Research Article Open Access
SIRT1 Protects Dendrites, Mitochondria and Synapses from Aβ Oligomers in Hippocampal Neurons
Juan A Godoy1, Claudio Allard1, Macarena S Arrázola1, Juan M Zolezzi2 and Nibaldo C Inestrosa1*
1Centro de Envejecimiento y Regeneración (CARE); Departamento de Biología Celular, Molecular; Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile
2Departamento de Biología, Facultad de Ciencias, Universidad de Tarapacá, Arica, Chile
- Corresponding Author:
- Nibaldo C Inestrosa
CARE, Biomedical Research Center
Pontificia Universidad Católica de Chile
Av. Alameda 340, Santiago, Chile
Tel: +(56)-2-6862724
Fax: +(56)-2-6862959
E-mail: ninestrosa@bio.puc.cl
Received date: September 04, 2013; Accepted date: October 15, 2013; Published date: October 25, 2013
Citation: Godoy JA, Allard C, Arrázola MS, Zolezzi JM, Inestrosa NC (2013) SIRT1 Protects Dendrites, Mitochondria and Synapses from Aβ Oligomers in Hippocampal Neurons. J Alzheimers Dis Parkinsonism 3:126. doi: 10.4172/2161-0460.1000126
Copyright: © 2013 Godoy JA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Aging is a major risk factor in the onset of neurodegenerative diseases, such as Alzheimer’s disease (AD). SIRT1, a β-NAD+-dependent histone deacetylase activity, holds great potential for promoting longevity, preventing against disease and increasing cell survival. We report here, that SIRT1 protects against the damage caused by Aβ oligomers at the level of synaptic contacts, dendritic branching and mitochondrial structure in cultured rat hippocampal neurons. Neurons overexpressing SIRT1 showed increased synaptic contacts, dendritic branching and preserved mitochondrial morphology, suggesting the prevention of the Aβ oligomer-mediated neurodegeneration. Such effects were not observed in neurons overexpressing a dominant negative form of SIRT1. The potential underlying signaling pathways involved in the SIRT1 neuroprotective mechanism are discussed in the context of the peroxisome proliferator-activated receptors (PPARs), peroxisome proliferator activated receptor co-activator 1α (PGC-1α), mTOR, and the Wnt signaling pathway. Our results suggest that SIRT1 modulation might well be a therapeutic agent to protect against neurodegenerative diseases, like AD.
Keywords
Neurodegeneration; Alzheimer’s disease; Cell signaling; PPARs, PGC-1α, Wnt signaling pathway
Abbreviations
AD: Alzheimer’s Disease; Ara C: Cytosine Arabinoside; APP: Amyloid Precursor Protein; BACE1: β-Secretase; BSA: Bovine Serum Albumin; β-NAD: Nicotinamide Adenine Dinucleotide; CAT: Catalase; CR: Calorie Restriction; DIV: Days in vitro; DRP1: Dynamin-Related Protein 1; DMSO: Dimethyl Sulphoxide; EGFP: Enhanced Green Fluorescent Protein; GPx: Glutathione Peroxidase-1; IGF: Insulin Growth Factor; mTOR: Mammalian Target of Rapamycin; PBS: Phosphate Buffer Saline; PGC- 1α: Peroxisomal Proliferator Activated Receptor γ Co-activator-1α; PPAR: Peroxisomal Proliferator Activated Receptor; PPRE: Peroxisome Proliferator Responsive Element; ROS: Reactive Oxygen Species; RXR: PPAR: Retinoid X Receptor: Peroxisomal Proliferator Activated Receptor; SIRT1: Silent Information Regulator 1; S.E.M: The Standard Error of the Mean; SOD: Superoxide Dismutase; MTT: Tetrazolium Salt 3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide; Mfn: Mitofusin; mtTFA: Mitochondrial Transcription Factor A; NCoR: Nuclear Receptor Corepressor; YY1: Ying Yang 1
Introduction
Alzheimer’s disease is one of the most devastating neurodegenerative disorders and its underlying neuropathology includes, the extracellular deposits of amyloid β-peptide (Aβ), the intra-neuronal accumulation of hyper-phosphorylated tau forms, as well as synapse dysfunction and neuronal loss [1-3]. Compelling evidence supports the hypothesis that amyloid-β (Aβ) oligomers are a key factor in synaptic impairment and spatial memory decline associated with neuronal dysfunction [4-6]. This includes synaptic failure associated with the loss of synaptic proteins which contributes to the progression of the disease [7-9].
Sirtuins are a family of β-NAD+-dependent protein deacetylases, which requires β-NAD+ as a co-substrate. They act as a sensor of cellular energy and redox state, and might be regulated by different cellular metabolic conditions [10,11]. Studies suggest that the beneficial impact of calorie restriction (CR) in promoting longevity and cellular function may be mediated, in part, by SIRT1 through mechanisms involving a transcription co-activator of the PPARs, called PGC-1α, which plays important roles in the regulation of cellular metabolism and inflammatory and antioxidant responses [12]. Recent studies from our laboratory indicates, that treatment with different PPARs agonists prevent cognitive changes, amyloid deposition, tau phosphorylation and electrophysiological changes present in double APPswe/PS-1 transgenic mice model of AD [13]. Moreover, it has been proposed that the induction of SIRT1 expression also attenuates neuronal degeneration and death in animal models of AD and Huntington’s disease [14]. SIRT1 has emerged as a major regulator of mammalian transcription in response to cellular metabolic status and stress. It has been shown to positively regulate β-secretase promoter transcription (which controls non-amyloidogenic cleavage of the amyloid precursor protein (APP)), leading to reduced amyloid deposition [15].
SIRT1 also induces the expression of target genes involved in stress protection, while inhibiting genes involved in cell cycle arrest, senescence and/or apoptosis [10,11]. SIRT1 plays a major role in the calorie restriction (CR) response and life span control [10]. Indeed, like with exercise [16], it has been shown that CR mitigates the excessive amyloidogenesis in Tg2576 brains via the activation of β-secretase through SIRT1-mediated transcriptional activation of FOXO3a [17]. In addition, it reduces neuronal damage through potential inhibition of neuro-inflammatory (PPARs) signaling pathways [18,19]. The effect of CR on AD was first observed in studies where the Aβ plaques were reduced in the brains of AD mice that were calorie-restricted [20]. Furthermore, one of the first studies that showed that SIRT1 protects against AD and Amyotrophic Lateral sklerosis (ALS), used mouse models and primary neurons challenged with neurotoxic insults. These authors showed that resveratrol, a SIRT1 activator which is a phytocompound present in the red wine, have neuroprotective effects in vitro and in vivo against oxidative stress and Aβ toxicity [21], reducing hippocampal neurodegeneration, preventing learning impairment, and decreasing the acetylation of the known SIRT1 substrates, such as PGC- 1α and p53 [22]. In the same way, synaptotoxic effects have been close related to soluble Aβ oligomers [21]; moreover, even at low micromolar concentration, Aβ oligomers are able to impair excitatory synaptic transmissions, induce loss of dendritic spines and compromise spatial memory in rodents [23]. It has been demonstrated that Aβ oligomers alter the synaptic plasticity in vitro, their most reproducible effect being the inhibition of the long-term potentiation (LTP) in hippocampal slices, along with neuronal death, and the consequent memory malfunction and neurodegeneration [24]. Again, SIRT1 has also proved to promote memory and normal cognitive function through a distinct mechanism from the one described for its neuroprotective activity [25,26].
