Review Article Open Access
Silkworm Baculovirus Expression System for Molecular Medicine
Mizuho Kajikawa1, Kaori Sasaki-Tabata2, Hideo Fukuhara3, Masataka Horiuchi3, Yuki Okabe3 and Katsumi Maenaka3*1Laboratory for Infectious Immunity, RIKEN Research Center for Allergy and Immunology, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan
2Department of Pharmacognosy, Graduate School of Pharmaceutical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku Fukuoka, 812-8582, Japan
3Department of Biomolecular Chemistry, Graduate School of Pharmaceutical Sciences, Hokkaido University, N-12, W-6, Kita-ku, Sapporo 060-0812, Japan
- Corresponding Author:
- Katsumi Maenaka
Department of Biomolecular Chemistry
Graduate School of Pharmaceutical Sciences
Hokkaido University, N-12, W-6, Kitaku
Sapporo 060-0812, Japan
E-mail: maenaka@pharm.hokudai.ac.jp
Received date: March 11, 2012; Accepted date: March 24, 2012; Published date: March 26, 2012
Citation: Kajikawa M, Sasaki-Tabata K, Fukuhara H, Horiuchi M, Okabe Y, et al. (2012) Silkworm Baculovirus Expression System for Molecular Medicine. J Biotechnol Biomaterial S9:005 doi:10.4172/2155-952X.S9-005
Copyright: © 2012 Kajikawa M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Keywords
BEVS; BmNPV: Bacmid DNA; Silkworm; Immunoreceptors; GPCR
Recombinant Protein Production for Biomedical Sciences
The rapid evolution of biotechnology in recent years has enabled the identification of various proteins responsible for several diseases. To develop drugs or therapies targeting these proteins, precise understanding of their functions is crucially important. Therefore, many researchers are utilizing combinations of several methods to characterize the molecular basis of the functions of these proteins. However, such biomedical research generally requires large amounts of proteins, and thus efficient protein production is becoming more of a concern. In addition, medically important proteins themselves are expected to be employed as biopharmaceuticals [1]. In this regard, efficient recombinant protein production will presumably reduce the cost.
Among the several recombinant protein expression systems currently available, the Escherichia coli (E. coli) expression system is one of the attractive tools for large-scale recombinant protein expression. This system has the advantages of low cost, simplicity, and high expression level; however, it also has some disadvantages for the expression of many human proteins, such as the lack of posttranslational modifications (i.e. intramolecular disulfide-bond, glycosylation, and phosphorylation). Recently, some improved E. coli systems with phosphorylation or disulfide-bond ability have been developed [2], but they are not always successful. On the other hand, eukaryotic cells, including mammalian cells (i.e. human 293, hamster CHO etc.) and yeast (i.e. Pichia pastoris, Saccharomyces cerevisiae etc.), are appropriate hosts for the production of these “difficult-toexpress” proteins [3-6]. Many human proteins with post-translational modifications and proper conformations have been produced by these systems. However, the costs are usually quite high and the expression levels are generally low.
Among the available expression systems, the baculovirus expression vector system (BEVS) has many advantages for the expression of these proteins, including 1) high level expression by strong promoters (Polyhedrin and P10), 2) post-translational modifications similar to those generated by mammalian cell expression, and 3) more reasonable cost than mammalian cell culture. The commercially available BEVS utilizes Autographa californica nucleopolyhedrovirus (AcNPV) and insect cell lines (High five™, Sf9 and Sf21) [7]. This system employs a very useful bacmid DNA comprising the AcNPV DNA genome with the E. coli origin and transposition sequences. Therefore, we can construct recombinant AcNPV viruses by simple molecular biological techniques, because the AcNPV bacmid DNA can replicate in E. coli, and the recombinant virus can be expressed by direct transfection into an insect cell line. However, this still requires the time-consuming procedures of virus amplification, handling, and large-scale cultivation.
