Small Lymphocytic Lymphoma Presenting as Multiple Lymphomatous Polyposis
Received: 24-Oct-2012 / Accepted Date: 06-Dec-2012 / Published Date: 08-Dec-2012 DOI: 10.4172/2161-0681.1000132
308580Introduction
Multiple Lymphomatous Polyposis (MLP) is characterized by multiple polyps with lymphoma invasion involving long segments of the Gastrointestinal (GI) tract. [1] Most MLP are thought to represent Mantle Cell Lymphoma (MCL) of the GI tract. However, some follicular lymphomas, [2,3] mucosa-associated lymphoid tissue lymphomas, [3,4] and adult T-cell lymphomas [5] have been found to show a multiple polypoid appearance throughout long segments of the GI tract. We report a case with MLP associated with Small Lymphocytic Lymphoma (SLL). SLL in extranodal sites is difficult to diagnose and the clinical course is different from that of MCL. MCL has a more aggressive clinical course than Chronic Lymphocytic Leukemia or SLL (CLL/SLL). A median survival time of CLL/SLL is 7 to 10 years, [6] while patients with MCL have a median survival of 3 years [7].
Case Report
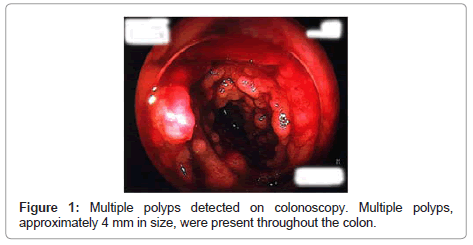
A 45-year-old man presented with a 6 × 12 cm left axillary mass. Needle biopsy of the axillary mass was performed and revealed malignant lymphoma. He was then referred for further investigation to our hospital. He denied weight loss, fever, or night sweats. Lymphocyte counts in peripheral blood were 2.5 × 10^9/L and atypical lymphocyte was 12%. Serological tests showed no evidence of EBV reactivation, and anti-HTLV1-antibody was negative. Computed tomography scans demonstrated lymphoadenopathy in the right axilla, mediastium, and paraaorta, but hepatosplenomegary was not found. Bone marrow aspiration showed atypical lymphocyte infiltration. Colonoscopy was performed to evaluate diarrhea, and multiple polyposis was detected throughout the colon (Figure 1). Five different colonic lesions were biopsied. Moreover, axillary lymph node was biopsied, since the first biopsy was too small due to the needle biopsy. After diagnosis of SLL, the patient received CHOP therapy and achieved partial remission. Four years after the first diagnosis, the patient had diarrhea and lymphadenopathy again. Colonoscopy revealed lymphoma relapse, which was confirmed by the biopsy. Fludarabine plus cyclophosphamide therapy was performed with the recovery of the patient. He was alive at the time of this report, 12 years after the initial diagnosis.
Histopathology of Colon and Lymph Node
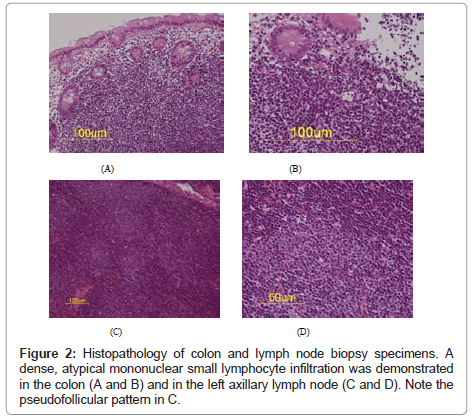
All colonic biopsies showed a dense, atypical mononuclear small lymphocyte infiltration that populated the lamina propria with normal glands (Figures 2A and 2B). In the lymph node, small to medium lymphocytes proliferated to form pseudofollicules (Figures 2C and 2D).