On the other hand, mitochondria are critical for energy, metabolism, apoptosis regulation, and plays a central role in aging; in fact, mitochondrial dysfunction increases with age and produces harmful levels of reactive oxygen species (ROS) which leads to cellular oxidative damage. More importantly, oxidative stress is highly damaging to macromolecules and is also a major cause of neuronal loss and impairment in neurodegenerative disorders, such as in AD [27]. Interestingly, during neuronal differentiation SIRT1 has proven to be involved in the redox potential sensing [28] and, under normal conditions, SIRT1 is able to increases mitochondrial function and reduces the oxidative stress that often occurs during neuronal degeneration [29,30].
We report here that SIRT1 is able to protect dendritic branching from Aβ oligomer damage in hippocampal neurons. Additionally, we provide evidence that SIRT1 overexpression allows neurons to be more resistant to the damage induced by Aβ-oligomer, an effect which was not observed in neurons overexpressing a dominant negative form of SIRT1. We also assess the role of SIRT1 on the mitochondrial status of hippocampal neuron challenged with Aβ oligomer, and discuss the potential underlying signaling pathways involved in neuroprotection that might emerge as attractive targets for future therapeutic intervention of several age-related neurodegenerative disorders.
Materials and Methods
Reagents
Resveratrol, β-NAD+ and hydrogen peroxide were obtained from Sigma-Chemical (St. Louis, MO, USA); Hoescht and Lipofectamine 2000 from Invitrogen (Carlsbad, CA, USA); rabbit anti-SIRT1 antibodies and monoclonal anti-PSD-95 from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and polyclonal anti-Synapsin from DAKO (Glostrup, Denmark); secondary antibodies labeled with 488Alexa, 543Alexa or 633Alexa were obtained from Affinity Bio Reagents Inc. (Golden, CO, USA). Aβ1-42 peptides corresponding to the human sequence (Genemed Synthesis Inc., South San Francisco, CA, USA) were dissolved in dimethyl sulphoxide (DMSO) at a concentration 15 μg/μl and immediately stored in aliquots at -20°C before assaying. Mitotracker for mitochondria staining was obtained from Molecular Probes (Carlsbad, CA, USA).
Ethics statement
Sprague-Dawley rats used in these experiments were housed at the Animal House facility of the Faculty of Biological Sciences, P. Universidad Católica de Chile, and handled according to guidelines outlined and approved by the Institutional Animal Care and Use Committee of the Faculty of Biological Sciences, P. Universidad Católica de Chile. Animals were euthanized by anesthesia overdose.
Rat hippocampal neurons, primary cultures and transfection
Briefly, hippocampal neurons were obtained from 18-day-old Sprague-Dawley rat embryos. Neurobasal medium containing 1% B27 supplement from Invitrogen, plus streptomycin and penicillin were used. On culture-day 3, hippocampal neurons were treated with 2 μM 1-β-D-arabinofuranosylcytosine (AraC) for 24 h to reduce the number of proliferating non-neuronal cells [31]. Hippocampal neurons were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), three days after seeded on covers slips (DIV 3) [32] in 24 well culture plates at a density of 6 × 104 cells per well; experiments were performed at DIV14. Constructs used for the transfection were: the enhanced green fluorescent protein (EGFP), from Clontech (Mountain View, CA, USA), and the SIRT1 and the SIRT1-H363Y (SIRT1-DN), which were a kind gift from Dr. Tso-Pang Yao (Duke University, Durham, NC, USA) and Dr. Jeff Milbrandt (Washington University, St. Louis, MO, USA). Briefly, DNA and Lipofectamine 2000 were mixed in 100 μl of OptiMEM (GIBCO) according to the manufacturer’s instructions. After 30 min the DNA-Lipofectamine 2000 Reagent complex was added to the cells. Neurons were incubated for 2 h at 37°C and then, the media was replaced with Neurobasal growth medium (GIBCO) plus B27. EGFP expressing neurons were considered as positive for the transfection process.
Aβ oligomers preparation
Aβo were obtained as previously described [33,34]. Stock solutions were prepared by dissolving freeze-dried aliquots of Aβ in Me2SO. Peptide stock aliquots were diluted in 0.1 M Tris-HCl (pH 7.4) to a final concentration of 100 μM Aβ. The solutions were stirred continuously (210 rpm) at 4°C for 24 h to obtain oligomers. A 5 μl aliquot was stained with 2% uranyl acetate and photographed with an electron microscope and analyzed by Tris-Tricine SDS gel electrophoresis, as previously described [34].
Cell viability assay (MTT reduction)
MTT assays were performed as described previously [35]. Hippocampal neurons (1×105 cells/100 μl/well) were assayed in B27- and phenol red-free medium. Neurons were pre-incubated for 2 h with different concentration of Resveratrol, β-NAD+ or medium (control); then, Aβo was added. Cells were incubated for 12 h at 37°C and cell viability was measured by the MTT method. Color variations were determined in a Lab Systems Uniskam I spectrophotometer at 540 and 650 nm. For apoptotic nuclei neurons were stained with Hoechst.
Mitochondrial staining
Hippocampal neurons were labeled with 50 nM Mitotracker orange for 20 min at 37°C prior to the different treatments were carried out [36]. Different experimental conditions were performed at 37°C and photographed under confocal microscopy.
Immunofluorescence
Hippocampal neurons were rinsed twice in ice-cold PBS and fixed with a freshly prepared solution of 4% paraformaldehyde/4% sucrose in PBS for 5 min. Cells were permeabilized and then blocked with 1% BSA in PBS for 60 min at room temperature, followed by an overnight incubation at 4°C with primary antibodies [35,36]. Cells were incubated with Alexa-conjugated secondary antibodies (Molecular Probes) for 60 min at 37°C.
Neuronal dendrite tree analysis
Sholl analysis was performed with the Image J software, using a semiautomated method on neurons expressing EGFP (positive for transfection), in which the soma boundary was approximated by an ellipsoid and dendrite arborization was assessed at radial distances from the soma. Each 5 μm increment of radial distance the dendritic tree was assessed, as the overlap of dendrites and the racial circumference. Statistical analysis was done with ANOVA followed by the appropriate post hoc test [21,37].
Statistical analysis
Statistical analyses were performed using GraphPad Prism Software.