Silkworm Expression System
The silkworm (Bombyx mori) expression system is a BEVS that uses the silkworm, in place of cell lines, as a bioreactor for the production of recombinant proteins. A quarter-century ago, Maeda et al. [8] reported the secreted production of human interferon α in the haemolymph of silkworm larvae, using recombinant Bombyx mori nucleopolyhedrovirus (BmNPV) encoding human α-interferon driven by the Polyhedrin promoter, as in the AcNPV system. That was the first report of the recombinant production of a medically relevant protein in silkworm. The expression levels of recombinant proteins in silkworm are generally higher than those in cultured cells [9], and this provides the biggest advantage. Moreover, the procedures required for this system are relatively easy, because the silkworm larvae do not require sterile facilities and large-scale cultivation in flasks or culture bags. The recent progress in artificial diet production is also helpful for feeding larvae, and eliminates the need for prior experience in silkworm breeding and cultivation.
However, the construction, amplification, and purification of recombinant BmNPV virus using the silkworm cell line are also timeconsuming, and require specific techniques as well as the AcNPV system. As a solution to this problem, transgenic silkworm technology is available without virus handling, for the stable expression of recombinant proteins [10], although it requires special skills and substantial time is needed to establish the transgenic lines.
We recently developed BmNPV bacmid DNA systems, based on BmNPV genomic DNA, to solve these labor problems [11-14]. The BmNPV bacmid DNA is the simplest tool among silkworm systems, because the recombinant proteins are expressed in the silkworm by the direct injection of recombinant bacmid DNA in larvae or pupae, without the preparation of a recombinant BmNPV virus [11].
In order to use recombinant proteins in biomedical studies, the profiling of post-translational N-glycosylation is an important analysis. The population of N-linked glycans on recombinant proteins expressed by silkworms is often investigated. Misaki et al. [15] reported that mouse interferon-β, expressed in silkworm larvae using the BmNPV virus, had variable sets of high and paucimannose-type N-linked sugars. On the other hand, in the case of the BmNPV-bacmid expression system, Ishikiriyama et al. [16] demonstrated that the IgG protein consisted of only two paucimannose-type oligosaccharides, Manα1-6Manβ1-4GlcNAcβ1-4(Fucα1-6)GlcNAc (77.5%) and Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4(Fucα1-6)GlcNAc (12.7 %), and Sasaki et al. [17] showed that the human KIR2DL1 ectodomain also had only two paucimannose-types, Manα1-6Manβ1-4GlcNAcβ1- 4(Fucα1-6)GlcNAc and Manα1-6Manβ1-4GlcNAcβ1-4GlcNAc. Thus, these sugar profiles on proteins expressed in the silkworm are nearly identical to those produced by other insect cell lines [18,19]. In human, sialylated complex type N-linked glycans are predominant in glycoproteins, while proteins expressed in insect cells and silkworm lack the sialylated complex, but have high-mannose or paucimannose-type oligosaccharides [19]. Glycosylation assists in both the adoption of the proper conformation and stabilization after purification. However, if glycosylation is not necessary for their functions, then the recombinant proteins with heterologous glycans will have endogenous activity. In addition, the small and relatively homogeneous N-linked sugar modifications in the BmNPV bacmid-silkworm system would be an advantage for structural studies.
