Research Article Open Access
Simultaneous Determination of Morphine, Morphine Glucuronides (M3G, M6G) and Oxycodone in Human Plasma by High-performance Liquid Chromatography
Tomoya Sakurada1*, Seiji Zusi2, Eriko Kobayashi1, Nobunori Satoh3 and Shiro Ueda11Department of Drug Information and Communication, Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba, 260-8675, Japan
2Yell Pharmacy Group Co., Ltd, 1503-2-10-45 Hijima-cho, Kochi, 780-0066, Japan
3Department of Clinical Education and Research, Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba, 260-8675, Japan
- *Corresponding Author:
- Tomoya Sakurada
Department of Drug information and Communication
Graduate School of Pharmaceutical Sciences
Chiba University,1-8-1 Inohana
Chuo-ku, Chiba, 260-8675, Japan
Tel: (81-43)226-2884
Fax: (81-43)226-2884
E-mail: sakurat@p.chiba-u.ac.jp
Received date: August 31, 2010; Accepted date: September 29, 2010; Published date: October 01, 2010
Citation: Sakurada T, Zusi S, Kobayashi E, Satoh N, Ueda S (2010) Simultaneous Determination of Morphine, Morphine Glucuronides (M3G, M6G) and Oxycodone in Human Plasma by High-performance Liquid Chromatography. J Anal Bioanal Tech 1:101. doi: 10.4172/2155-9872.1000101
Copyright: © 2010 Sakurada T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Morphine and oxycodone are widely used as analgesic drugs for cancer pain. Frequently, morphine and oxycodone are given alternately to avoid adverse drug reactions. Morphine is metabolized primarily into two glucuronide metabolites, morphine-3-glucuronide and morphine-6-glucuronide to be pharmacologically active. Morphine-3-glucuronide and morphine-6-glucuronide have neuroexcitatory action and analgesic activity, respectively. Oxymorphone, a metabolite of oxycodone, has an analgesic effect, however it is so small that it can be neglected when considering oxycodone. The pharmacological effects of these drugs and also their metabolites have been reported in experimental papers, but in humans, the relationships between these plasma concentrations and the clinical effects remain unclear. Also the necessity for simultaneous determination of both drugs has been suggested because opioid rotation is performed clinically. However, to date there is no study which has simultaneously determined these four drugs, and also achieved a high recovery. In this paper, in order to perform a reliable pharmacokinetic study of cancer pain patients receiving morphine and oxycodone, an easy, rapid, sensitive and selective analytical method was proposed and validated.
Keywords
High-performance liquid chromatography; Human plasma; Morphine; Morphine-3-glucuronide; Morphine-6-glucuronide; Oxycodone; Simultaneous determination
Abbreviations
CV: Coefficient of Variation; HPLC: High- Performance Liquid Chromatography; IS: Internal Standard; MOR: Morphine; M3G: Morphine-3-glucuronide; M6G: Morphine-6- glucuronide; OXY: Oxycodone; QC: Quality Control; RE: Relative Error
Introduction
Morphine (MOR) and oxycodone (OXY) are the only oral strong opioid preparations currently available in Japan. The 3-step analgesic ladder recommended by the World Health Organization has been widely used in cancer pain management [1-3], and guidelines based on these steps have also been recommended for the treatment of non-cancer pain [4,5]. MOR and OXY are on the third step of the analgesic ladder. However, since there is small specification of 5mg in Japan, OXY is used from second step of the analgesic ladder. MOR is metabolized primarily to two glucuronide metabolites, morphine- 3-glucuronide (M3G) and morphine-6-glucuronide (M6G) to be pharmacologically active [6,7]. M3G and M6G have neuroexcitatory action and analgesic activity, respectively; MOR administration contributes to the analgesic effect however adverse drug reactions have been reported [8-11]. Therefore, in patients administered MOR, it is of concern to assess these metabolites as well as the plasma concentration of MOR.