Results
Activation of SIRT1 with β-NAD+ and RES protects neurons from the damage triggered by Aβo
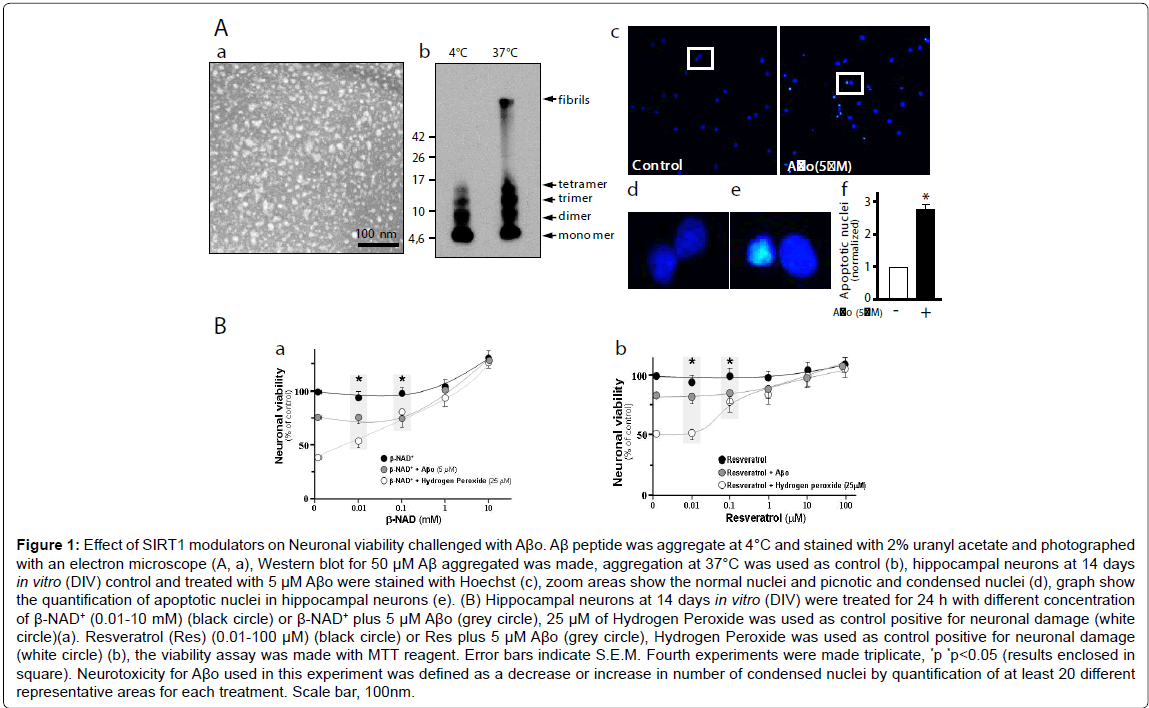
SIRT1 has been shown to catalyze β-NAD+-dependent protein deacetylation, and this activity is stimulated by RES. Thus, we evaluated hippocampal neurons viability under β-NAD+ and RES stimulus, and challenged with Aβo. Figure 1A,a shows the Aβo under the electron microscopy. They appear as spheres, probably reflecting amylospheroids. By western blot the Aβo appears mainly as monomers, dimers and trimers whit a small proportion of tetramers when prepared at 4°C (Figure 1A,b). This Aβo preparation was used to measure toxicity in hippocampal neurons. Apoptotic nuclei were stained with Hoechst (Figure 1A,c; magnification inset, d; graph, e). Different concentration of β-NAD+ (0.01-10 mM) improves the neuronal viability evaluated with MTT; at 10 mM β-NAD+ results shown a 20% increase in survival over control (Figure 1B,a: black circle). When the neurons were challenged with 5 μM Aβo, the viability decrease 25% compared to control. Interestingly, neuronal viability was improved when we used 1 to 10 mM of β-NAD+ (Figure 1B,a: grey circle). Similarly, when hydrogen peroxide (25 μM), a positive control for oxidative damage, was used a 60% neuronal viability decrease was observed, and this damage was reverted by 0.1 mM of β-NAD+ (Figure 1B,a: white circle). When 1 μM RES, a polyphenolic compound that significantly increase SIRT1 activity, was used a close to 20% general neuron protection from Aβo-damage was achieved compared with control (Figure 1B,b: grey circle). Against 25 μM hydrogen peroxide, cell viability was improved in the presence of 1 μM of RES (Figure 1B,b: white circle). The ability of neurons to reduce MTT provides information of mitochondrial integrity and activity, which in turn, is interpreted as an indicator of cell viability. These results together suggest that SIRT1 activators can mediate neuroprotective effects and that have the potential to preserve the cellular viability through the protection of healthy mitochondria.
SIRT1 protects neuronal morphology and dendritic tree from Aβo
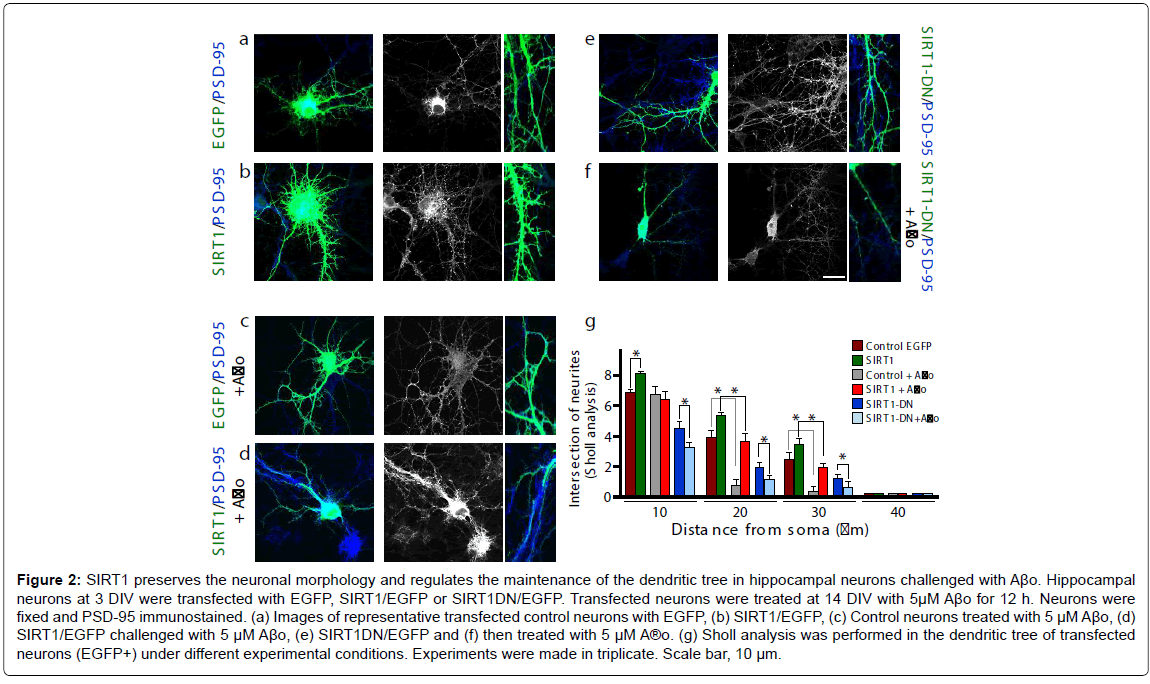
To evaluate the effects of SIRT1 on the dendritic tree and its ability to protect neurons from Aβ-oligomer-damage, this deacetylase was overexpressed in hippocampal neurons. Different conditions were evaluated (Figure 2); control neurons (a: left first row), transfected SIRT1 (b: left second row); control plus Aβo 5 μM (c: left third row); SIRT1 plus Aβo 5 μM (d: left fourth row); SIRT1-DN (e: right first row) and SIRT1-DN plus Aβo 5 μM (f: right second row). The dendritic arborization was analyzed in transfected neurons by Sholl analysis, and indicates that the dendritic tree was improved when SIRT1 was overexpressed compared with the control (brown bar vs. green bar, *p<0.01). The neurons transfected only with EGFP and challenged with Aβo showed more than 70% arborization decrease 20-30 μm from the soma (Figure 2g: brown bar vs. EGFP plus Aβo, grey bar, *p<0.01 at 20-30 μm). SIRT1 transfection control, challenged with Aβo, significantly protect the dendrites at the 20-30 μm intersection from the soma (Figure 2g: green bar vs. red bar, *p<0.01 at 20-30 μm). On the other hand, neurons transfected with SIRT1-DN exhibit an already diminished dendritic tree, which was accentuated when challenged with Aβo (Figure 2g: blue bar vs. light blue, *p<0.01 at 10-20-30 μm). These results suggest that SIRT1 allows neurons to preserve their morphology, dendritic tree and protect them against Aβ-oligomer-damage.
