Research Article Open Access
Simultaneous Estimation of Aceclofenac, Paracetamol and Tizanidine in Their Combined Dosage Forms by Spectrophotometric and RP- HPLC Method
Chandrashekhar Narajji1*, Hardik R Patel1, Manohar D Karvekar2 and Suresh Babu AR31Dept of Quality Assurance, Acharya & B.M Reddy College of Pharmacy, Soldevanahali, Bengaluru -560 090, India
2Dept of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bengaluru- 560 035, India
3G7 Synergon Private Limited, Bengaluru– 560054, India
- *Corresponding Author:
- Dr. Chandrashekhar Narajji
Dept of Quality Assurance
Acharya & B.M Reddy College of Pharmacy
Soldevanahali, Bengaluru -560 090, India
E-mail: chandrashekharn@acharya.ac.in
Received date: July 13, 2011; Accepted date: October 03, 2011; Published date: October 07, 2011
Citation: Chandrashekhar N, Patel HR, Karvekar MD, Suresh BAR (2011) Simultaneous Estimation of Aceclofenac, Paracetamol and Tizanidine in Their Combined Dosage Forms by Spectrophotometric and RP- HPLC Method. J Anal Bioanal Tech 2:123. doi: 10.4172/2155-9872.1000123
Copyright: © 2011 Chandrashekhar N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A rapid and sensitive RP-HPLC method with UV detection and UV spectrophotometric method for routine Pharmaceutical quality control of aceclofenac, paracetamol and tizanidine in tablets formulation was developed. Chromatography was performed by using Phenomenex-Luna C 18 (250 x 4.6 mm, 5μ) with a mobile phase containing Methanol: Water (90:10v/v), flow rate 1mL/min, wavelength at 256 nm. Linearity observed over the concentration range between 5-30μg/mL, 10-60μg/mL and 2-12μg/mL for aceclofenac, paracetamol and tizanidine respectively. The UV spectrophotometric method was performed at 277, 248 and 323 nm by derivative spectrophotometry with simultaneous equation method. The entire three drugs obey Beer’s law in the concentration range between 5-30 μg/mL, 2-16 μg/mL and 2-30 μg/mL respectively. The proposed methods were simple, rapid, precise, accurate and sensitive and can be used for routine quality control in pharmaceuticals.
Keywords
Derivative spectrophotometry; RP-HPLC; Aceclofenac; Paracetamol; Tizanidine
Introduction
Aceclofenac (ACO) is 2-[2-[2-[(2,6diclorophenyl) amino] phenyl] acetyl] oxyacetic acid and non-steroidal anti inflammatory drug [1]. Aceclofenac is used for the relief of pain and inflammation in rheumatoid arthritis, osteoarthritis. Paracetamol (PCM) is N-(4 hydroxyphenyl) acetamide [2] is a potent analgesic and anti-pyretic drug, Tizanidine (TZN) is 5-choloro N(4,5dihydro-1H-imidazol-2-yl)- 2,1,3-benzothiadiazol-4-amine, used as a muscle relaxant [3]. It used to treat the spasms, cramping and tightness of muscles etc. (Figure 1).
A tablet dosage form containing all the three (ACO 100 mg, PCM 500 mg and TZN 2 mg) is commercially available and used as nonsteroidal anti-inflammatory, analgesic, muscle relaxant. ACO, PCM and TZN are official in Indian pharmacopoeia. ACO has been analysed separately and in combination with other drugs by UV [4-7] and HPLC [8-12]. PCM has been analysed separately and combination with other drug by UV [13-15], HPLC [16], RP-HPLC [17]. TZN has been analysed separately and combination with other drug by UV [18-20], RP-HPLC [21-22], HPTLC [23-24] and spectrophotometric method has been reported for ACO and TZN [25]. Spectrophotometric method has been reported for estimation of PCM, NIMS (Nimesulide) and TZN [26]. However no spectrophotometric method for simultaneous analysis of ACO, PCM and TZN in combination dosage form has been reported yet, hence the present study is essential to develop simultaneous estimation method for all three drugs in tablet formulation.