On the other hand, oxymorphone, a metabolite of OXY has an analgesic effect, but it is produced in such a small amount it can be neglected when we considering OXY [12]. OXY is reported to have an analgesic potency 1.5 fold that of morphine after oral administration for chronic cancer related pain [13]. Additionally, it has high oral bioavailability (60-87%) [14,15] in humans and fewer adverse effects (hallucinations, pruritus, vomiting) [16,17]. Also, an opioid rotation is often carried out between MOR and OXY in clinic. Opioid rotation is a method of pain management in which one strong opioid analgesic is switched to another in the treatment of chronic pain when side effects (e.g. nausea, vomiting, drowsiness and constipation) are uncontrollable and/or pain relief is inadequate despite dose titration, or for other reasons including cognitive failure, delirium, myoclonus, local toxicity, severe sedation [18-21]. Therefore, in order to perform a reliable pharmacokinetic study of cancer pain patients receiving MOR and OXY a simultaneous determination method was necessary.
Several methods of analysis have been reported for MOR, M3G, M6G and OXY. These methods include high-performance liquid chromatography (HPLC) combined with electrochemical, ultraviolet or fluorescence detection [22-26]. More analysis methods with gas chromatography and liquid chromatography coupled to mass spectrometric detection have been developed [27,28]. However, these methods are intended for MOR or OXY solely, MOR and its active metabolite, and they do not determine the four drugs simultaneously. Recently, a method employing HPLC combined with electro spray ionization-tandem mass spectrometry for low level quantitation of MOR, M3G, M6G and OXY has been described [29]. The novel methods are exceptionally robust and sensitive, however access to the necessary instrumentation is often limited and they commonly include derivatization, multiple extraction steps, solvent evaporation and expensive equipment.
The most common analytical techniques currently used are HPLC with UV detectors. For the analysis of MOR, M3G, M6G and OXY the published reports focus on HPLC with UV detection [23,25], however, it’s not simultaneous determination and often short concentration intervals are used. Here, we describe an analytical method based on HPLC which enables simultaneous determination of these four plasma concentrations, and it satisfies the range of assay concentration to treat clinical samples.
Experimental Section
Chemicals and reagents
MOR was provided by Takeda Pharmaceutical Company (Osaka, Japan). M3G, M6G, OXY and Naloxone as internal standards were provided by Sigma-Aldrich (Tokyo, Japan). Acetonitrile for the mobile phase was HPLC-grade (Wako, Osaka, Japan). All other reagents were analytical grade (Wako, Osaka, Japan). HPLC-grade water was obtained by purifying distilled water in a Milli-Q filtration system (Millipore, Bedford, MA, USA).
Preparation of stock solutions
Primary stock solutions of MOR, M3G, M6G, OXY (100 μg/ mL) and the internal standard (IS) for naloxone (100 μg/ mL) were prepared in deionized water and stored at -20°C. Working solutions of MOR, M3G, M6G, OXY and naloxone were prepared daily by diluting primary stock solutions with deionized water. Human plasma calibration standards of MOR, M3G, M6G and OXY were prepared by spiking appropriate aliquots of the working standard solution of MOR to drug-free human plasma to give final concentrations (MOR, M6G, OXY from 10 to 600 ng/ mL, M3G from 50 to 3000 ng/ mL). Each solution was stored in a plastic 1.5 ml tube and kept at -20°C.
HPLC conditions
The HPLC system consisted of Shimadzu (Kyoto, Japan) equipment (LC-10AD pump, SIL-10A DVP autosampler, SCL-10A system controller and a SPD-10A UV detector) and a CTO-10AC column thermostat (Kyoto Chromato, Kyoto, Japan). The UV detector wavelength was set at 210 nm. The separation was performed on a CAPCELL PAK SCX UG 80 (Shiseido, 5 μm, 2.0 mm i.d.× 150 mm), and a CAPCELL PAK SCX UG 80 precolumn (Shiseido, 5 μm, 2.0 mm i.d.× 35 mm), using a mixture of potassium dihydrogen phosphate buffer (pH 3.0; 50 mM) and acetonitrile (40: 60, v/v) at a flow rate of 0.2 mL/min and at a column temperature of 40°C. The mobile phase was filtered through 0.45 μm pore size membranes (Millipore) and degassed in an ultrasonic bath. A volume of ten microliters was injected into the HPLC column.