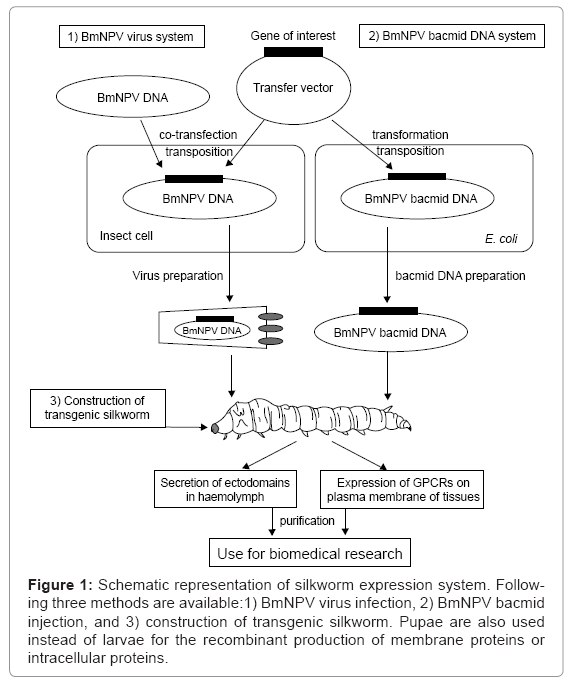
The silkworm is expected to become one of the attractive systems for the expression of recombinant proteins with posttranslational modifications for biomedical sciences, and in fact, many human proteins have already been expressed using silkworm [9,20]. The following three silkworm systems are available now: 1) infection with recombinant BmNPV virus, 2) injection of BmNPV bacmid DNA, and 3) construction of a transgenic silkworm (Figure 1). In this review, we describe the successful silkworm expression of the following typical biomedical targets, the ectodomains of human immunoreceptors and human guanine nucleotide-binding protein (G-protein) coupled receptors (GPCRs).
Figure 1: Schematic representation of silkworm expression system. Following three methods are available:1) BmNPV virus infection, 2) BmNPV bacmid injection, and 3) construction of transgenic silkworm. Pupae are also used instead of larvae for the recombinant production of membrane proteins or intracellular proteins.
Ectodomains of Immunoreceptors
Human immunoreceptors expressed on the cell surface regulate various immunological functions via specific binding to their physiological ligands. Immunoreceptors are often directly involved in immune-related diseases. Therefore, they are important for drug development, and moreover, they have potential not only as effective immunotherapeutic targets but also as biopharmaceuticals by themselves. In fact, the soluble ectodomains of such immunoreceptors or specific antibodies against them could become attractive biopharmaceutical drugs for immune regulation [21-23]. However, it is generally difficult to obtain sufficient amounts of functional immunoreceptors for biomedical research, because they frequently require some posttranslational modifications, such as glycosylation, as described above. Moreover, even if these modifications are not required, many immunoreceptor ectodomains require intramolecular-disulfide bonds to form the proper conformation; therefore, these proteins are expressed as inclusion bodies in the reductive environment of the E. coli cytoplasm. In this case, an appropriate refolding procedure is required to utilize these proteins in subsequent experiments. On the other hand, the BEVS are quite useful for the preparation of post-translationally modified recombinant ectodomains of immunoreceptors. We will introduce successful examples of the use of the silkworm system for the expression of immunoreceptor ectodomains.
Interleukin-13 Receptor Using BmNPV Virus
Interleukin (IL)-4Rα and IL-13Rα1 form a heterodimeric receptor on the surface of immune cells, and mediate signal transduction to regulate inflammatory responses [24,25]. In 2008, Honjo et al. [26] reported the expression and purification of the extracellular region of the human IL-4 receptor α chain (IL-4Rα) and human IL-13 receptor α1 chain (IL-13Rα1) by a silkworm-baculovirus system. They constructed the recombinant BmNPV virus [27] encoding the cDNA of IL-4Rα or IL13Rα1 with the Fc domain of murine IgG2a, and injected it into the body cavity of silkworm larvae. Each recombinant protein was purified from haemolymph by sequential chromatography using Protein-A resin and anion exchange resin. Finally, 0.19 mg of IL13Rα1 and 0.014 mg of IL-4Rα without the Fc domain were successfully purified from 1 ml of haemolymph (Table 1). As reported previously [28], these receptors formed a heterodimer and bound to IL-13. In addition, a surface plasmon resonance (SPR) analysis was performed, and the Kd value of IL-13 against IL-13Rα1-Fc was determined (2.3 nM) [26]. This was consistent with the previously reported Kd value (≈ 4 nM) calculated by a radiolabeled IL-13 binding assay, using IL-13Rα1 expressed on mammalian CHO cells [29].