Experimental
Reagents and chemicals
The ACO, PCM and TZN reference standards were obtained from Micro labs Pvt. Ltd. Bangalore, Karnataka and Excel Parma Pvt. Ltd, Mehsana, Gujarath. Pharmaceutical dosage forms (Zerodol MRR) containing 500 mg PCM, 100 mg ACO and 2 mg TZN were obtained commercially (Manufactured by IPCA Laboratories Ltd. Mumbai). Acetonitrile and Methanol for chromatography were purchased from Merck and S.D. fine chemicals. HPLC Grade water from Milli-Q Gradient A10 (Milli-pore), were used to prepare the solutions for the UV spectrophotometric experiments.
Apparatus / Instrumentation and analytical conditions
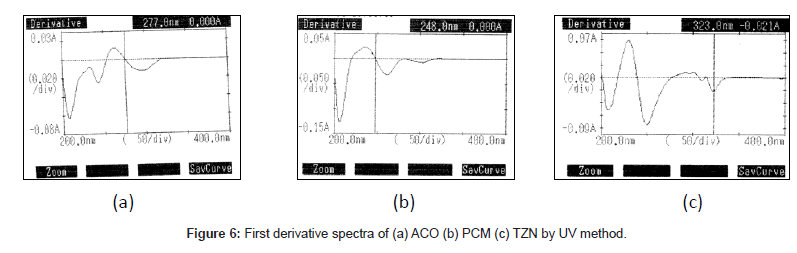
Chromatographic analysis was performed using Shi-madzu LC- 20AT HPLC system equipped with LC-20AT pump and SPD-M20A detector, Chromatography was performed on Phenomenex Luna C18 column (250 × 4.6 mm i.d, 5μ) maintained at 40ºC. The mobile phase composition was Methanol: Water 90:10 v/v (Binary flow) which was pumped at flow rate of 1 mL/min without splitter. The mobile phase was prepared freshly before use and filtered through 0.45 μm membrane filter (Milli-pore) and degassed by ultra sonication bath before use .The auto sampler temperature was set at 10ºC. The wavelength of the UV detector was set to 256 nm, The UV spectrophotometric method was performed on a UV/Visible double beam spectrophotometer (Shimadzu model-1700) at 277 nm, 248 nm and 323 nm using 1cm Quartz cells.
Preparation of the standard solution
For the HPLC method, ACO, PCM and TZN (10 mg each) were weighed accurately and transferred to separate 100 mL volumetric flasks. All drugs were dissolved in 100 mL methanol to prepare standard stock solution of 100 μg/ mL. For the UV spectrophotometric method, ACO, PCM and TZN (10 mg each ) were weighed and transferred to 100 mL volumetric flask, dissolved and made up to the volume-using methanol. Accurately pipette out 10 ml of above solution separately into 100 ml standard flask and the volume was made up to 100 ml using methanol to get a concentration of 100 μg/ml of ACO, PCM and TZN respectively.
Preparation of the sample solutions
For both UV and HPLC analysis of the tablet dosage form, twenty tablets of Zerodol MRR were weighed individually and their average weight was determined. The tablets were then crushed to a fine powder and powder equivalent to the weight of one tablet was transferred to a 100 mL volumetric flask and dissolved in 50 mL methanol. The solution was shaken vigorously for 15 min and filtered through Whatman #41 filter paper.
Construction of calibration plots
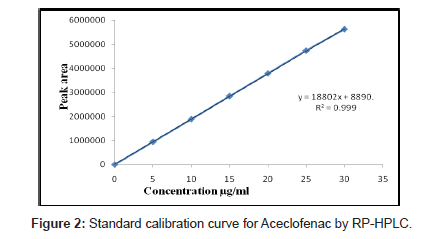
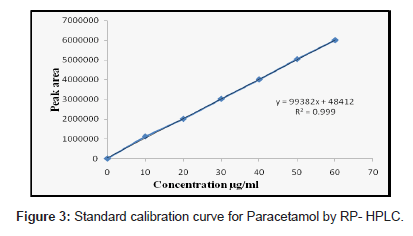
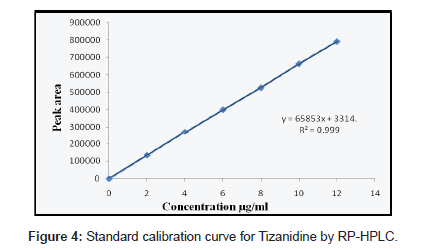
Solutions of ACO, PCM and TZN drugs having different concentrations were prepared by dilution of the standard solutions. These solutions (20 μL) were chromatographed and the peak areas were measured. Peak areas were then plotted against the respective concentrations for ACO, PCM and TZN. From the plots it was found that the linear range for ACO, PCM and TZN was between 5-30, 10- 60 and 2-12 μg/mL for HPLC method whereas for UV method was between 5-30, 2-16 and 2-30 μg/mL.