Five calibration curves of MOR, M6G and M3G were determined in a concentration range of 10-600 ng/mL (MOR, M6G, OXY: 10, 40, 150, 300, 600 ng/mL) and 50-3000 ng/mL (M3G: 50, 200, 750, 1500, 3000 ng/mL). Peak-height ratios of MOR, M3G, M6G and OXY to the internal standard were used to generate standard calibration curves by plotting the peak-height ratio of MOR/naloxone, M3G/naloxone, M6G/naloxone and OXY/naloxone versus the concentration of each drug in the plasma samples. A least squares linear regression analysis was performed to determine slope and coefficient of variation (CV).
Quality control (QC) samples were prepared from pooled drugfree human plasma, in advance. (QC1: 10ng/ml MOR, 50ng/ml M3G, 10ng/ml M6G, 10ng/ml OXY; QC2: 40ng/ml MOR, 200ng/ml M3G, 40ng/ ml M6G, 40ng/ml OXY; QC3: 300ng/ml MOR, 1500ng/ml M3G, 300ng/ml M6G, 300ng/ml OXY; QC4: 600ng/ml MOR, 3000ng/ml M3G, 600ng/ml M6G, 600ng/ml OXY). QC samples were stored deep-frozen at -20°C. Intra- and inter-assay precisions were evaluated by QC samples. The naloxone was added to each QC sample just prior to sample processing. The intra-day variation was analyzed on the same day, and the inter-day variation was measured on three different days.
The CV served as a measure of precision. According to another report, the CV should be less than 15%, except at the lower limit of quantification where it should not exceed 20% [30].
The accuracy of the assay was determined on the above samples, by comparing the means of the measured MOR, M3G, M6G and OXY concentrations with the specified concentrations either in standard samples (intra-day accuracy) or in QC samples (inter-day accuracy). The percentage deviation of the mean from true values, expressed as relative error (RE) served as a measure of accuracy. The mean value of RE should be within ±15% of the nominal value, except at the lower limit of quantification where it should not exceed 20% [30].
The absolute recovery of each drug was determined by extracting standard solutions of the drugs which added to drug-free human plasma by the proposed method, the peak heights obtained were compared to those obtained after direct injection of non-extracted standard solutions, at the same concentrations.
Solid-phase extraction
A solid-phase extraction cartridge (Oasis HLB, Waters Co., Milford, MA) was used for solid-phase extraction. Cartridges were preconditioned by flushing with 1mL of acetonitrile and 1 mL of distilled water. Plasma samples and calibration standards of 0.95mL were added to the internal standard solution (naloxone 0.4 mg/mL) of 50 μL. After precipitation of plasma proteins with 0.7 N perchloric acid, the supernatant of 1 mL was passed through the column and then washed with 0.5 mL of water and were eluted with an elution solvent (acetonitrile : water = 2 : 1 ) of 1 mL and 10 μL were injected into the HPLC system.
