Research Article Open Access
Spectrophotometric Estimation of Metaxalone and Diclofenac Potassium by Multicomponent Analytical Method from Tablet Dosage Form
Chintan R Patel1, Ritu V Kimbahune2, Prachi V Kabra1*, Harish AR3 and Nargund LVG1
1Department of Quality Assurance, Nargund College of Pharmacy, Karnataka, India
2Department of Quality Assurance, Al-Ameen College of Pharmacy, Karnataka, India
3Department of Pharmaceutical Analysis, S.A.C. College of Pharmacy, Karnataka, India
- *Corresponding Author:
- Prachi V Kabra
Department of Quality Assurance
Nargund College of Pharmacy
Dattatreya Nagar, II-main, 100ft ring road
BSK IIIstage, Bangalore-85
Karnataka, India
E-mail: prachi.v.kabra@gmail.com
Received date: May 28, 2012; Accepted date: July 13, 2012; Published date: July 16, 2012
Citation: Patel CR, Kimbahune RV, Kabra PV, Harish AR, Nargund LVG (2012) Spectrophotometric Estimation of Metaxalone and Diclofenac Potassium by Multicomponent Analytical Method from Tablet Dosage Form. J Anal Bioanal Tech 3:137. doi: 10.4172/2155-9872.1000137
Copyright: © 2012 Patel CR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple Multicomponent Spectrophotometric method for simultaneous estimation of Metaxalone and Diclofenac Potassium in combined tablet dosage form had been proposed. Considering the absorption spectra of both analytes in the range of 220-350 nm in methanol, the four wavelengths of equal intervals of 40 nm were selected as 350 nm, 310 nm, 270 nm and 230 nm to produce calibration curves of mixture of analytes. The present method used inbuilt application of instrument (Shimadzu Pharm Spec 1700, UV spectrophotometer) to quantify Metaxalone and Diclofence potassium in formulation. The method had been validated in accordance with ICH guidelines for accuracy and precision.
Keywords
Metaxalone; Diclofenac potassium; Multi component
Introduction
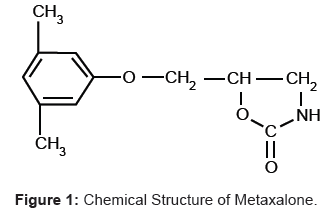
Metaxalone (MET) is a muscle relaxant used to relax muscles and relieve pain caused by strains, sprains, and other musculoskeletal conditions. Chemically MET is 5-[(3, 5-dimethylphenoxy) methyl]-1, 3-oxazolidin-2-one (Figure 1). It is a white to almost white, odorless crystalline powder freely soluble in chloroform, soluble in methanol, 96% ethanol, deionized water and in propylene glycol, but practically insoluble in ether and water [1].
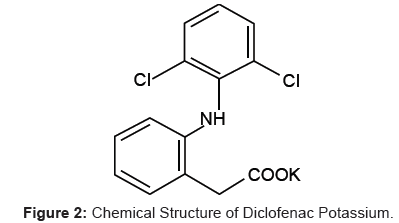
Diclofenac Potassium (DP), a Non-Steroidal Anti-Inflammatory Drug (NSAID), is 2-[(2, 6-dichlorophenyl) amino] benzene acetic acid, monopotassium salt (Figure 2). It is a faintly yellowish white to light beige, virtually odorless, slightly hygroscopic crystalline powder. It is freely soluble in methanol, soluble in ethanol, sparingly soluble in water and practically insoluble in chloroform and in dilute acid [2]. It is used to reduce inflammation and as an analgesic reducing pain in conditions such as arthritis or acute injury.
Metaxalone (400 mg) and Diclofenac potassium (50 mg) is available in combination as tablet dosage form and prescribed for muscle relaxant and pain relief. Literature survey reveals that few analytical methods like chromatographic methods [3,4], RP-HPLC [5,6] are available to determine MET and DP individually or in combination with other drugs. An attempt has been made to develop and validate new UVVisible spectrophotometric method for simultaneous estimation MET and DP in tablet dosage form.