| Classification | Name | Method | Tissue | Tag | Yield | Ligand-binding affinity (value from other hosts) | Reference |
|---|---|---|---|---|---|---|---|
| Immunoreceptor | IL4-Rα | virus | haemolymph | Fc | 0.19 mg/ml haemolymph | N.D. (Binding activity was confirmed by gel filtration analysis) | [26] |
| IL13-Rα | virus | haemolymph | Fc | 0.014 mg/ml haemolymph | Kd = 2.3 nM (≈ 4 nM, CHO cells [29]) | [26] | |
| KIR2DL1 | bacmid | haemolymph | 6xHis | ~0.2 mg/larva | Kd = 8.6 ± 0.69 μM (33 μM, E .coli [32]) | [17] | |
| FasR | virus | haemolymph | Fc | 22.5 mg/26 ml haemolymph | N.D. (Binding activity was confirmed by immunoprecipitation and size exclusion chromatography) |
[39] | |
| FasR | virus | haemolymph | Fc | 13.5 mg/25 ml haemolymph | N.D. (Binding activity was confirmed by size-exclusion chromatography) | [40] | |
| GPCR | u-opioid receptor | transgenic | fat body | 6xHis | 150–250 ng/larva* | Kd = 1.4~2.1 nM (0.37 ± 0.09 nM, Sf9 [55]) | [54] |
| nociceptin receptor | bacmid | fat body | 6xHis | N.D. | EC50 = 9.3~24 nM (12 nM, Sf9 [62]) | [56] |
Table 1: Human immunoreceptors and GPCRs expression using silkworm.
KIR2DL1 Using BmNPV Bacmid DNA
Human killer cell immunoglobulin-like (KIR) family genes, encoding transmembrane-type Ig-like glycoproteins, are expressed on natural killer cells and some T cell subsets [30] and mediate several forms of immune regulation [31]. Our group succeeded in the highlevel expression of a KIR family ectodomain, KIR2DL1, by using the BmNPV bacmid DNA-silkworm larvae system [17]. Using one-step affinity chromatography with a hexahistidine tag, ~ 0.2 mg of highly purified KIR2DL1 ectodomain was successfully obtained from the haemolymph of one larva (Table 1). The SPR analysis revealed that the KIR2DL1 bound to its physiological ligand, HLA-Cw4, with a Kd of 8.6 ± 0.69 μM [17]. This was consistent with the previous report, using refolded KIR2DL1 from an E. coli expression and refolding system (Kd ≈ 3.3 μM) [32]. In addition, a circular dichroism spectrum analysis suggested that the secondary structure is β-sheet rich, indicating the proper folding of the KIR2DL1 ectodomain [33]. The high level expression of the KIR2DL1 ectodomain was previously established with the E. coli system and refolding technique. However, the silkworm expression system can provide a sufficient amount of protein for several investigations, without large-scale cultivation and refolding.
Fas Receptor Using BmNPV Virus
The Fas receptor (FasR)-Fas ligand (FasL) system is an effective apoptotic system used by immune cells. The recombinant expression of the ectodomains of these proteins, using various expression systems, was previously reported [34-38]. Muraki and Honda [39,40] applied the silkworm expression system to produce the FasR ectodomain by a simple procedure using silkworm larvae. They generated the recombinant BmNPV virus encoding the fusion protein, composed of the human FasR ectodomain and the Fc domain of human IgG1 (hFasRECD-Ig), based on the BmNPV genome lacking the cysteine protease gene [27]. The recombinant virus was injected into silkworm larvae, and hFasRECD-Ig was successfully secreted as a disulfide-linked dimer in the haemolymph. Using protein G affinity chromatography and anion exchange chromatography, they purified 22.5 mg of hFasRECD-Ig from 26 ml of haemolymph (Table 1). As compared with the Sf9 system, the expression level of hFasRECD-Ig in silkworm haemolymph was 150 times higher. Using the recombinant human FasL ectodomain produced from a Pichia pastoris expression system [41,42], they confirmed the binding activity of hFasREDC-IG to hFasL by immunoprecipitation and size exclusion chromatography.