Results and Discussion
Chromatography
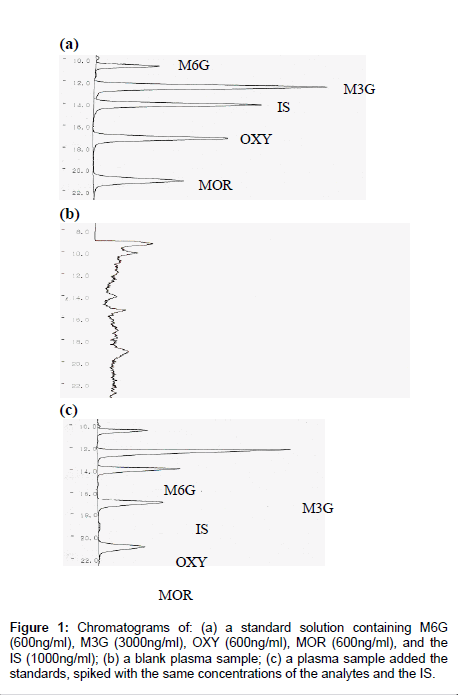
Sample preparation for this study included a modification of the extraction methods proposed for the separation of MOR from plasma by other authors [31] including a single step extraction in solid-phase. A reversed-phase solid-phase extraction cartridge (Oasis® HLB, Waters Co., Milford, MA) was used for solid-phase extraction. The material used in this kind of column is a copolymer designed to have a hydrophilic-lipophilic balance that gives high and reproducible recoveries for acidic, basic and neutral compounds, even if the cartridge runs dry. Good sensitivity and short retention times were obtained with this HPLC system. The proposed method enables the simultaneous quantification of MOR, M3G, M6G and OXY in a human plasma sample on the same HPLC run. Retention times for MOR, M3G, M6G, OXY and IS were 21.0, 12.4, 10.6, 17.1 and 14.1min, respectively, and the total run time of analysis was 25min. MOR, M3G, M6G, OXY and IS gave well resolved, sharp peaks. Endogenous compounds interfering with retention times of the compounds or that of the IS could not be seen in the chromatograms and the chromatographic background after extraction was clean. A typical chromatogram after extraction from plasma is shown in Figure 1. Separations with a CAPCELL PAK SCX UG 80 column 5μm, 150mm×2.0mm ( Shiseido Co., Tokyo) gave good resolution of the compounds. In order to protect the column and improve its lifetime, a guard column packed with the same material was used. Adopting these precautions, the analytical column was maintained in a good condition for around 500 times injections. The number of injected samples the column was capable of resolving satisfactorily before accuracy and precision was compromised to an extent requiring replacement of the column.
Method validation
Selectivity: The extraction method also allowed for adequate separation among these compounds. Additionally, when plasma samples which did not contain these compounds were also analyzed by the proposed method, no interference was found. Based on the system suitability test, the mean retention times and S.D. for MOR were 21.19 (0.24), M3G 12.85 (0.44), M6G 10.85 (0.28), OXY 17.18 (0.09), and for the IS 14.13 (0.09) min. On the basis of five replicate determinations, the reproducibility (CV) of the retention time was 1.12, 3.99, 2.61, 0.55% for the compounds and 0.63% for IS.
Calibration curves and linearity: Calibration curves were constructed from the peak height ratios of MOR, M3G, M6G, OXY to IS versus the respective concentrations. Linearity of the calibration curves for the four compounds was achieved for concentrations between 10 and 600ng/mL (MOR, M6G and OXY) and between 50 and 3000ng/mL (M3G). Calibration curves showed good linearity, the correlation coefficients were consistently greater than 0.995 for all compounds.