Figure 1: Effect of SIRT1 modulators on Neuronal viability challenged with Aβo. Aβ peptide was aggregate at 4°C and stained with 2% uranyl acetate and photographed with an electron microscope (A, a), Western blot for 50 μM Aβ aggregated was made, aggregation at 37°C was used as control (b), hippocampal neurons at 14 days in vitro (DIV) control and treated with 5 μM Aβo were stained with Hoechst (c), zoom areas show the normal nuclei and picnotic and condensed nuclei (d), graph show the quantification of apoptotic nuclei in hippocampal neurons (e). (B) Hippocampal neurons at 14 days in vitro (DIV) were treated for 24 h with different concentration of β-NAD+ (0.01-10 mM) (black circle) or β-NAD+ plus 5 μM Aβo (grey circle), 25 μM of Hydrogen Peroxide was used as control positive for neuronal damage (white circle)(a). Resveratrol (Res) (0.01-100 μM) (black circle) or Res plus 5 μM Aβo (grey circle), Hydrogen Peroxide was used as control positive for neuronal damage (white circle) (b), the viability assay was made with MTT reagent. Error bars indicate S.E.M. Fourth experiments were made triplicate, *p *p<0.05 (results enclosed in square). Neurotoxicity for Aβo used in this experiment was defined as a decrease or increase in number of condensed nuclei by quantification of at least 20 different representative areas for each treatment. Scale bar, 100nm
Figure 2: SIRT1 preserves the neuronal morphology and regulates the maintenance of the dendritic tree in hippocampal neurons challenged with Aβo. Hippocampal neurons at 3 DIV were transfected with EGFP, SIRT1/EGFP or SIRT1DN/EGFP. Transfected neurons were treated at 14 DIV with 5μM Aβo for 12 h. Neurons were fixed and PSD-95 immunostained. (a) Images of representative transfected control neurons with EGFP, (b) SIRT1/EGFP, (c) Control neurons treated with 5 μM Aβo, (d) SIRT1/EGFP challenged with 5 μM Aβo, (e) SIRT1DN/EGFP and (f) then treated with 5 μM A®o. (g) Sholl analysis was performed in the dendritic tree of transfected neurons (EGFP+) under different experimental conditions. Experiments were made in triplicate. Scale bar, 10 μm.
SIRT1 protects neuronal synapses from damage produced by Aβ-oligomers
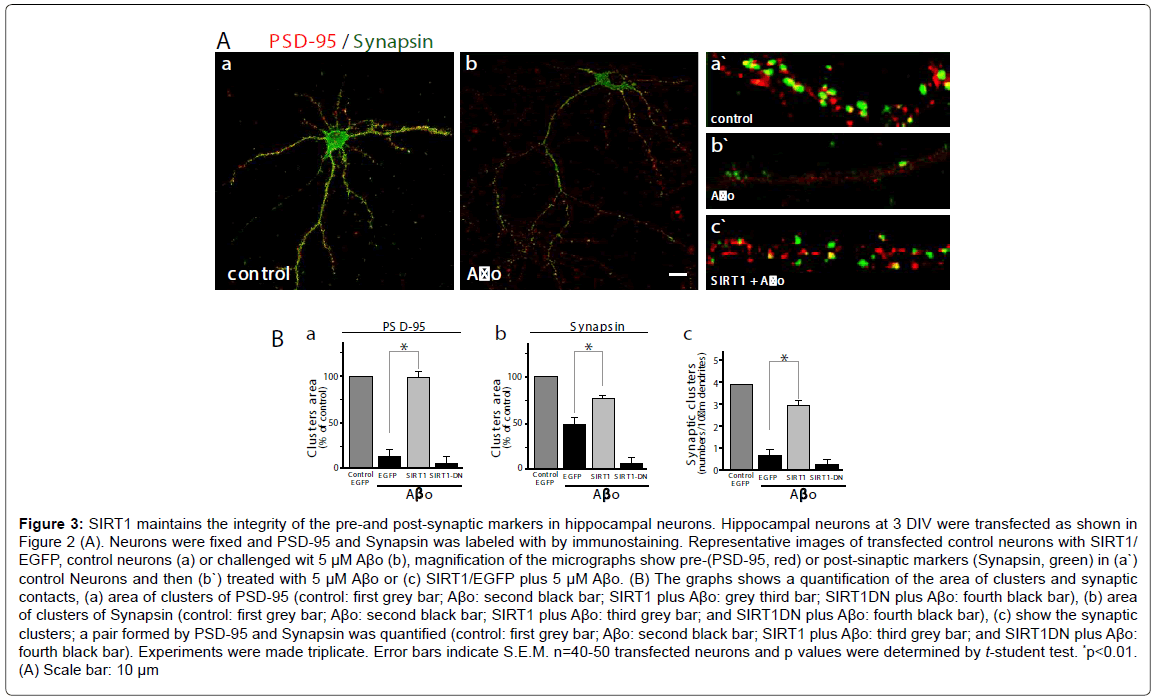
To evaluate the effects of SIRT1 on neuronal synapsis, we used a postsynaptic marker (PSD-95, red) and a pre-synaptic marker (Synapsin, green), and measured the cluster area and the synaptic clusters (a pair formed by PSD-95/Synapsin). A representative micrograph showing neurons stained with both antibodies; control neurons (Figure 3A,a: control neurons) and challenged with Aβo 5 μM (Figure 3A,b: neurons plus Aβo). The quantification of both markers clusters and synaptic clusters were made in magnified segments (as shown in Figure 3A: a`-c`). The PSD-95 clusters area decreases markedly in neurons transfected only with GFP and when treated with Aβo, the loss is over 80% compared with the control (Figure 3B, a: control, grey bar v/s neurons plus Aβo , black bar, *p<0.01); on the other hand, neurons transfected with SIRT1 and challenged with 5 μM Aβo, exhibits a recovery in the area of PSD95 cluster (Figure 3B,a: neurons transfected wit SIRT1 plus Aβo , grey bar), but when the neurons were transfected with SIRT1-DN, the PSD-95 clusters recovery did not occurs (Figure 3B,a: neurons transfected wit SIRT1-DN plus Aβo, fourth black bar). Regarding Synapsin, the presynaptic markers evaluated, the clusters area was reduced to half when neurons were challenged with Aβo compared with the control (Figure 3B,b: control, grey bar; transfected with EGFP alone plus with Aβo, black bar). When the neurons were transfected with SIRT1 and challenged with 5 μM Aβo, the area of Synapsin clusters was recovered significantly as compared with the EGFP alone (Figure 3B,b: black bar vs. grey bar SIRT1). As supposed, the dominant negative construct was unable to recover the cluster of Synapsin under Aβo challenge (Figure 3B,b: transfected wit SIRT1- DN, fourth black bar). We further evaluated the pair PSD-95/Synapsin (synaptic clusters). Aβo treated neurons showed decreased synaptic clusters in EGFP alone transfected neurons, but when transfected with SIRT1, neurons recovered the synaptic cluster up to 75% compared to the control (Figure 3B,c: control neurons, first grey bar, GFP alone plus Ao 5 μM, second black bar; transfected with SIRT1 plus Aβo, third grey bar, transfected with SIRT1-DN plus 5 μM Aβo, fourth black bar). On the other hand, the neurons transfected with the dominant negative of SIRT1 were unable to maintain or recover the synaptic clusters. These experiments indicate that SIRT1 is necessary for the maintenance of functional synapses in hippocampal neurons.