These examples of IL-4R, IL13R, KIR2DL1, and FasR expression clearly showed that the BmNPV bacmid DNA-silkworm expression system is quite useful for the efficient production of ectodomains, as secreted, functional recombinant proteins, in comparison with the insect cell expression system.
GPCRs
GPCRs are seven transmembrane-type proteins with cytoplasmic regions that associate with a trimeric G protein, composed of alpha (Gα), beta (Gβ), and gamma (Gγ) subunits [43]. The binding of specific ligands (peptides, lipids, steroids, etc.) to the extracellular region of GPCRs causes the activation of the GTPase activity of the trimeric G protein, which is released from the GPCRs. This dissociation triggers the following signal transduction to induce several biological events related to diseases [44,45]. Thus, although approximately ~50% of commercial drugs target GPCRs [46], the molecular basis of their effects on GPCRs remains unclear.
To develop new effective drugs as agonists or antagonists of GPCR ligands, many researchers are trying to elucidate the molecular basis of drug effects on GPCRs by physicochemical methods, including the determination of three-dimensional structures. In addition, despite the presence of several hundred GPCRs in the human genome [47], the physiological ligands of many GPCRs remain unknown, and thus these GPCRs are designated as “orphan receptors” [48]. To understand orphan GPCRs, many researchers are searching for physiological GPCR ligands by ligand screening assays. For this purpose, large amounts of recombinant GPCRs are often required; however, GPCRs are quite difficult to express, due to their low solubility and instability. To successfully express sufficient amounts of functional GPCRs, several expression hosts have been employed [49]. Among them, the BEVS are a powerful tool for the large-scale expression of functional GPCRs. In fact, the first-determined three-dimensional structure of a human GPCR, β adrenergic receptor, utilized the recombinant protein produced by BEVS with insect cell lines [50-52]. Silkworm technology is expected to be a simpler, user-friendly alternative method to express GPCRs.
μ-Opioid Receptor Using Transgenic Silkworm
In 2009, Tateno et al. [53] generated a transgenic silkworm expressing the μ-opioid receptor, one of the human GPCRs related to the analgesic action of morphine [54], in the silk glands and fat body of larvae. To our knowledge, this is the first report of the successful expression of a recombinant human GPCR in silkworm. The recombinant human μ-opioid receptor exhibited a similar level of diprenorphine-binding activity (Kd = 1.4 ± 0.95 ~ 2.1 ± 1.4 nM) to those produced from Sf9 cells (Kd = 0.37 ± 0.09 nM) and HEK293 cells (Kd = 0.29 ± 0.26 nM) [55]. They estimated that one transgenic larva expresses ~250 ng of μ-opioid receptor (Table 1), equivalent to ~20– 30 ml of Sf9 culture. The establishment of transgenic strains is timeconsuming and requires laborious technical skills. However, this report clearly showed the potential of the silkworm as an attractive host for the expression of recombinant human GPCRs.
Nociceptin Receptor Using BmNPV Bacmid DNA
Using the BmNPV bacmid system, the human nociceptin receptor was expressed in silkworm larvae [56]. Nociceptin receptor, a member of the opioid receptor family, is expressed in the central nervous system, and its physiological ligand, called nociceptin peptide, binds to the extracellular region to control several neurological responses [57,58]. Upon the injection of the recombinant BmNPV bacmid DNA, silkworm larvae expressed human nociceptin receptor on their fat body. The microsomal fraction expressing recombinant human nociceptin receptor, fused with the Giα subunit at C-terminus [59-62], exhibited [35S] GTPγS-binding activity dependent on the nociceptin concentration. The EC50 value (9.3 ± 3.4 nM) was consistent with the previous report using recombinant Giα-fused nociceptin in Sf9 cells (EC50 = 12 nM) [62]. The expression level of nociceptin receptor was not determined; however, the microsome fraction from one larva was sufficient to perform 500 ligand-screening assays [56].