Precision and accuracy: Table 1 and 2 provide the results obtained for intra-assay and inter-assay precision and accuracy calculations for all analytes. The intra- and inter-precision and accuracy of the method were determined using QC samples at five different concentration levels and at three different concentration levels, respectively. Five replicate determinations were made at each concentration level. The intra- and inter-precision were in the ranges of 1.7-14.9% and 1.2- 17.2%, respectively, while the intra- and inter-accuracy were 1.1-16.1% and 0.1-18.5%, respectively. The variation between the calculated values for standards and the theoretical concentrations were well within the acceptance criterion of <15% for RE and CV, except for the OXY concentration of 10ng/mL. The CV and RE in inter-precision of OXY (10ng/mL) was 17.2% and 18.5, but this satisfied the lower limit of the quantification where it should not exceed 20%. The recovery rate indicated more than 90% except for M3G (intra precision 200ng/ mL; 88.6%), and it was confirmed again that the repeatability was good (Table 3). This high performance chromatographic method with a simultaneous UV detection has been developed for the quantitative determination of MOR, its active metabolites and OXY in human plasma. Many published papers describe the need for multiple extraction procedures and complex chromatographic systems to ensure reproducibility and resolution, but there are few reports which describe the simultaneous measurement of MOR, M3G, M6G and OXY. An extremely sensitive method for the detection of MOR, M3G, M6G and OXY in human plasma using LC-ESI-MS/MS was described by Musshoff F et al. [29]. The determination range of MOR, M6G and OXY was suitable for some clinical specimens. However, because there were many clinical specimens with more than 1000ng/mL of M3G [32], the determination range (10-1000ng/ml) for M3G was not sufficient. Furthermore, its recovery rate was low with 40.5-69.2%. Our method enabled simultaneous measurement of an adapted range of concentration for the clinical specimen at a high recovery rate.
| Nominal concentration | Measured concentration (ng/mL) | CV (%) | RE (%)a | n |
| (ng/mL) | ||||
| MOR 10 | 10.8 | 7.2 | -7.9 | 5 |
| 40 | 38.9 | 1.7 | 2.8 | 5 |
| 150 | 153 | 2.3 | -2 | 5 |
| 300 | 288.8 | 2.8 | 3.7 | 5 |
| 600 | 586.2 | 3.7 | 2.3 | 5 |
| M6G 10 | 11.4 | 8.7 | -14.2 | 5 |
| 40 | 43.7 | 12 | -9.3 | 5 |
| 150 | 152 | 10 | -1.3 | 5 |
| 300 | 303.3 | 9.6 | -1.1 | 5 |
| 600 | 629.8 | 6.4 | -5 | 5 |
| M3G 50 | 55.8 | 9.7 | -11.6 | 5 |
| 200 | 177.3 | 2.4 | 11.4 | 5 |
| 750 | 738.2 | 2.9 | 1.6 | 5 |
| 1500 | 1519.9 | 6.4 | -1.3 | 5 |
| 3000 | 2741.3 | 5.9 | 8.6 | 5 |
| OXY 10 | 11.6 | 14.9 | -16.1 | 5 |
| 40 | 45.2 | 1.7 | -13 | 5 |
| 150 | 150.8 | 1.5 | -0.5 | 5 |
| 300 | 298.1 | 0.9 | 0.6 | 5 |
| 600 | 576.5 | 0.5 | 3.9 | 5 |
a RE (%) = [(nominal concentration-mean concentration found)/nominal concentration]×100.
Table 1: Intra-day precision and accuracy for analysis of MOR, M3G, M6G and OXY in human plasma.
| Nominal concentration | Measured concentration (ng/mL) | CV (%) | RE (%) | n |
| (ng/mL) | ||||
| MOR 10 | 9.6 | 3.6 | 4.1 | 5 |
| 150 | 155.1 | 14.3 | -3.4 | 5 |
| 600 | 600.8 | 12.7 | -0.1 | 5 |
| M6G 10 | 10.5 | 1.2 | -4.7 | 5 |
| 150 | 135.3 | 7.9 | 9.8 | 5 |
| 600 | 620.3 | 5.2 | -3.4 | 5 |
| M3G 50 | 56.2 | 5.7 | -12.4 | 5 |
| 750 | 761.1 | 3 | -1.5 | 5 |
| 3000 | 3253.1 | 12 | -8.4 | 5 |
| OXY 10 | 11.8 | 17.2 | -18.5 | 5 |
| 150 | 152.7 | 4.2 | -1.8 | 5 |
| 600 | 577.9 | 1.4 | 3.7 | 5 |
Table 2: Inter-day precision and accuracy for analysis of MOR, M3G, M6G and OXY in human plasma.