Materials and Methods
Instrument
A Shimadzu UV- Visible double beam spectrophotometer model 1700 (Japan) with 1 cm matched quartz cells connected to a PC computer running UV-Probe 2.32 software for absorbance measurements and treatment of data was used along with Sartorius digital balance for weighing.
Chemicals and reagents
The drug samples of MET and DP were obtained from Ra chem Pharma Ltd, Hyderabad and Wexford Laboratories Pvt Ltd, Bangalore respectively. Tablets containing MET and DP (Myospas™ D Win- Medicare Pvt.Ltd. New Delhi) were purchased from local pharmacy. Methanol (AR grade) and Whatmann filter paper (no.41) was used throughout the experiment.
Method
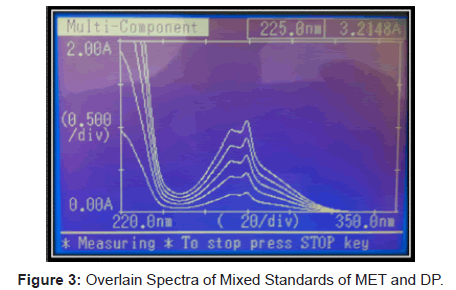
The mixed stock solution of analytes was prepared by transferring 200 mg of MET and 25 mg of DP in 200 ml volumetric flask, dissolved in few ml of methanol and making up the volume with methanol. This solution was further diluted to get five serial dilutions containing 20 to 100 μg/ml of MET and 2.5 to 12.5 μg/ml of DP in Methanol. All mixed standard solutions were scanned over the range of 350 nm to 220 nm in multi component mode of spectrophotometer at medium scanning speed. The absorption of solutions were measured at wavelength interval of 40 nm, stored in the instrument, and processed by instrument. An overlain spectrum of mixed standard solutions is as shown in (Figure 3) The spectral data of these scans stored and processed in the instrument is used to determine the concentration of MET and DP in the sample solution.
Analysis of commercial formulation
Twenty tablets were accurately weighed and crushed to fine powder. The tablet powder equivalent to 50 mg of MET (6.25 mg of DP) was accurately weighed, transferred to 50 ml volumetric flask, dissolved in small quantity of methanol with sonication and finally make up to mark with methanol. This solution was filtered through whattman filter paper No. 41. The filtrate was further diluted with methanol to get concentration of 60 μg/ml of MET 7.5 μg/ml of DP.
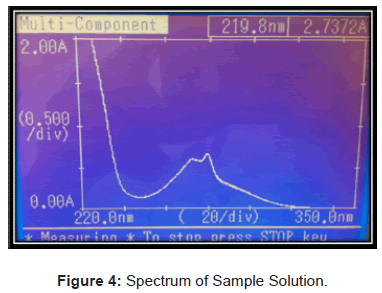
The sample solution was scanned over the range of 350 nm to 230 nm in multi component mode and concentration of each component was estimated by analysis of spectral data of sample solution with respect to that of mixed standards by the instrument. The spectrum of sample solution is given in Figure 4 and results of assay of marketed formulation are given in (Table 1).
| Analyte | Lable claim per tablet (mg) | Mean amount found in tablet (mg) | Mean amount found (%) | R.S.D. (%) n=6 |
|---|---|---|---|---|
| MET | 400 | 399.212 | 99.820 | 0.6297 |
| DP | 50 | 49.9124 | 99.824 | 0.5571 |
Table 1: Assay of Marketed Formulation (Myospas TM D).
Validation of method
The proposed method of analysis for MET and DP in combination were validated as per the recommendations of ICH guidelines [7] for accuracy and precision. Recovery studies were carried out by addition of pure drug to previously analyzed tablet sample at three different concentration levels (80%, 100%, and 120%). The results of recovery studies are reported in (Table 2). While, precision of the method was determined by repeatability and intermediate precision (intra-day, inter-day) expressed as % Relative Standard Deviation (% RSD). Intraday precision was evaluated by analyzing concentration of MET (60 μg/ml) and DP (7.5 μg/ml) of standard and sample solutions at three different time intervals under the same experimental conditions on the same day, while inter-day precision was determined by analyzing above mentioned concentrations of solutions on three consecutive days (Table 3).
