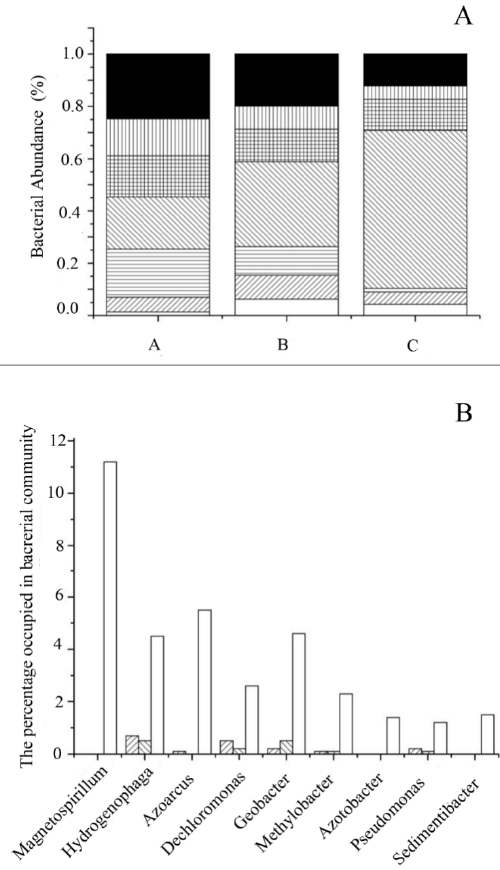
| To analyze the composition of microbial community in the soil samples, 454-pyrosequencing was performed. The distribution of bacteria in soil sample A, B and C was different from each other. The populations of Actinobacteria, Bacteroidetes, Firmicute and Chloroflexi in soil C were less than those in A and B, especially Chloroflexi, which almost did not exist in soil C with a percentage of 1.438%. In contrast, Proteobacteria represented the most abundant microbial phylum in soil C with a percentage of 60.4298%, which was about as three times as that in soil A (Figure 3). |
| To further analyze the structural differences of microbial community in three soil samples, the differences at genus level were compared among soil A, B and C (Figure 4). The population percentages of Magnetospirillum, Hydrogenophaga, Azoarcus, Dechloromonas, Geobacter, Methylobacter, Azotobacter, Pseudomonas, and Sedimentibacter in sample C were very high, while these numbers were low in sample A and B, for example, the percentages of genus Magnetospirillum, Azotobacter and Sedimentibacter were 0%. All these genera belonged to phylum Proteobacteria except for Sedimentibacter, which belonged to phylum Firmicute. It’s worth noting that the quantity of genus Magnetospirillum in sample C was very high, with a percentage of 11.2%, suggesting that this genus may play an important role in oil degradation. Although there are differences at phylum and genus level, the diversity indices of communities in soil A, B and C were very close. Shannon's diversity index and Simpson's diversity index of the three soil samples were similar (Table 2). |
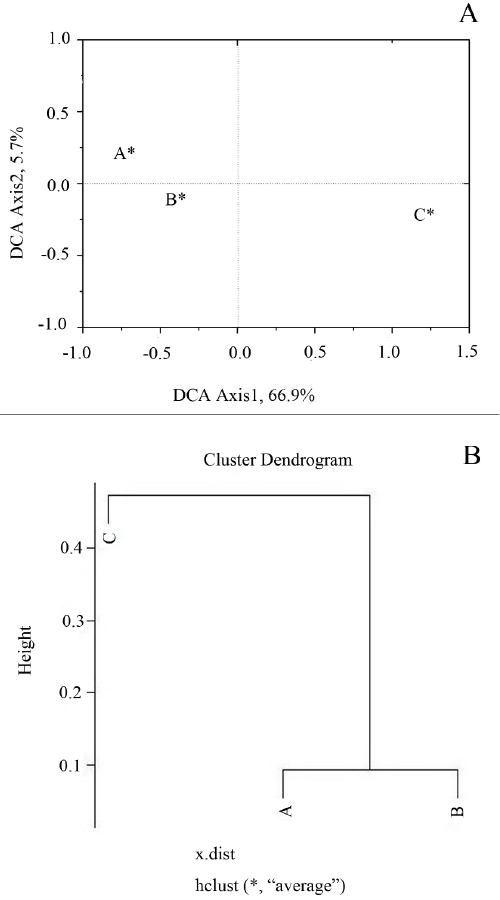
| Detrended correspondence analysis (Figure 4A) and hierarchical clustering analysis (Figure 4B) were performed to evaluate the similarity of the microbial community among different samples. The results indicated that the microbial communities in sample A and B shared a higher similarity, and the community in sample C had more differences. As soil A and B were collected in areas both contaminated by long- time heavy metal exposure, and soil C was collected near restaurants, where soil was contaminated by WCO, the results suggested that the structure of soil microbial communities could change dynamically in response to environmental stresses. |
| Functional analysis of relationship between microbial community and environmental variables |
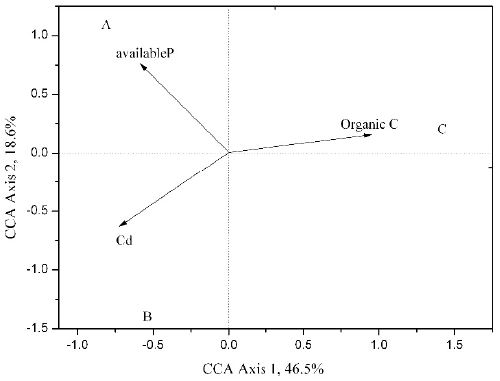
| Canonical correspondence analysis (CCA) was applied to discern possible relations between microbial community and physicochemical parameters of soil (Figure 5). Initially, correlations between ten environmental variable (organic carbon, available nitrogen, available phosphorus, pH, K, Ca, Mg, Fe, Cd, Cr and Cu) and microbial community were analyzed in CCA. Variance inflation factors analysis demonstrated that the factors of five of the ten variables were greater (data not shown), indicating that there was significant correlations between those five environmental variables and microbial communities. Rest of the variables were further selected based on P-value analysis, and finally three variables (organic carbon, available phosphorus and cadmium) in CCA model explained 65.1% of the relationship between environmental variables and microbial communities. In the CCA model, the first canonical axis was positively correlated with organic carbon and negatively correlated with cadmium and available phosphorus. The second axis was negatively correlated with cadmium. The length of the biplot showed the importance of each environmental variable [37]. For instance, organic carbon may be the most important environmental variable to the community in soil sample C, whereas the community in soil B was positively correlated with cadmium and negatively correlated with organic carbon. |