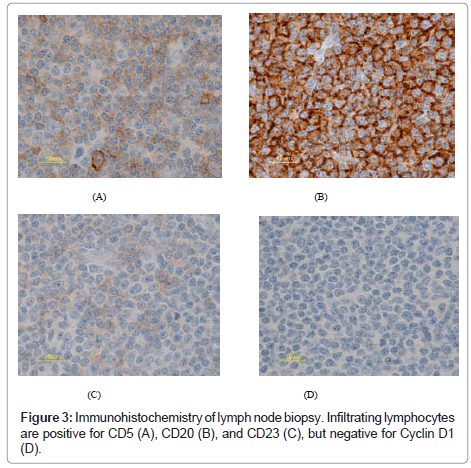
Immunohistochemical staining (avidin-biotin-peroxidase complex method) was performed with monoclonal antibodies, L26/ CD20 and CD5 (DAKO, Glostrup, Denmark), and CD23 and cyclin D1 (P2D11F11) (Novocastra, Newcastle, UK). Immunohistochemistry revealed a predominantly B-cell infiltration in both the colon and the lymph node biopsy specimens. The lymphocytes were also positive for CD5 and CD23, but negative for cyclin D1 (Figure 3).
Molecular Analyses
To further confirm that both lesions were derived from the identical clone, molecular analysis was performed. DNA was extracted from the formalin-fixed, paraffin-embedded colonic and lymph node specimens. The semi-nested Polymerase Chain Reaction (PCR) was performed [8-12] for the immunoglobulin heavy chain Variable Region (VH) using consensus primers spanning the recombined VH to JH segment. PCR was initially performed with sense primer (VHFR1c: AGGTGCAGCTG(C/G)(A/T)G(C/G)AGTC(A/T/G)GG) and antisense primer (VH-LJH: TGAGGAGACGGTGACC). The second PCR was performed with sense primer (VH-FR1c) and antisense primer (VH-VLJH: GTGACCAGGGT(A/C/G/T)CTTTGGCCCCAG). The resulting products were directly sequenced in both directions with primers VH-FR1c and VH-VLJH. The detected nucleotide sequence was aligned with the closest germline sequence derived from the NCBI database. The molecular analyses demonstrated clonal IgH gene rearrangements in both colonic and lymph node specimens. The two sequences were identical and the alignments between this case and germline V gene (VH3-23) were as follows: FWR1 (79/89, 88.8%), CDR1 (12/15, 80%), FWR2 (40/42, 95.2%), CDR2 (42/51, 82.4%), FWR3 (84/94, 89.4%), and total (257/291, 88.3%) (Figure 4).
Discussion
MLP is a very rare presentation of CLL/SLL, and most MLP cases have MCLs. To our knowledge, there has been only one case report in the English literature, [13] but the number of polyps was limited compared to that in the present case. The patient in that report showed three polyps in the rectum by colonoscopy. These lesions were diagnosed as CLL, which diffusely infiltrated to the bone marrow. On the other hand, in the current case there were multiple polyps, and its presentation showed far more similarities with MLP in MCL. GI tract involvement is common in MCL, and according to Romaguera et al. [14], 88% of MCL patients were revealed to have GI invasion histologically. MCL is an aggressive lymphoma, which usually has a poor prognosis.These clinical features are in contrast to those of CLL/SLL, which has a better clinical course. Therefore, it is important to discriminate CLL/SLL from MCL, especially when the clinical symptoms and signs appear to be similar, such as in this MLP case. Furthermore, it remains difficult to rule out the possibility of cyclinD1-negative MCL. Although cyclinD1 is believed as a hallmark of MCL, 5% of the cases with morphological features of MCL lack expression of cyclinD1. In the present case, we diagnosed as CLL/SLL, rather than cyclinD1-negative MCL, on the basis of the CD23 positivity and morphological pattern of the lymph node. Moreover, we exclude very rare composite lymphoma(cyclinD1-negative MCLin the colon and CLL/SLL in the lymph node) by the molecular analysis.
The diagnosis of CLL/SLL was confirmed in the present case by the lymph node biopsy specimen, showing the morphology and immunohistochemistry typical of SLL. Further molecular analysis demonstrated the identical sequences of the immunoglobulin heavy chain gene, confirming the identical clone in both lesions. In the two MCL case reports by Medicott et al. [15], the patients had CLL presenting as MLP-like lesions of the appendix and colonic diverticula, respectively. Molecular analysis enabled us to exclude such a rare possibility in our present case.