| Nominal concentration | Recovered (%) | |||
| (ng/mL) | MOR | M6G | M3G | OXY |
| 10 | 108.95±7.76 | 118.80±24.03 | â?? | 104.62±4.63 |
| 40 | 97.22±1.64 | 109.29±13.16 | â?? | 97.97±2.96 |
| 50 | â?? | â?? | 111.64±10.80 | â?? |
| 200 | â?? | â?? | 83.24±6.34 | â?? |
| 300 | 96.26±2.70 | 105.50±11.80 | â?? | 96.15±1.92 |
| 600 | 97.70±3.63 | 104.96±6.67 | â?? | 94.55±1.57 |
| 1500 | â?? | â?? | 101.33±6.45 | â?? |
| 3000 | â?? | â?? | 91.38±5.38 | â?? |
| ã?? | ã?? | ã?? | ã?? | ã?? |
Table 3: Percentage recovery of MOR, M3G, M6G and OXY from human plasma.
Conclusions
In conclusion, a relatively simple HPLC method for the simultaneous determination of MOR, its active metabolites and OXY in human plasma is characterized by high sensitivity and appropriate validation parameters, allowing for routine monitoring of opioid therapy including therapy employing opioid rotation. Furthermore, it can be applied in many clinical laboratories because it uses standard apparatus and has the advantages of speed, as well as economy, as only one cartridge is used per extraction. The main advantage of this method is the ability to analyze four compounds simultaneously under similar conditions, thereby saving time and expenses for sample preparation.
References
- World Health Organization (1996) Cancer Pain Relief. (2nd Ed), World Heath Organization, Geneva, Switzerland.
- Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA (1995) Validation of world health organization guidelines for cancer pain relief: a 10-year prospective study. Pain 63: 65-76.
- Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, et al. (2001) Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 93: 247-257.
- Kalso E, Allan L, Dellemijn PL, Faura CC, Ilias WK, et al. (2003) Recommendations for using opioids in chronic non-cancer pain. Eur J Pain 7: 381-386.
- Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, et al. (2006) Opioid guidelines in the management of chronic non-cancer pain. Pain Physician 9: 1-39.
- Iwamoto K, Klaassen CD (1977) First-pass effect of morphine in rats. J Pharmacol Exp Ther 200: 236-244.
- Bock KW, Brunner G, Hoensch M, Huber E, Josting D (1978) Determination of microsomal UDP-glucuronyltransferase in needle-biopsy specimens of human liver. Eur J Clin Pharmacol 14: 367-373.
- Frances B, Gout R, Monsarrat B, Cros J, Zajac JM (1992) Further evidence that morphine-6-glucuronide is a more potent opioid agonist than morphine. J Pharmacol Exp Ther 262: 25-31.
- Wright AW, Nocente ML, Smith MT (1998) Hydromorphone-3-glucuronide: Biochemical synthesis and preliminary pharmacological evaluation. Life Sci 63:401-411.
- Bartlett SE, Cramond T, Smith MT (1994) The excitatory effects of M3G are attenuated by LY274614, a competitive NMDA receptor antagonist, and by midazolam, an agonist at the benzodiazepine site on the GABAA receptor complex. Life Sci 54: 687-694.
- Smith MT,Watt JA, Cramond T (1990) Morphine-3-glucuronide - a potent antagonist of morphine analgesia. Life Sci 47: 579-585.
- Reder RF, Oshlack B, Miotto JB, Benziger DD, Kaiko RF (1996) Steady-state bioavailability of controlled-release oxycodone in normal subjects. Clin Ther 18: 95-105.
- Foley KM, (1985) The treatment of cancer pain. N Engl J Med 313: 84-95.
- Pöyhiä R, Seppälä T, Olkkola KT, Kalso E (1992) The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol 33: 617-621.