| Discussion |
| At present, how to properly dispose WCO becomes a serious problem in China [38]. The traditional way to process WCO is chemical transesterification, whereas this approach is comparatively expensive and energy intensive, and may also easily cause secondary pollution [20]. In this study, we used soil microbes to degrade WCO, which might be a potentially more efficient alternative to chemical method. |
| Initially, we collected three different soil samples and analyzed their physicochemical parameters (Table 1). As described in the results, the physicochemical parameters and total heavy metal content of the three soil samples were different from each other. The concentrations of heavy metals in soil A and B samples were higher than those in soil C sample, which may be correlated with the collection sites. Soil A sample was collected from an industrial waste water discharge site [39]. Soil B sample was collected near the bank of the Yellow River, where the river may also contaminated by heavy metals since multiple heavy industrial plants are located along the upstream of the river. Soil C sample was collected near restaurants and might be contaminated by WCO. It is known that environments have huge impact on the soil microbial community structure and function [40]. The microbial community has to adapt to the local environment to survive and develop the ability to remediate the stress [41]. It has been reported that dispersed hydrocarbon plume in deep sea can select for those oil-degrading microbes [42]. Therefore, we speculated that the indigenous bacteria in the soil contaminated by catering waste could develop an ability to degrade WCO and use it as carbon source, which was demonstrated in our fermentation experiment. GC-MS was applied to determine the chemical composition of the oil before and after fermentation. The result showed that primitive WCO were mainly composed of hexadecanoic and octadecadienoic acid, while the degradation products in sample C were similar to diesel. Additionally, the caloric value of the fermented oil in site C sample was 17.2% higher than unfermented WCO and almost equal to diesel, indicating that the microbes in sample C were able to efficiently convert WCO to fatty acid-derived fuel, a substitute of fossil oil [10]. This implied that high concentration of oil in the soil changed the microbial community structure, and enriched those bacteria that could utilize WCO better. |
| 16S meta sequencing was performed to analyze the structure of microbial community in the three different soil samples to reveal whether the structure is related to the WCO degradation ability. |