Method validation
The methods were validated according to International Conference on Harmonization (ICH) guidelines for validation of analytical procedures [27]. The System suitability test was carried through for both the methods evaluating theoretical plates and asymmetry.
Linearity
The calibration curve was obtained with five concentrations of the standard solution in the range 5 to 30, 10 to 60 and 2 to 12 μg/mL for HPLC method and for UV spectrophotometric method the linearity range was optimized with 5 to 30, 2 to 16 and 2 to 30 μg/mL for ACO, PCM and TZN respectively (Figure 2-4).
Precision
The assay precision was carried out by repeatability (intra-day) and intermediate precision (inter-day). Repeatability was evaluated by assaying sample solutions of three different concentrations of ACO, PCM and TZN (5, 10 and 15 μg/mL, 10, 20 and 30 μg/mL, 6, 8 and 10 μg/ mL for HPLC method and 5, 10 and 15 μg/mL, 6, 8 and 10 μg/mL, 5, 10 and 15 μg/mL for UV spectrophotometric method) were prepared and assayed at same concentration and during the same day. The Percentage relative standard deviation (% RSD) values of peak area for repeated injections of ACO, PCM and TZN were found to be 0.08 %, 0.17%, 0.023 % and 0.023%, 0.014 %, 0.027% respectively. Intermediate precision of the method was checked by repeating studies on three different days. Percentage relative standard deviation (%RSD) was found to be less than acceptable limit of 2% for within a day and day to day variations, which proves that method is precise.
Accuracy
The accuracy of an analytical method is the closeness of the test results obtained by the method to the true value. For the spectrophotometric method, the accuracy was determined by recovery of known amounts of ACO, PCM and TZN reference standard added to the samples at the beginning of the process. The accuracy was assessed from five replicate determinations of three concentration levels and the absolute means obtained were shown in Table 3, and it is evident that the method is accurate within the desired range. For the HPLC method, the accuracy was determined by the assay of three concentrations of the sample solution in triplicate and the absolute means obtained were shown in Table 3.
Specificity
The specificity of the HPLC and UV spectrophotometric methods was determined. This parameter was performed to assess and ensure that the impurities, degraded products and diluents do not affect the samples analyzed. ACO, PCM and TZN were injected into the system and chromatograms are recorded. For the UV spectrophotometric method, the specificity was obtained by the absorption spectra of the ACO, PCM and TZN reference standard 10μg/mL and by the use of the simulated sample of excipients.
Robustness
The effect of small, deliberate variation of the analytical conditions on the peak areas and retention time of the ACO, PCM and TZN were examined, such as small changes in the percentage (±5%) in the mobile phase and flow rate (±0.1mL/min) [28]. The robustness of the UV spectrophotometric method was determined by small changes in the solvent lot.
Limit of detection (LOD) and quantification (LOQ)
The LOD and LOQ of ACO, PCM and TZN by proposed methods were determined using calibration standards. LOD and LOQ of the drugs were calculated as 3.3σ/S and 10σ/S, respectively, where σ is the standard deviation of the response (y-intercept) and S is the slope of the calibration plot and showed in Table 2.