Conclusion
The silkworm expression system is a powerful tool for recombinant protein production [9]. In this review, we described the successful expression of human immunoreceptor ectodomains and human GPCRs with posttranslational modifications in silkworm. These proteins are considered to be important targets of biomedical research; however, it is generally difficult to express them by using bacterial and mammalian expression systems. The proteins were expressed in silkworm BEVS at higher expression levels and with simpler methodology than the other BEVS using insect cell lines. Therefore, the silkworm system will open the door to the biomedical analyses of these medicinal targets.
References
- Tomlinson IM (2004). Nat Biotechnol 22: 521-522.
- Salinas G, Pellizza L, Margenat M, Flo M, Fernandez C (2011) Tuned Escherichia coli as a host for the expression of disulfide-rich proteins. Biotechnol J 6: 686-699.
- Cockett MI, Bebbington CR, Yarranton GT (1990) High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology (NY) 8: 662-667.
- Aricescu AR, Assenberg R, Bill RM, Busso D, Chang VT, et al. (2006) Eukaryotic expression: developments for structural proteomics. Acta Crystallogr D Biol Crystallogr 62: 1114-1124.
- Durocher Y, Butler M (2009) Expression systems for therapeutic glycoprotein production. Curr Opin Biotechnol 20: 700-707.
- Mattanovich D, Branduardi P, Dato L, Gasser B, Sauer M, et al. (2012) Recombinant protein production in yeasts. Methods Mol Biol 824: 329-358.
- Ikonomou L, Schneider YJ, Agathos SN (2003) Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol 62: 1-20.
- Maeda S, Kawai T, Obinata M, Fujiwara H, Horiuchi T, et al. (1985) Production of human alpha-interferon in silkworm using a baculovirus vector. Nature 315: 592-594.
- Kato T, Kajikawa M, Maenaka K, Park EY (2010) Silkworm expression system as a platform technology in life science. Appl Microbiol Biotechnol 85: 459-470.
- Tamura T, Thibert C, Royer C, Kanda T, Abraham E, et al. (2000) Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 18: 81-84.
- Motohashi T, Shimojima T, Fukagawa T, Maenaka K, Park EY (2005) Efficient large-scale protein production of larvae and pupae of silkworm by Bombyx mori nuclear polyhedrosis virus bacmid system. Biochem Biophys Res Commun 326: 564-569.
- Hiyoshi M, Kageshima A, Kato T, Park EY (2007) Construction of a cysteine protease deficient Bombyx mori multiple nucleopolyhedrovirus bacmid and its application to improve expression of a fusion protein. J Virol Methods 144: 91-97.
- Park EY, Abe T, Kato T (2008) Improved expression of fusion protein using a cysteine- protease- and chitinase-deficient Bombyx mori (silkworm) multiple nucleopolyhedrovirus bacmid in silkworm larvae. Biotechnol Appl Biochem 49: 135-140.
- Xiang X, Yang R, Yu S, Cao C, Guo A, et al. (2010) Construction of a BmNPV polyhedrin-plus Bac-to-Bac baculovirus expression system for application in silkworm, Bombyx mori. Appl Microbiol Biotechnol 87: 289-295.
- Misaki R, Nagaya H, Fujiyama K, Yanagihara I, Honda T, et al. (2003) N-linked glycan structures of mouse interferon-beta produced by Bombyx mori larvae. Biochem Biophys Res Commun 311: 979-986.
- Ishikiriyama M, Nishina T, Kato T, Ueda H, Park EY (2009) Human single-chain antibody expression in the hemolymph and fat body of silkworm larvae and pupae using BmNPV bacmids. J Biosci Bioeng 107: 67-72.