- Leow KP, Smith MT, Williams B, Cramond T (1992) Single-dose and steady-state pharmacokinetics and pharmacodynamics of oxycodone in patients with cancer. Clin Pharmacol Ther 52: 487-495.
- Ordóñez Gallego A, González Barón M, Espinosa Arranz E (2007) Oxycodone: a pharmacological and clinical review. Clin Transl Oncol 9: 298-307..
- Lauretti GR, Oliveira GM, Pereira NL (2003) Comparison of sustained-release morphine with sustained-release oxycodone in advanced cancer patients. Br J Cancer 89: 2027-2030.
- de Stoutz ND, Bruera E, Suarez-Almazor M (1995) Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage 10: 378-384.
- Mercadante S (1999) Opioid rotation for cancer pain: rationale and clinical aspects. Cancer 86: 1856-1866.
- MacDonald N, N, Der L, Allan S, Champion P (1993) Opioid hyperexcitability: the application of alternate opioid therapy. Pain 53: 353-355.
- Doyle D, Hanks G, Cherny N, Calman K (2005) Oxford Textbook of Palliative Medicine. (3rd Ed), Oxford University Press, Oxford, UK.
- Groenendaal D, Blom-Roosemalen MC, Danhof M, Lange EC (2005) Highperformance liquid chromatography of nalbuphine, butorphanol and morphine in blood and brain microdialysate samples: application to pharmacokinetic/ pharmacodynamic studies in rats. J Chromatogr B Analyt Technol Biomed Life Sci 822: 230-237.
- Cheremina O,Bachmakov I, Neubert A, Brune K, Fromm MF, et al. (2005) Simultaneous determination of oxycodone and its major metabolite, noroxycodone, in human plasma by high-performance liquid chromatography. Biomed Chromatogr 19: 777-782.
- Meng QC, Cepeda MS, Kramer T, Zou H, Matoka DJ, et al. (2000) Highperformance liquid chromatographic determination of morphine and its 3- and 6-glucuronide metabolites by two-step solid-phase extraction. J Chromatogr B Biomed Sci Appl 742: 115-123.
- Ary K,Róna K (2001) LC determination of morphine and morphine glucuronides in human plasma by coulometric and UV detection. J Pharm Biomed Anal 26: 179-187.
- Bogusz MJ, Maier RD, Erkens M, Driessen S (1997) Determination of morphine and its 3- and 6-glucuronides, codeine, codeine-glucuronide and 6-monoacetylmorphine in body fl uids by liquid chromatography atmospheric pressure chemical ionization mass spectrometry. J Chromatogr B Biomed Sci Appl 703: 115-127.
- Fryirs B, Dawson M, Mather LE (1997) Highly sensitive gas chromatographicmass spectrometric method for morphine determination in plasma that is suitable for pharmacokinetic studies. J Chromatogr B Biomed Sci Appl 693: 51-57.
- Zheng M,McErlane KM, Ong MC (1998) High-performance liquid chromatographymass spectrometry-mass spectrometry analysis of morphine and morphine metabolites and its application to a pharmacokinetic study in male Sprague- Dawley rats. J Pharm Biomed Anal 16: 971-980.
- Musshoff F, Trafkowski J, Kuepper U, Madea B (2006) An automated and fully validated LC-MS/MS procedure for the simultaneous determination of 11 opioids used in palliative care, with 5 of their metabolites. J Mass Spectrom 41: 633-640.
- Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, CDER, CVM, May 2001.
- López P, Bermejo AM, Tabernero MJ, Fernández P, Alvarez I (2007) Determination of cocaine and heroin with their respective metabolites in meconium by gas chromatography-mass spectrometry. J Appl Toxicol 27: 464-471.
- Fredheim OM, Borchgrevink PC, Klepstad P, Kaasa S, Dale O (2007) Long term methadone for chronic pain: a pilot study of pharmacokinetic aspects. Eur J Pain 11: 599-604.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16278
- [From(publication date):
October-2010 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 11711
- PDF downloads : 4567