CLL/SLL is genetically classified into two groups, mutated and un-mutated CLL/SLL, based on the homology of the IgH V region sequence with the counterpart germline gene. When the sequence shows less than 98% homology with the closest germline gene sequence, the case is classified as having mutated CLL/SLL. Hamblin et al. [16] demonstrated that patients with mutated CLL/SLL had a better prognosis than those with unmutated CLL/SLL. Thus, the molecular diagnosis was mutated CLL/SLL, and our patient remained alive for 12 years, to date.
MLP is a very rare presentation of CLL/SLL. Although it has major similarities with MCL, based on morphology and immunohistochemistry, it is very important to discriminate these lymphomas from each other as they have different prognoses. Molecular analysis provides further concrete information facilitating the correct diagnosis and subtyping of MLP.
References
- Cornes JS (1961) Multiple lymphomatous polyposis of the gastrointestinal tract. Cancer 14: 249-257.
- LeBrun DP, Kamel OW, Cleary ML, Dorfman RF, Warnke RA (1992) Follicular lymphomas of the gastrointestinal tract. Pathologic features in 31 cases and bcl-2 oncogenic protein expression. Am J Pathol 140: 1327-1335.
- Kodama T, Ohshima K, Nomura K, Taniwaki M, Nakamura N, et al. (2005) Lymphomatous polyposis of the gastrointestinal tract, including mantle cell lymphoma, follicular lymphoma and mucosa-associated lymphoid tissue lymphoma. Histopathology 47: 467-478.
- Yatabe Y, Nakamura S, Nakamura T, Seto M, Ogura M, et al. (1998) Multiple polypoid lesions of primary mucosa-associated lymphoid-tissue lymphoma of colon. Histopathology 32: 116-125.
- Hokama A, Tomoyose T, Yamamoto Y, Watanabe T, Hirata T, et al. (2008) Adult T-cell leukemia/lymphoma presenting multiple lymphomatous polyposis. World J Gastroenterol 14: 6584-6588.
- Rozman C, Montserrat E (1995) Chronic lymphocytic leukemia. N Engl J Med 333: 1052-1057.
- Weisenburger DD, Armitage JO (1996) Mantle cell lymphoma--an entity comes of age. Blood 87: 4483-4494.
- Uchiyama M, Maesawa C, Yashima A, Itabashi T, Ishida Y, et al. (2003) Consensus primers for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell lymphomas. J Clin Pathol 56: 778-779.
- Wan JH, Trainor KJ, Brisco MJ, Morley AA (1990) Monoclonality in B cell lymphoma detected in paraffin wax embedded sections using the polymerase chain reaction. J Clin Pathol 43: 888-890.
- Ramasamy I, Brisco M, Morley A (1992) Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol 45: 770-775.
- Tarusawa M, Yashima A, Endo M, Maesawa C (2002) Quantitative assessment of minimal residual disease in childhood lymphoid malignancies using an allele-specific oligonucleotide real-time quantitative polymerase chain reaction. Int J Hematol 75: 166-173.
- Aubin J, Davi F, Nguyen-Salomon F, Leboeuf D, Debert C, et al. (1995) Description of a novel FR1 IgH PCR strategy and its comparison with three other strategies for the detection of clonality in B cell malignancies. Leukemia 9: 471-479.
- Khaled S, Gotlieb V, Schuster IP, Saif MW (2008) Multiple lymphomatous polyposis associated with small lymphocytic lymphoma: a unique presentation. J Gastrointestin Liver Dis 17: 461-463.
- Romaguera JE, Medeiros LJ, Hagemeister FB, Fayad LE, Rodriguez MA, et al. (2003) Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer 97: 586-591.
- Medlicott SA, Brown HA, Roland B, Beck PL, Auer I, et al. (2007) Multiple lymphomatous diverticulosis and comorbid chronic lymphocytic leukemia: novel manifestations of ileocolic mantle cell lymphoma. Int J Surg Pathol 15: 408-413.
- Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK (1999) Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 94: 1848-1854.
Citation: Ota Y, Takeuchi K, Kurokawa M, Fukayama M (2012) Small Lymphocytic Lymphoma Presenting as Multiple Lymphomatous Polyposis. J Clin Exp Pathol 2:132. DOI: 10.4172/2161-0681.1000132
Copyright: © 2012 Ota Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14793
- [From(publication date): 11-2012 - Dec 04, 2024]
- Breakdown by view type
- HTML page views: 10327
- PDF downloads: 4466