Results and Discussion
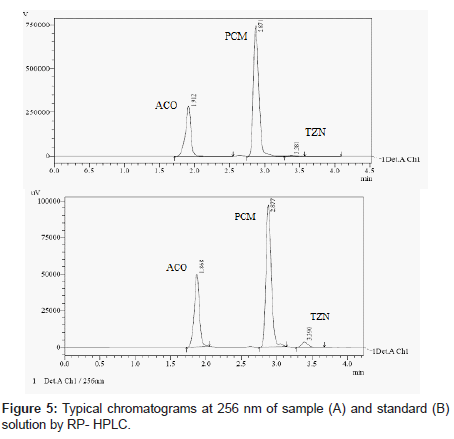
HPLC method
The mobile phase selection was based on peak parameters (symmetry, tailing), run time, ease of preparation and cost. Figure 5 shows a typical chromatogram which was obtained from the analysis of a standard and sample of ACO, PCM and TZN using the proposed method (Table 1) and were eluted forming a symmetrical peak. The retention time observed (1.87, 2.85 and 3.38 min) allowed a rapid determination of the drug, which is important for routine analysis. The calibration curves for ACO, PCM and TZN were constructed by plotting peak area verses concentration and showed good linearity within 5-30, 10-60 and 2-12 μg/ mL range. The representative linear equation was y = 18802 x + 8890, y = 99382x + 48412 and y = 65853x + 3314 with a correlation coefficient (r2 = 0.9999) highly significant for the method (Table 2). LOD and LOQ were found to be 0.0194, 0.2246, 0.111μg/ mL and 5, 2, 10μg/mL respectively, indicating high method sensitivity. The method precision was determined by repeatability (intra-day) and intermediate precision (inter-day) and was expressed as RSD (%) of a series of measurements. The result obtained showed RSD of 0.08, 0.17 and 0.023% indicating good precision (intra-day). Inter-day variability was calculated in three different days and it showed a mean RSD of 0.35, 0.54 and 0.047% respectively. The accuracy of the method was determined and the mean recovery was found to be 101.68, 100.55 and 100.32%, indicating an agreement between the true value and the found value (Table 3). The HPLC method is specific to the evaluation of ACO, PCM and TZN in tablets. No interfering peaks were observed when simulated samples of excipients were added in the sample solutions. The method was found to be robust when the mobile phase and flow rate were varied.
| Formulation | Drug | Label claim mg/tablet |
HPLC method | UV method |
|---|---|---|---|---|
| % label claim found* ± % RSD |
% label claim found* ± % RSD |
|||
| Zerodol- MRR | ACO | 100 | 101.68±0.99 | 99.78±0.98 |
| PCM | 500 | 100.55±0.64 | 99.84±0.48 | |
| TZN | 2 | 100.32±0.34 | 99.70±0.63 |
*Average of five readings
Table 1: Result of assay by HPLC and UV method.
| Parameter | HPLC method | UV method | ||||||
|---|---|---|---|---|---|---|---|---|
| ACO | PCM | TZN | ACO | PCM | TZN | |||
| Retention time (min) | 1.89 | 2.87 | 3.34 | - | - | - | ||
| Working λmax (nm) | - | - | - | 277 | 248 | 323 | ||
| Linearity (µg/mL) | 5-30 | 10-60 | 2-12 | 5-30 | 2-16 | 2-30 | ||
| LOD (µg/mL) | 0.019 | 0.224 | 0.111 | 0.81 | 0.21 | 0.29 | ||
| LOQ (µg/mL) | 0.588 | 0.678 | 0.338 | 1.4 | 0.717 | 0.582 | ||
| Precision(%RSD*) | Intra-day precision | 0.08 | 0.17 | 0.023 | 0.023 | 0.014 | 0.027 | |
| Inter-day precision | 0.35 | 0.54 | 0.047 | 0.047 | 0.023 | 0.029 | ||
| Theoretical plate/meter | 20186.48 | 37466.62 | 42965.18 | - | - | - | ||
| Asymmetry | 0.9 | 1.2 | 1.2 | - | - | - | ||
| Resolution | 6.914 | 3.175 | - | - | - | |||
| Sandell’s sensitivity | - | - | - | 0.029 | 0.011 | 0.027 | ||
| Slope | 18802 | 99382 | 65853 | 0.033 | 0.092 | 0.0189 | ||
| Intercept | 8890 | 48412 | 3314 | 0.008 | 0.005 | 0.0295 | ||
| Regression coefficient (r2) | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | ||
*Average of three readings
Table 2: Validation parameter of HPLC and UV Method
| Drug | HPLC METHOD | UV METHOD | ||||||
|---|---|---|---|---|---|---|---|---|
| Amount of sample (µg) | Amount of standard (µg) |
Total conc. found (µg) |
% Recovery± %RSD* |
Amount of sample (µg) | Amount of standard (µg) |
Total conc. found (µg) |
% Recovery± %RSD* |
|
| ACO | 8 | 6 | 14.02 | 100.04±0.34 | 10 | 10 | 19.8 | 99.92±0.87 |
| 8 | 8 | 16.06 | 100.08±0.74 | 10 | 12 | 21.05 | 101.15±0.15 | |
| 8 | 10 | 18.15 | 101.53±0.69 | 10 | 14 | 24.3 | 101.38±0.84 | |
| PCM | 40 | 5 | 45.03 | 101.79±0.79 | 50 | 10 | 59.9 | 99.13±0.07 |