| LEVEL OF RECOVERY | |||
|---|---|---|---|
| Analyte | 80% (± RSD) | 100% (± RSD) | 120% (± RSD) |
| MET | 99.15 ± 0.056 | 99.30 ± 0.302 | 99.70 ± 0.088 |
| DP | 101.98 ± 0.139 | 100.63 ± 0.533 | 100.14 ± 0.843 |
Table 2: Results of Recovery Studies.
| Analyte | Concentrations of sample solution (μg/ml) | Intra-day precision % RSD (n=3) | Inter-day precision % RSD (n=3) |
|---|---|---|---|
| MET | 60 | 0.0352 | 0.1732 |
| DP | 7.5 | 0.0622 | 0.0992 |
Table 3: Results of Precision Studies (Intra-Day and Inter-Day).
Results and Discussion
The stock solutions of both drugs were prepared in methanol and further dilutions were made in methanol, 0.1 N hydrochloric acid, 0.1N sodium hydroxide, and water. The overlay spectra of drugs in each solvent were studied carefully. The absorptions of both drugs were found to be satisfactory in methanol. Hence methanol was used as solvent.
As the proposed method is specific to instrument having software for provision of such determination, selection of proper sampling wavelength and concentrations of mixed standard solutions are critical. Hence overlay spectra of analytes were studied carefully. DP has shown absorption in the range of 200-340 nm while MET was found to be absorbing in the region 230-300 nm. Considering common absorption of drugs, the wavelength range of 220-350 nm was selected for the study. The various wavelength intervals were tried to measure and process data by the instruments to determine the exact concentration of standard and sample solutions. The wavelength interval of 40 nm was found to effective to quantify both drugs in formulation. The concentrations of mixed standard solutions were selected on the basis of linearity of each analyte at their wavelengths of absorption. The care was taken while selecting the concentrations of mixed solutions such that the absorbance at four selected wavelengths was not more than 1.5 (considering % relative error by instruments).
The mean content of analytes in the marketed formulation were found to be 99.82 and 99.824% for MET and DP respectively, while recovery was found in the range of 99.15 to 99.70% for MET and 100.14 to 101.98% for DP respectively. The values of relative standard deviations of inter-day and intra-day studies were found to be less than 1%. Intra-day study also indicated the standard and sample solutions were stable for measurement for longer time. The limitation of the present study is need of inbuilt software and retaining the standards of both analytes for spectra of mixed standards. The assay and validation results confirmed that the contents of MET and DP estimated in the tablet dosage form were free from the interference of excipients.
Conclusion
The developed multi component spectroscopy method for simultaneous estimation of Metaxalone and Diclofenac Potassium in combined tablet dosage form is simple, economical, accurate and reproducible and can be conveniently adopted for the routine quality control analysis from its pharmaceutical formulations and bulk drug.
References
- http://www.rxlist.com/skelaxin-drug.html
- http://www.drugs.com/pro/diclofenac-potassium.html
- Subramanian G, Musmade P, Agarwal S, Udupa N (2004) Simultaneous RP-HPLC estimation of tizanidine, diclofenac potassium and paracetamol in tablets. Indian J Pharm Sci 66: 694-696.
- Elkady EF (2010) Simultaneous determination of diclofenac potassium and methocarbamol in ternary mixture with guaifenesin by reversed phase liquid chromatography. Talanta 82: 1604-1607.
- Sahu PK, Annapurna MM, Kumar SD (2011) Development and Validation of Stability Indicating RP-HPLC Method for the Determination of Metaxalone in Bulk and its Pharmaceutical Formulations. E-Journal of Chemistry 8: S439-S447.
- Gowramma B, Rajan S, Muralidharan S, Meyyanathan SN, Suresh B (2010) A validated rp-hplc method forsimultaneous estimation of paracetamol anddiclofenac potassium in pharmaceuticalformulation. Int J Chem Tech Res 2: 676-680.
- ICH Guidance on Analytical Method Validation, in: Proceedings of the International Convention on Quality for the Pharmaceutical Industry, Toronto, Canada, and September, 2002.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18965
- [From(publication date):
July-2012 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 14287
- PDF downloads : 4678