- Sasaki K, Kajikawa M, Kuroki K, Motohashi T, Shimojima T, et al. (2009) Silkworm expression and sugar profiling of human immune cell surface receptor, KIR2DL1. Biochem Biophys Res Commun 387: 575-580.
- Kubelka V, Altmann F, Kornfeld G, Marz L (1994) Structures of the N-linked oligosaccharides of the membrane glycoproteins from three lepidopteran cell lines (Sf-21, IZD-Mb-0503, Bm-N). Arch Biochem Biophys 308: 148-157.
- Kost TA, Condreay JP, Jarvis DL (2005) Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 23: 567-575.
- Miyajima A, Schreurs J, Otsu K, Kondo A, Arai K, et al. (1987) Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene 58: 273-281.
- Fernandez-Botran R, Chilton PM, Ma Y (1996) Soluble cytokine receptors: their roles in immunoregulation, disease, and therapy. Adv Immunol 63: 269-336.
- Nabel G J (2004) Genetic, cellular and immune approaches to disease therapy: past and future. Nat Med 10: 135-141.
- Bremer E, de Bruyn M, Wajant H, Helfrich W (2009) Targeted cancer immunotherapy using ligands of the tumor necrosis factor super-family. Curr Drug Targets 10: 94-103.
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE (1999) The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 17: 701-738.
- Pan PY, Rothman P (1999) IL-4 receptor mutations. Curr Opin Immunol 11: 615-620.
- Honjo E, Shoyama Y, Tamada T, Shigematsu H, Hatanaka T, et al. (2008) Expression of the extracellular region of the human interleukin-4 receptor alpha chain and interleukin-13 receptor alpha1 chain by a silkworm-baculovirus system. Protein Expr Purif 60: 25-30.
- Suzuki T, Kanaya T, Okazaki H, Ogawa K, Usami A, et al. (1997) Efficient protein production using a Bombyx mori nuclear polyhedrosis virus lacking the cysteine proteinase gene. J Gen Virol 78: 3073-3080.
- Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, et al. (1996) Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci U S A 93: 497-501.
- Miloux B, Laurent P, Bonnin O, Lupker J, Caput D, et al. (1997) Cloning of the human IL-13R alpha1 chain and reconstitution with the IL4R alpha of a functional IL-4/IL-13 receptor complex. FEBS Lett 401: 163-166.
- Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH (2002) Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol 20: 853-885.
- Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH (1998) Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391: 703-707.
- Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, et al. (2003) Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A 100: 8856-8861.
- Fan QR, Long EO, Wiley DC (2001) Crystal structure of the human natural killer cell inhibitory receptor KIR2DL1-HLA-Cw4 complex. Nat Immunol 2: 452-460.
- Schneider P, Bodmer JL, Holler N, Mattmann C, Scuderi P, et al. (1997) Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J Biol Chem 272: 18827-18833.
- Mahiou J, Abastado JP, Cabanie L, Godeau F (1998) Soluble FasR ligand-binding domain: high-yield production of active fusion and non-fusion recombinant proteins using the baculovirus/insect cell system. Biochem J 330: 1051-1058.
- Rooney I, Butrovich K, Ware CF (2000) Expression of lymphotoxins and their receptor-Fc fusion proteins by baculovirus. Methods Enzymol 322: 345-363.
- Li Y, Yang X, Nguyen AH, Brockhausen I (2007) Requirement of N-glycosylation for the secretion of recombinant extracellular domain of human Fas in HeLa cells. Int J Biochem Cell Biol 39: 1625-1636.
- Wisniewski P, Master A, Kaminska B (2008) Cloning and purification of functionally active Fas ligand interfering protein (FIP) expressed in Escherichia coli. Acta Biochim Pol 55: 51-56.
- Muraki M, Honda S (2010) Efficient production of human Fas receptor extracellular domain-human IgG1 heavy chain Fc domain fusion protein using baculovirus/silkworm expression system. Protein Expr Purif 73: 209-216.