| Through the analysis of the abundance of bacteria in soil sample, phylum Proteobacteria in soil C sample were rich with a percentage of 60.43%, which is consistent with the previous report that the proportion of phylum Proteobacteria in marine microbial community in the oil-contaminated sea sediments had a significant increase [42]. On the other hand, phylum Chloroflexi, was barely detectable in the microbial community of soil sample C, but relatively rich in A and B, which is in accordance with previous studies, that Chloroflexi can be found in heavy-metal-contaminated soil [43-45]. Furthermore, as described in the analysis of genus differences of soil samples, the percentages of Magnetospirillum, Hydrogenophaga, Azoarcus, Dechloromonas, Geobacter, Methylobacter, Azotobacter, Pseudomonas, and Sedimentibacter in microbial community in sample C were higher. As reported in previous studies, these nine genera all have the abilities of degrading aromatic compounds and hydrocarbons, such as benzene, toluene, ethylbenzene, xylene hydroxyl benzene and polychlorinated biphenyls [46-53]. |
| GC-MS indicated that the oil degraded by sample C contains different kinds of alkanes. However, the process of converting WCO to alkanes is still not clear. In a previous study, cyanobacteria were proven to have the abilities of converting fatty acid to alkanes and alkenes by an acyl-acyl carrier protein reductase and an aldehyde decarbonylase [54]. Therefore, one or more genera in those nine genera might have the abilities of decarboxylating and secreting alkanes, and the others might be able to breakdown long chain alkanes or utilize alkanes as carbon source, such as Azoarcus, which was abundant in sample C [55]. The process of producing varieties of alkanes is complicated and involves different microorganisms instead of one single strain. Further studies are needed to find out which genus has the ability of decarboxylating. Among those nine genera, Magnetospirillum was more abundant than others. It is known that members of genus Magnetospirillum play a key role in the anaerobic breakdown of aromatic compounds through two key enzymes, benzylsuccinate synthase and benzoyl-CoA reductase [51,56,57]. Thus, we speculated that genus Magnetospirillum may play an important role in the process of degrading WCO, especially decarboxylating. Isolation of Magnetospirillum or related organisms from this community to determine their anaerobic oil degradation ability requires future study. |
| At the same time, CCA of the three soil samples was performed to find out whether some environmental variables can affect the structure of microbial community. The CCA model indicated that the community in soil sample C was positively correlated with organic carbon, which may be derived primarily from the catering waste oil soaked into the soil. While in site A and B, there was far less oil, which might be the reason that the microbe communities of soil samples A and B were much different from those of soil sample C, agreeing with the results of detrended correspondence analysis and hierarchical clustering analysis. |
| Our experiments suggest that a microbial community instead of a single strain might be an attractive reagent to reprocess WCO. And this is the first report exemplifying the ability of a microbial community to convert WCO to alkanes. Lanzhou is located in the northwestern China which has typical geographic location and climate [58]. The structure of soil microbial community investigated may also provide guidance on exploring the co-operation of different strains in degrading WCO or long chain fatty acid in northwestern China. |
| Acknowledgements |
| This work was supported by National Natural Science Foundation grant 31200085, PRC and Hui-Chun Chin and Tsung-Dao Lee Chinese Undergraduate Research Endowment. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14339
- [From(publication date):
August-2013 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 9568
- PDF downloads : 4771