| 40 | 10 | 50.17 | 100.30±0.47 | 50 | 12 | 62.6 | 99.09±0.97 | |
| 40 | 15 | 55.05 | 100.04±0.34 | 50 | 14 | 63.4 | 98.94±0.62 | |
| TZN | 0.16 | 1 | 1.15 | 99.75±0.32 | 0.2 | 10 | 9.8 | 100.73±1.00 |
| 0.16 | 2 | 2.14 | 99.12±0.89 | 0.2 | 12 | 12.09 | 99.77±0.50 | |
| 0.16 | 3 | 3.13 | 99.06±0.12 | 0.2 | 14 | 13.8 | 99.90±0.95 | |
*Average of three readings
Table 3: Result of recovery study
UV Spectrophotometric method
The proposed first derivative spectrophotometric method allowed a rapid and accessible quantitation of ACO, PCM and TZN in tablets without any time consuming sample preparation. Moreover, the spectrophotometric method involved simple instrumentation compared with other instrumental techniques. The absorption spectra of ACO, PCM and TZN showed λmax was 277 nm, 248 nm and 323 nm, which was the wavelength used (Figure 3). The calibration curves were constructed in the range of expected concentrations (5-30, 2-16 and 2-30 μg/mL). The representative equation analysis was y = 0.033x + 0.006, y = 0.092x - 0.005 and y = 0.0189x + 0.0296 with a correlation coefficient of 0.9999 (Table 2). LOD and LOQ were found to be 0.81, 0.21, 0.29 μg/mL and 1.4, 0.71, 0.58μg/mL respectively, showing that the experimental values obtained for the determination of ACO, PCM and TZN in the samples indicated a satisfactory intra-day variability (RSD of 0.023, 0.014 and 0.027%) and inter-day variability (R.S.D. of 0.047, 0.023 and 0.029%). A good accuracy of the method was verified with a mean recovery of 99.78, 99.84 and 99.70% (Table 3). Finally, the method showed to be specific for the determination of ACO, PCM and TZN in tablets.
Comparison between HPLC, UV Spectrophotometric and the official method
The HPLC method and the UV method developed and validated for the analysis of ACO, PCM and TZN in tablets were found to be reliable, simple, fast, precise, accurate and sensitive. Results of UV spectrophotometric method showed no significant difference from those obtained with the method of HPLC and the official method (P > 0.01) but when LOD, LOQ and assay parameter of both the methods were compared and HPLC method was found to be more sensitive than the UV method. Hence these HPLC methods can be used for simultaneous estimation of ACO, PCM and TZN routinely in tablet. In summary, the proposed method can be used for the routine of quality control in pharmaceuticals.
Acknowledgements
The author expresses gratitude to Rajiv Gandhi University of Health science, Bangalore, for providing research grant and also thanks to Micro Lab Pvt. Ltd. and Excel Pharma Pvt. Ltd for providing ACO, PCM and TZN. The authors also thankful to chairman, principal and staff member of Acharya and B.M. Reddy College of Pharmacy, Bangalore, for providing required facilities to carry out this research work.
References
- The Indian pharmacopoeia commission "Indian Pharmacopoeia" (2010) Ghaziabad 1859, 2230.
- Medicines and Health Care Products Regulatory Agencies "British Pharmacopeia" (2009) Stationary Office London 44: 1554.
- Maryadele JO, Smith A (1996) "The Merck Index", Merck Research Laboratories. NJ, USA 1174.
- Rohit S, Chandrakant M, Shital KP, Dhayan KC, Nilofar N (2008) Validated spectroscopic method for estimation of aceclofenac from tablet formulation. Research J Pharm and Tech 1: 430.
- Vivek SR, Santoss VG, Upasana PP, Mahima RS (2009) Simultaneous determination of drotaverine hydrochloride and aceclofenac in tablet dosage form by spectrophotometry. Eurasian. J Anal Chem 4: 184.
- Shanmugam S, Cendil AK, Vetrichelvan T, Manavalan R, Venkappayya D, et al. (2005) Spectrophotometric method for estimation of aceclofenac in tablets. Indian Drugs 42: 106.
- Bose A, Dash PP, Sahoo MK (2010) Simple spectrophotometric methods for estimation of aceclofenac from bulk and formulations. Pharmaceutical method 1: 57.