SIRT1 protects mitochondria from the damage induced by Aβ-oligomers
The potential role of SIRT1 on mitochondrial morphology was assessed in hippocampal neuron overexpressing SIRT1 and treated under different conditions. We evaluated the number of mitochondria with Mitotracker, which stain mitochondria with normal membrane potential, on the EGFP dendrites positive, (Figure 4A, SIRT1 transfected neurons, first row; mitochondria are shown in second row and mitochondria magnification are shown in third row). Neurons transfected only with EGFP and challenged with Ao, exhibit reduced mitochondrial count (Figure 4B,a: grey bar vs. black bar, *p<0.01), while the SIRT1 overexpressing neurons shows two fold increase in the numbers of mitochondria, (Figure 4B,a: first grey bar vs. third grey bar bar, *p<0.01). On the other hand, the neurons transfected with SIRT1- DN shows decreased number of mitochondria which decreased even more with the Aβo treatment. Controls mostly evidenced elongated mitochondria (about 0.5-2 μm) with a small percentage of smaller mitochondria (Figure 4B,b: white bar vs. grey bar). When neurons were challenged with Aβo, the number of elongated mitochondria decreases and the small mitochondria was raised (Figure 4B,b: third white bar vs. fourth grey bar, *p<0.01). However, SIRT1 transfected neurons exhibit increased number of elongated mitochondria compared with the control (Figure 4B,b: first white bar v/s sixth white bar, *p<0.01); interestingly SIRT1-neurons challenged with Aβo did not evidenced any significative change in the number or proportion of the mitochondria (Figure 4B,b: SIRT1 transfected neurons). On the other hand, neurons transfected with SIRT1-DN, control and/or challenged with Aβo, showed a decreased number of elongated mitochondria and increased in the smaller mitochondria fraction in the dendritic tree (Figure 4B,b: SIRT1-DN transfected neurons, *p<0.01). These results indicate that the deacetylase activity of SIRT1 is implicated in the development of the dendritic tree and in the maintenance of the integrity and number of the mitochondrial population in hippocampal neurons.
Figure 3: SIRT1 maintains the integrity of the pre-and post-synaptic markers in hippocampal neurons. Hippocampal neurons at 3 DIV were transfected as shown in Figure 2 (A). Neurons were fixed and PSD-95 and Synapsin was labeled with by immunostaining. Representative images of transfected control neurons with SIRT1/ EGFP, control neurons (a) or challenged wit 5 µM AÃÂ?o (b), magnification of the micrographs show pre-(PSD-95, red) or post-sinaptic markers (Synapsin, green) in (a`) control Neurons and then (b`) treated with 5 µM AÃÂ?o or (c) SIRT1/EGFP plus 5 µM AÃÂ?o. (B) The graphs shows a quantification of the area of clusters and synaptic contacts, (a) area of clusters of PSD-95 (control: first grey bar; AÃÂ?o: second black bar; SIRT1 plus AÃÂ?o: grey third bar; SIRT1DN plus AÃÂ?o: fourth black bar), (b) area of clusters of Synapsin (control: first grey bar; AÃÂ?o: second black bar; SIRT1 plus AÃÂ?o: third grey bar; and SIRT1DN plus AÃÂ?o: fourth black bar), (c) show the synaptic clusters; a pair formed by PSD-95 and Synapsin was quantified (control: first grey bar; AÃÂ?o: second black bar; SIRT1 plus AÃÂ?o: third grey bar; and SIRT1DN plus AÃÂ?o: fourth black bar). Experiments were made triplicate. Error bars indicate S.E.M. n=40-50 transfected neurons and p values were determined by t-student test. *<0.01. (A) Scale bar: 10 µm
Figure 4:SIRT1 maintains the integrity of the mitochondria in hippocampal neurons. Hippocampal neurons at 3 DIV were transfected as shown in Figure 2. Mitochondria was stained with orange mitotracker, then fixed and photographed in confocal microscope. (A) Representative images of transfected neurons under different conditions (first row), the neurons transfected were stained with Mitotrackers (second row), a zoom showing the mitochondrial morphology under the treatments is shown (third row). (B) Numbers of mitochondria in transfected neurons was quantified (a), different subpopulation of mitochondria under different conditions was quantified (b). Experiments were made triplicate, p values were determined by Kruskal-Wallis/Dunn (*p<0.01, **p<0.001). Error bars indicate S.E.M. n=40-50 transfected neurons. Scale bar, 10 μm.