- Muraki M, Honda S (2011) Improved isolation and purification of functional human Fas receptor extracellular domain using baculovirus - silkworm expression system. Protein Expr Purif 80: 102-109.
- Muraki M (2006) Secretory expression of synthetic human Fas ligand extracellular domain gene in Pichia pastoris: influences of tag addition and N-glycosylation site deletion, and development of a purification method. Protein Expr Purif 50: 137-146.
- Muraki M (2008) Improved secretion of human Fas ligand extracellular domain by N-terminal part truncation in Pichia pastoris and preparation of the N-linked carbohydrate chain trimmed derivative. Protein Expr Purif 60: 205-213.
- Wess J (1997) G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J 11: 346-354.
- Liebmann C, Bohmer FD (2000) Signal transduction pathways of G protein-coupled receptors and their cross-talk with receptor tyrosine kinases: lessons from bradykinin signaling. Curr Med Chem 7: 911-943.
- Kristiansen K (2004) Molecular mechanisms of ligand binding, signaling, and regulation within the super family of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther 103: 21-80.
- Gudermann T, Nurnberg B, Schultz G (1995) Receptors and G proteins as primary components of transmembrane signal transduction. Part 1. G-protein-coupled receptors: structure and function. J Mol Med (Berl) 73: 51-63.
- Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S (2002) Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett 520: 97-101.
- Wise A, Jupe SC, Rees S (2004) The identification of ligands at orphan G-protein coupled receptors. Annu Rev Pharmacol Toxicol 44: 43-66.
- McCusker EC, Bane SE, O'Malley MA, Robinson AS (2007) Heterologous GPCR expression: a bottleneck to obtaining crystal structures. Biotechnol Prog 23:540-547
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, et al. (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318: 1258-1265.
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, et al. (2007) Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450: 383-387.
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, et al. (2007) GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318: 1266-1273.
- Tateno M, Toyooka M, Shikano Y, Takeda S, Kuwabara N, et al. (2009) Production and characterization of the recombinant human mu-opioid receptor from transgenic silkworms. J Biochem 145: 37-42.
- Waldhoer M, Bartlett SE, Whistler JL (2004) Opioid receptors. Annu Rev Biochem 73: 953-990.
- Kempf J, Snook LA, Vonesch JL, Dahms TE, Pattus F, et al. (2002) Expression of the human mu opioid receptor in a stable Sf9 cell line. J Biotechnol 95: 181-187.
- Kajikawa M, Sasaki K, Wakimoto Y, Toyooka M, Motohashi T, et al. (2009) Efficient silkworm expression of human GPCR (nociceptin receptor) by a Bombyx mori bacmid DNA system. Biochem Biophys Res Commun 385: 375-379.
- Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, et al. (1999) Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol 406: 503-547.
- Mogil JS, Pasternak GW (2001) The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53: 381-415.
- Bertin B, Freissmuth M, Jockers R, Strosberg AD, Marullo S (1994) Cellular signaling by an agonist-activated receptor/Gs alpha fusion protein. Proc Natl Acad Sci U S A 91: 8827-8831.
- Molinari P, Ambrosio C, Riitano D, Sbraccia M, Gro MC, et al. (2003) Promiscuous coupling at receptor-Galpha fusion proteins. The receptor of one covalent complex interacts with the alpha-subunit of another. J Biol Chem 278: 15778-15788.
- Takeda S, Okada T, Okamura M, Haga T, Isoyama-Tanaka J, et al. (2004) The receptor-Galpha fusion protein as a tool for ligand screening: a model study using a nociceptin receptor-Galphai2 fusion protein. J Biochem 135: 597-604.
- Akuzawa N, Takeda S, Ishiguro M (2007) Structural modelling and mutation analysis of a nociceptin receptor and its ligand complexes. J Biochem 141: 907-916.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16452
- [From(publication date):
specialissue-2012 - Jan 30, 2025] - Breakdown by view type
- HTML page views : 11907
- PDF downloads : 4545