- Zawilla NH, Mohammad M, Azim A (2002) Spectrophotometric methods for the determination of acediasulfone in mixture with cinchocaine. J Pharm Biomed Anal 7: 243.
- Lee HS, Jeong CK, Choi SJ, Kim SB, Lee MH, et al. (2000) Simultaneous determination of aceclofenac and diclofenac in human plasma by narrowbore HPLC using column-switching, J Pharm Biomed Anal 23: 775.
- Hinz B, Auge D, Rau T, Rietbrock S, Brune K, et al. (2003) Simultaneous determination of aceclofenac and three of its metabolites in human plasma by high-performance liquid chromatography. Biomed Chromatogr 17: 268.
- Raja RK, Sankar GG, Rao AL, Seshagiri RJ (2005) Development and validation of RP-HPLC method for the estimation of aceclofenac in tablet dosage form. Indian Drugs 2: 693.
- Jin J, Chen H, Gu S (2009) Determination of aceclofenac in human plasma by reversed-phase high performance liquid chromatography. Chinese J Chromatography 22: 252.
- Karla K, Naik S, Jurmal G, Mishra N (2009) Spectrophotometric method for simultaneous estimation of paracetamol and domperidone in tablet formulation. Asian J Research Chem 2: 112.
- Sridhar N, Pradeep K, Rakesh S, Ayushman T, Manik G (2009) Simultaneous analysis of paracetamol and tramadol - analytical method development & validation. Der Pharma Chemica 1: 72.
- Wafaa SH (2008) Determination of ibuprofen and paracetamol in binary mixture using chemometric-assisted spectrophotometric methods. Am J Applied Sci 5: 1005.
- Karthik A, Subramanian G, Ranjith Kumar A, Udupa N (2007) Simultaneous estimation of paracetamol and domperidone in tablets by reverse phase HPLC method. Indian J Pharm Sci 69: 142.
- Joshi R, Sharma R (2008) Development and validation of RP-HPLC method for simultaneous estimation of three-component tablet formulation containing acetaminophen, chlorzoxazone and aceclofenac. Anal Lett 41: 3297.
- Devarajan, Lakshami S (2006) Simultaneous spectrophotometric determination of valdecoxib and tizanidine in tablets Indian. J Pharm Sci 68: 240.
- Sujatha K, Chitra K, Hettiarachchi DS, Krishna MV, et al. (2003) Spectrophotometric determination of tizanidine hydrochloride. Indian J Pharm Sci 65: 519.
- Shankar MB, Shah DA, Geeta M, Mehta MF, Mehta RS, et al (2004) Simultaneous spectrophotometric determination of tizanidine and diclofenac in tablets. Indian J Pharm Sci 66: 332.
- Manisha P, Wadher SJ, Seema D, Yeole PG (2006) Simultaneous estimation of valdecoxib and tizanidine hydrochloride in tablets by RP-HPLC. Indian J Pharm Sci 68: 670.
- Nimje H, Wate SP, Dharkar DP, Razdam P (2007) Simultaneous RPHPLC determination of nimesulide and tizanidine in tablets. Indian J Pharm Sci 69: 281.
- Senthil MK, Subramanian G, Vasudevan M (2005) HPTLC estimation of tizanidine and diclofenac sodium in combination tablets. Indian drugs 42: 465.
- Ravi TK, Gandhimathi M, Sireesha KR (2006) HPTLC method for the simultaneous estimation of tizanidine and rofecoxib in tablets. Indian J Pharm Sci 68: 234.
- Kumar RS, Nathan PS, Nallasivan PK, Sam Solomon WD, Venkatnarayanan R (2010) Validated RP-HPLC method for the simultaneous estimation of aceclofenac and diacerein in bulk and formulation. Int J Pharm Tech Res 2: 945.
- Chandratrey A, Sharm R (2010) Simulatanous spectrophotometric estimation and validation of three components tablet formulation containing paracetamol, nimesulide and tizanidine. Indian J Chem Technol 17: 229.
- ICH Harmonised Tripartite Guidelines "Validation of Analytical Procedures: Text and Methodology Q2 (R1)", 2005.
- Sistla R, Tata VSSK, Kashyap YV, Chandrasekar D, Diwan PV (2005) Development and validation of a reversed-phase HPLC method for the determination of ezetimibe in pharmaceutical dosage forms. J Pharm Biomed Anal 39: 517.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17277
- [From(publication date):
September-2011 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 12438
- PDF downloads : 4839