Discussion
In the present study, we provide evidence that SIRT1 overexpression in rat hippocampal neurons is able to protect dendritic branching from A-oligomer-damage in hippocampal neurons. Additionally, we provide evidence that SIRT1 overexpression allows neurons to be more resistant to the damage induced by Aβ oligomers, in particular protecting the pre- and post-synaptic regions, an effect which was not observed in neurons overexpressing a dominant negative form of SIRT1. We also assessed the role of SIRT1 on mitochondria of hippocampal neurons challenged with Aβ-oligomers; our results indicated that SIRT1 is able to preserve the mitochondrial morphology despite the presence of the Aβ-species.
Although a proper dendrite remodeling and shape underlies the normal mammalian brain function, including cognition and memory formation, abnormal dendritic development closely correlates with mental retardation and a number of central nervous system disorders including Down’s, Rett and Fragile X syndromes [38,39]. Therefore, the fact that SIRT1 was able to prevent most of the damage induced by Aβ-oligomers, clearly suggest that neuro-protection is an attractive target for therapeutic intervention in neurodegenerative disorders. Dendrite growth is a very dynamic process and the pattern of dendritic trees is believed to be regulated by an interplay between an intrinsic genetic program, extrinsic factors, and neuronal activity [40-42]. Many extracellular factors have been identified as regulators of dendritic growth and branching, including Wnt ligands [43,44]. It is therefore possible that the effect observed with SIRT1 might be associated with potential underlying signaling pathways involved in the general control of dendritogenesis, such as the Wnt signaling. Further studies are necessary to clarify this issue.
Together with the protection of dendritic branching, our results indicate that SIRT1 overexpression or agonist-mediated stimulation constitutes a key element for neuronal function. Moreover, considering that the cognitive impairment corresponds to the physiological manifestation of neurodegeneration and synaptic failure, our results agreed with previous studies which have been demonstrated that SIRT1 stimulation rescue the cognitive decline often observed in several in vivo models of AD [45]. Additionally, we have observed that enhanced SIRT1 stimulation, after resveratrol treatment, increases survival rate of neurons challenged with Aβ oligomers. This latter finding suggests that SIRT1 stimulation it is also able to trigger several signaling mechanisms which allow cells to counteract Aβ oligomer-damaging pathways. Indeed, it have been well established that part of the damaging effects of Aβ oligomer challenge are due to increased ROS production [46,47], and that resveratrol is able to prevent: accumulation of ROS, depletion of cellular glutathione, DNA oxidative damage [48] and reduced apoptotic neuronal cell death [49]. In mouse models of AD, brain-specific knockout of SIRT1 caused a significant elevation in amyloid plaques and reactive gliosis [50]. On the other hand, overexpression of SIRT1 caused a decrease in these parameters, possibly due to deacetylation by SIRT1 of retinoic acid receptor-β [11,15]. A very recent study shows that metabolic stress modulates Alzheimer’s β-secretase/BACE1 gene transcription via SIRT1-PPARγ-PGC-1α [50]. Since, calorie restriction promotes longevity and cellular function, in part mediated by SIRT1 through mechanisms involving a transcription co-activator of the PPARs, called PGC-1α [12]; it is interesting to mention that treatment with different PPARα and PPARγ agonists, reduce spatial memory impairment, synaptic failure and neurodegeneration in brains of a double APPswe/PS-1 transgenic mice model of AD [13].
On the other hand, mitochondria are particularly important to neurons, owing to their high energy demand because of their specialized functions, complex morphology and synaptic activity. Mitochondrial function is directly linked to mitochondrial dynamics, a control processes associated with the biogenesis, subcellular localization and distribution of mitochondria as well as their morphology, and vice versa; additionally, mitochondrial dysfunction has been strongly associated with neurodegenerative diseases [51,52]. Our results showed that SIRT1 overexpression increases mitochondrial number and maintains their normal morphology when challenged with Aβ oligomers. These findings suggest that overexpression of SIRT1 is able to prevent mitochondrial dysfunction due to Aβ oligomers, and induce mitochondrial dynamic events which account for the maintenance of mitochondrial number. In fact, Cunningham et al. [53] have suggested that the mitochondrial oxidative metabolism could be down regulated through mTOR-mediated inhibition of the ying-yang 1 (YY1)/PGC- 1α complex, resulting in PGC-1α release. This is interesting because a critical role has been suggested for PGC-1α in mitochondrial dynamics regulation [47]; in addition, Wnt signaling regulates mitochondrial physiology [54] and mitochondrial dynamics, moreover activation with Wnt ligands prevents changes induced by Aβ oligomers in mitochondrial fission-fusion dynamics [36]. In this context, it is important to mention that the activation of Wnt signaling both in vitro and in vivo protects from Aβ oligomers neurotoxicity [44,55-59]. At the same time, resveratrol treatment stimulates the nuclear accumulation of β-catenin and induces the transcription of -catenin-TCF/LEF target genes; at the same time, resveratrol reduces the level of glycogen synthase kinase- 3β (GSK-3β) [60]. As a whole, the above studies suggest that SIRT1 might exert its neuroprotective activity through the activation of Wnt signaling pathway.
In the same way, several authors have described a regulatory role for SIRT1 on mTOR [61,62], a key element which plays a critical role in neurons, affecting synaptic health, synaptic plasticity and dendritic branching [63,64], and which have been also observed to response under calorie restriction [65,66]. The neuroprotective effect of SIRT1 in Huntington’s disease models appears to be mediated by the activation of multiple targets, including CREB, CREB-regulated transcription co-activator 1, and FOXO3a [67]. In consequence, it is probably that mTOR could acts as a SIRT1 effector regarding the modulation of critical cellular processes that increased neuronal survival. Whether the protective effect of SIRT1 against Aβ oligomers observed in the present work is mediated by mTOR requires further studies.
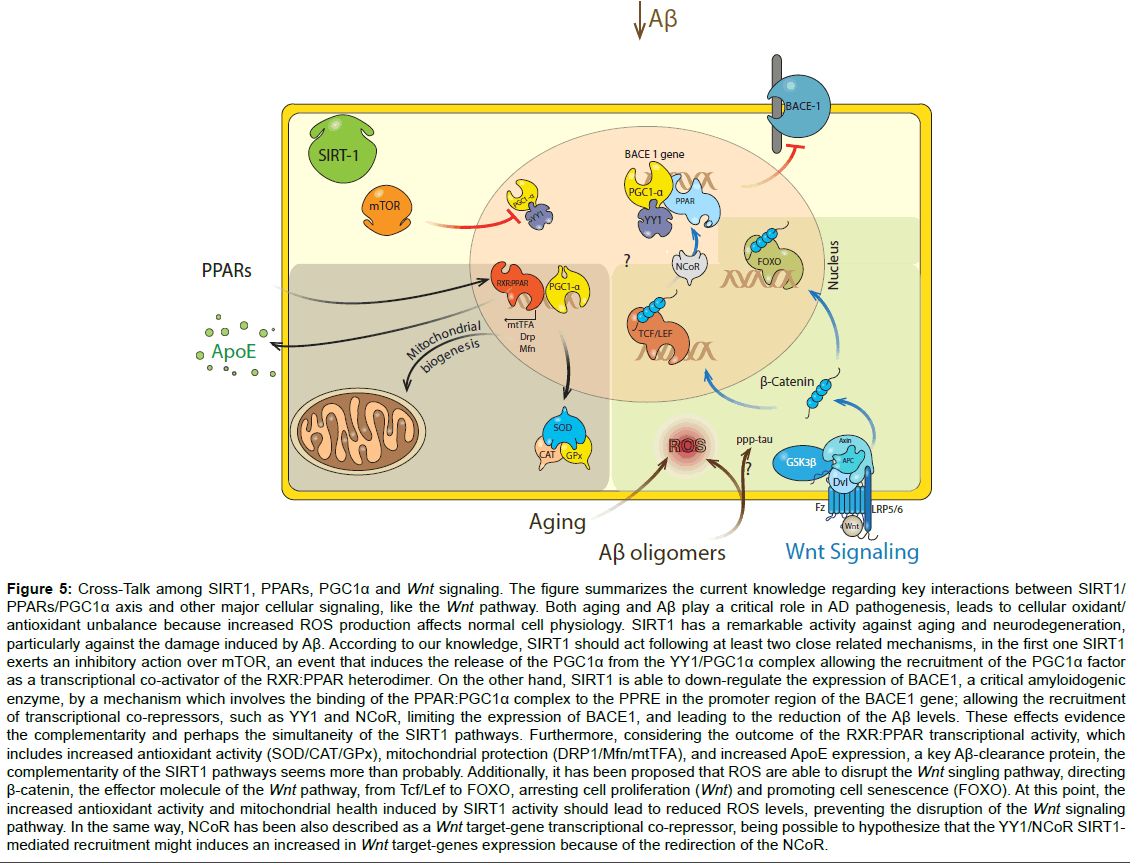
Figure 5:Cross-Talk among SIRT1, PPARs, PGC1α and Wnt signaling. The figure summarizes the current knowledge regarding key interactions between SIRT1/ PPARs/PGC1α axis and other major cellular signaling, like the Wnt pathway. Both aging and Aβ play a critical role in AD pathogenesis, leads to cellular oxidant/ antioxidant unbalance because increased ROS production affects normal cell physiology. SIRT1 has a remarkable activity against aging and neurodegeneration, particularly against the damage induced by Aβ. According to our knowledge, SIRT1 should act following at least two close related mechanisms, in the first one SIRT1 exerts an inhibitory action over mTOR, an event that induces the release of the PGC1α from the YY1/PGC1α complex allowing the recruitment of the PGC1α factor as a transcriptional co-activator of the RXR:PPAR heterodimer. On the other hand, SIRT1 is able to down-regulate the expression of BACE1, a critical amyloidogenic enzyme, by a mechanism which involves the binding of the PPAR:PGC1α complex to the PPRE in the promoter region of the BACE1 gene; allowing the recruitment of transcriptional co-repressors, such as YY1 and NCoR, limiting the expression of BACE1, and leading to the reduction of the Aβ levels. These effects evidence the complementarity and perhaps the simultaneity of the SIRT1 pathways. Furthermore, considering the outcome of the RXR:PPAR transcriptional activity, which includes increased antioxidant activity (SOD/CAT/GPx), mitochondrial protection (DRP1/Mfn/mtTFA), and increased ApoE expression, a key Aβ-clearance protein, the complementarity of the SIRT1 pathways seems more than probably. Additionally, it has been proposed that ROS are able to disrupt the Wnt singling pathway, directing β-catenin, the effector molecule of the Wnt pathway, from Tcf/Lef to FOXO, arresting cell proliferation (Wnt) and promoting cell senescence (FOXO). At this point, the increased antioxidant activity and mitochondrial health induced by SIRT1 activity should lead to reduced ROS levels, preventing the disruption of the Wnt signaling pathway. In the same way, NCoR has been also described as a Wnt target-gene transcriptional co-repressor, being possible to hypothesize that the YY1/NCoR SIRT1- mediated recruitment might induces an increased in Wnt target-genes expression because of the redirection of the NCoR.
Importantly, Aβ oligomers are a key factor in the synaptotoxicity that alters the cognitive process in AD [4,6]. Aβ oligomers trigger postsynaptic failures, loss of synaptic proteins and finally a loss of spatial memory and learning [9,68]. Our results show that SIRT1 prevents the loss of synaptic proteins and synaptic clusters (pair of pre-, post-synaptic markers), allowing the conservation of the synaptic structure. Considering that several signaling pathways (PPARs, PGC- 1α, mTOR and Wnt) play a role in the neuroprotective effects of SIRT1, a general scheme is presented in (Figure 5), which includes the progress in understanding the neurobiological benefits of SIRT1, in particular, showing all the putative factors that might be involve in the neurotrophic and neuroprotective roles of SIRT1 [69]. We conclude that the stimulation of SIRT1 activity by different modulators, including resveratrol, might be a therapeutic strategy to reduce mitochondrial fragmentation, dendrite abnormalities, synaptic damage and eventually cognitive decline in patients with AD.
Acknowledgements
This work was supported by grants from MIFAB Foundation and Fundación Ciencia por la Vida, FONDECYT 1120156 and Basal Centre for Excellence in Science and Technology (PFB 12/2007) to NCI and a pre-doctoral fellowship from CONICYT to CA and MSA.
References
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, et al. (2011) Alzheimer's disease. Lancet 377: 1019-1031.
- Selkoe D, Mandelkow E, Holtzman D (2012) Deciphering Alzheimer disease. Cold Spring Harb Perspect Med 2: a011460.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1: a006189.
- Cerpa W, Dinamarca MC, Inestrosa NC (2008) Structure-function implications in Alzheimer's disease: effect of Abeta oligomers at central synapses. Curr Alzheimer Res 5: 233-243.
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101-112.
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, et al. (2004) Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci 24: 10191-10200.
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68: 1501-1508.
- Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2: a006338.
- Borlikova GG, Trejo M, Mably AJ, Mc Donald JM, Sala Frigerio C, et al. (2013) Alzheimer brain-derived amyloid ÃÂ?ŽÃÂ?²-protein impairs synaptic remodeling and memory consolidation. Neurobiol Aging 34: 1315-1327.
- Duan W (2013) Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci 5: 36.
- Hall JA, Dominy JE, Lee Y, Puigserver P (2013) The sirtuin family's role in aging and age-associated pathologies. J Clin Invest 123: 973-979.
- Wang J, Fivecoat H, Ho L, Pan Y, Ling E, et al. (2010) The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer's disease neuropathology. Biochim Biophys Acta 1804: 1690-1694.
- Inestrosa NC, Carvajal FJ, Zolezzi JM, Tapia-Rojas C, Serrano F, et al. (2013) Peroxisome proliferators reduce spatial memory impairment, synaptic failure, and neurodegeneration in brains of a double transgenic mice model of Alzheimer's disease. J Alzheimers Dis 33: 941-959.
- Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5: 344-352.
- Donmez G, Wang D, Cohen DE, Guarente L (2010) SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell 142: 320-332.
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, et al. (2005) Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120: 701-713.
- Qin W, Zhao W, Ho L, Wang J, Walsh K, et al. (2008) Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer's disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci 1147: 335-347.
- Tang BL, Chua CE (2008) SIRT1 and neuronal diseases. Mol Aspects Med 29: 187-200.
- Zolezzi JM, Inestrosa NC (2013) Peroxisome Proliferator-activated Receptors and Alzheimer's Disease: Hitting the Blood-Brain Barrier. Mol Neurobiol.
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, et al. (2005) Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging 26: 995-1000.
- Codocedo JF, Allard C, Godoy JA, Varela-Nallar L, Inestrosa NC (2012) SIRT1 regulates dendritic development in hippocampal neurons. PLoS One 7: e47073.
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, et al. (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 26: 3169-3179.
- Shankar GM, Walsh DM (2009) Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener 4: 48.
- Krafft GA, Klein WL (2010) ADDLs and the signaling web that leads to Alzheimer's disease. Neuropharmacology 59: 230-242.
- Gao J, Wang WY, Mao YW, GrÃÂ?¤ff J, Guan JS, et al. (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466: 1105-1109.
- Michán S, Li Y, Chou MM, Parrella E, Ge H, et al. (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30: 9695-9707.
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9: 505-518.
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, SchrÃÂ?¶ter F, et al. (2008) Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol 10: 385-394.
- Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, et al. (2008) Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A 105: 15599-15604.
- Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, et al. (2012) SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front Cell Neurosci 6: 63.
- Banker GA, Cowan WM (1977) Rat hippocampal neurons in dispersed cell culture. Brain Res 126: 397-342.
- KÃÂ?¶hrmann M, Haubensak W, Hemraj I, Kaether C, Lessmann VJ, et al. (1999) Fast, convenient, and effective method to transiently transfect primary hippocampal neurons. J Neurosci Res 58: 831-835.
- Dinamarca MC, Sagal JP, Quintanilla RA, Godoy JA, Arrazola MS, et al. (2010) Amyloid-beta-Acetylcholinesterase complexes potentiate neurodegenerative changes induced by the Abeta peptide. Implications for the pathogenesis of Alzheimer's disease. Mol Neurodegener 5: 4.
- Santos MJ, Quintanilla RA, Toro A, Grandy R, Dinamarca MC, et al. (2005) Peroxisomal proliferation protects from beta-amyloid neurodegeneration. J Biol Chem 280: 41057-41068.
- FarÃÂ?Âas GG, VallÃÂ?©s AS, Colombres M, Godoy JA, Toledo EM, et al. (2007) Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci 27: 5313-5325.
- Silva-Alvarez C, Arrázola MS, Godoy JA, Ordenes D, Inestrosa NC (2013) Canonical Wnt signaling protects hippocampal neurons from Aβ oligomers: role of non-canonical Wnt-5a/Ca(2+) in mitochondrial dynamics. Front Cell Neurosci 7: 97.
- Charych EI, Akum BF, Goldberg JS, JÃÂ?¶rnsten RJ, Rongo C, et al. (2006) Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. J Neurosci 26: 10164-10176.
- Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10: 981-991.
- Miller FD, Kaplan DR (2003) Signaling mechanisms underlying dendrite formation. Curr Opin Neurobiol 13: 391-398.
- Cline HT (2001) Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11: 118-126.
- Whitford KL, Dijkhuizen P, Polleux F, Ghosh A (2002) Molecular control of cortical dendrite development. Annu Rev Neurosci 25: 127-149.
- Jan YN, Jan LY (2003) The control of dendrite development. Neuron 40: 229-242.
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC (2005) Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci 8: 34-42.
- Rosso SB, Inestrosa NC (2013) WNT signaling in neuronal maturation and synaptogenesis. Front Cell Neurosci 7: 103.
- Lalla R, Donmez G (2013) The role of sirtuins in Alzheimer's disease. Front Aging Neurosci 5: 16.
- Paula-Lima AC, Adasme T, San MartÃÂ?Ân C, Sebolleda A, Hetz C, et al. (2011) Amyloid ÃÂ?Ÿ-peptide oligomers stimulate RyR-mediated Ca2+ relÃÂ?©ase inducing mitocondrial fragmentation in hippocampal neurons and prevents RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid Redox Signal 14: 1209-1223.
- Zolezzi JM, Silva-Alvarez C, Ordenes D, Godoy JA, Carvajal FJ, et al. (2013) Peroxisome proliferator-activated receptor (PPAR) γ and PPARα agonists modulate mitochondrial fusion-fission dynamics: relevance to reactive oxygen species (ROS)-related neurodegenerative disorders? PLoS One 8: e64019.
- Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, et al. (2007) Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol 73: 550-560.
- Bureau G, LongprÃÂ?© F, Martinoli MG (2008) Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res 86: 403-410.
- Wang R, Li JJ, Diao S, Kwak YD, Liu L, et al. (2013) Metabolic stress modulates Alzheimer's β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons. Cell Metab 17: 685-694.
- Cho DH, Nakamura T, Lipton SA (2010) Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci 67: 3435-3447.
- Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, et al. (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67: 103-118.
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, et al. (2007) mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450: 736-740.
- Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, et al. (2010) Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev 24: 1507-1518.
- De Ferrari GV, ChacÃÂ?³n MA, BarrÃÂ?Âa MI, Garrido JL, Godoy JA, et al. (2003) Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry 8: 195-208.
- Toledo EM, Colombres M, Inestrosa NC (2008) Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol 86: 281-296.
- Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11: 77-86.
- Toledo EM, Inestrosa NC (2010) Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry 15: 272-285, 228.
- Purro SA, Dickins EM, Salinas PC (2012) The secreted Wnt antagonist Dickkopf-1 is required for amyloid ÃÂ?ŽÃÂ?²-mediated synaptic loss. J Neurosci 32: 3492-3498.
- Zhou H, Shang L, Li X, Zhang X, Gao G, et al. (2009) Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells. Exp Cell Res 315: 2953-2962.
- Ghosh HS, McBurney M, Robbins PD (2010) SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5: e9199.
- Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, et al. (2011) Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest 121: 4477-4490.
- Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, et al. (2010) Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One 5.
- Urbanska M, Gozdz A, Swiech LJ, Jaworski J (2012) Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J Biol Chem 287: 30240-30256.
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelárová H, Meijer AJ (1997) The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243: 240-246.
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451: 1069-1075.
- Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, et al. (2011) Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med 18: 159-165.
- Palop JJ, Mucke L (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci 13: 812-818.
- Choi HK, Choi KC, Yoo JY, Song M, Ko SJ, et al. (2011) Reversible SUMOylation of TBL1-TBLR1 regulates β-catenin-mediated Wnt signaling. Mol Cell 43: 203-216.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15551
- [From(publication date):
December-2013 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 10978
- PDF downloads : 4573