Research Article Open Access
Sex-Specific Regulation of Depression, Anxiety-Like Behaviors and Alcohol Drinking in Mice Lacking ENT1
Christina L. Ruby1, Denise L. Walker2, Joyce An1, Jason Kim1 and Doo-Sup Choi1,2*1Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic College of Medicine, USA
2Department of Psychiatry and Psychology, Mayo Clinic College of Medicine, USA
- *Corresponding Author:
- Doo-Sup Choi, Ph.D
Department of Molecular Pharmacology and Experimental Therapeutics
Mayo Clinic College of Medicine, 200 First Street SW
Rochester, Minnesota 55905, USA
Tel: (507) 284-5602
Fax: (507) 266-0824
E-mail: choids@mayo.edu
Received November 08, 2011; Accepted December 21, 2011; Published December 25, 2011
Citation: Ruby CL, Walker DL, An J, Kim J, Choi DS (2011) Sex-Specific Regulation of Depression, Anxiety-Like Behaviors and Alcohol Drinking in Mice Lacking ENT1. J Addict Res Ther S4:004. doi:10.4172/2155-6105.S4-004
Copyright: © 2011 Ruby CL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Objectives: Adenosine signaling has been implicated in the pathophysiology of several psychiatric disorders including alcoholism, depression, and anxiety. Adenosine levels are controlled in part by transport across the cell membrane by equilibrative nucleoside transporters (ENTs). Recent evidence showed that a polymorphism in the gene encoding ENT1 is associated with comorbid depression and alcoholism in women. We have previously shown that deletion of ENT1 reduces ethanol intoxication and elevates alcohol intake in mice. Interestingly, ENT1 null mice display decreased anxiety-like behavior compared to wild-type littermates. However, our behavioral studies were performed only in male mice. Here, we extend our research to include female mice, and test the effect of ENT1 knockout on other behavioral correlates of alcohol drinking, including depressive and compulsive behavior, in mice.
Methods: To assess depression-like behavior, we used a forced swim test modified for mice. We examined anxiety-like behavior and locomotor activity in open field chambers, and perseverant behavior using the marble-burying test. Finally, we investigated alcohol consumption and preference in female mice using a two-bottle choice paradigm.
Results: ENT1 null mice of both sexes showed reduced immobility time in the forced swim test and increased time in the center of the open field compared to wild-type littermates. ENT1 null mice of both sexes showed similar locomotor activity levels and habituation to the open field chambers. Female ENT1 null mice displayed increased marble-burying compared to female wild-types, but no genotype difference was evident in males. Female ENT1 null mice showed increased ethanol consumption and preference compared to female wild-types.
Conclusions: Our findings suggest that ENT1 contributes to several important behaviors involved in psychiatric disorders. Inhibition of ENT1 may be beneficial in treating depression and anxiety, while enhancement of ENT1 function may reduce compulsive behavior and drinking, particularly in females.
Keywords
Adenosine; Depression; Anxiety; Alcohol; ENT1; Sex; Compulsivity
Introduction
Recent analysis of disability-adjusted life years (DALYs) indicates that depression and alcohol use disorders are the two largest contributors to the global health loss burden among mental, neurobiological and substance-abuse disorders. Notably, comorbidity between alcoholism and depression poses a major clinical problem in treating these two prevalent psychiatric disorders [1], and the economic burden and severity of this comorbidity surpasses depression alone [2]. Clinical studies have shown that abstinence from alcohol causes depression [3] and depression is also main trigger for excessive alcohol drinking. However, the causal relationship between alcoholism and depression remains unknown.
Adenosine has been implicated in the pathophysiology of several central nervous system (CNS) disorders including alcoholism [4-6] and depression [7]. Adenosine acts as an inhibitory neuromodulator in the central nervous system (CNS), controlling neuronal excitability, modulating neurotransmitter release, and regulating ion channel function, primarily through the activation of two subtypes of G-protein-coupled receptors (GPCRs), A1 and A2A receptors. Extracellular or synaptic adenosine levels are mainly regulated by plasma membrane equilibrative nucleoside transporters (ENTs). ENT1 is expressed ubiquitously, but mainly in astrocytes in the CNS [8]. Acute ethanol treatment increases extracellular adenosine levels in cultured cells by selectively inhibiting ENT1, while chronic exposure to ethanol no longer increases extracellular adenosine levels owing to downregulation of ENT1 gene expression [9]. Adenosine is known to mediate several effects of acute alcohol, including ethanol-induced ataxia and sedation. Interestingly, mice lacking ENT1 exhibit reduced ataxic and hypnotic responses to acute ethanol exposure and consume more alcohol compared to wild-type littermates [10]. Conversely, ENT1 overexpression in the brain increases ethanol intoxication in mice [11]. Several other studies also report an inverse correlation between ENT1 gene expression and alcohol consumption [12-14]. Furthermore, recent human genetic association studies demonstrate that variants of ENT1 are associated with an alcohol abuse phenotype in women [7] and alcoholism with a history of withdrawal seizures [15].
Adenosine signaling has also been implicated in depression and anxiety disorders. Numerous studies have linked polymorphisms in the A2A receptor gene, ADORA2A, with anxiety in many psychiatric disorders and in response to stimulant drugs (see [16] for review). Likewise, several adenosine-related genes have been associated with depression [7]. A very recent study reported a correlation between the consumption of coffee, which contains high amounts of the A1/ A2A receptor antagonist caffeine, and lowered risk for depressive symptoms in women [17]. Despite this and other evidence implicating adenosinergic mechanisms in depression, there has been little preclinical investigation on the contribution of adenosine signaling to depression models. Moreover, the scant preclinical evidence that does exist is unclear, with many studies reporting an antidepressant effect of caffeine [18,19] and other adenosine receptor antagonists [20], but others reporting an antidepressant effect of adenosine itself [21,22]. Female mice are seldom used in preclinical research due to the potential influence of estrous cycle fluctuations on behavior. However, since ENT1 expression or function appears to play a role in depression specifically in women, we examined the effect of genetic deletion of ENT1 on depression-like behavior in both male and female mice in this study. In addition, we extended our previous studies showing genotype differences in alcohol intake and anxiety-like behavior to include female mice, and assessed compulsive-like behavior in both sexes.
Materials and Methods
Animals
ENT1 null mice were generated on a C57BL/6J x 129X1/SvJ background. Chimeric mice were bred with C57BL/6J mice to generate F1 hybrids. These ENT1 heterozygous mutant F1 mice were intercrossed to generate F2 hybrid (50% C57BL/6J and 50% 129X1/SvJ) littermates for experiments. Mice were housed in standard Plexiglas cages with rodent chow and water available ad libitum. The colony room was maintained on a 12-h light/ dark cycle with lights on at 0700 h. Experiments were performed between 0800 and 1100 h using both male and female mice when they reached approximately 10 weeks of age. Estrous cycling was not monitored in female mice. Different animals were used for each experiment, consisting of no more than 3 mice per genotype from the same litter. Animal care and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committees in accordance with National Institutes of Health guidelines.
Forced Swim Test
To assess depression-like behavior, we performed a forced swim test modified for mice as described [23]. Briefly, each mouse was placed in a 2000 ml beaker filled with water (depth = 30 cm) for a total of 5 minutes, and the duration of time spent swimming vs. immobile (floating) was measured. The water temperature was warm (30°C) to avoid causing stress or hypothermia.
Open field locomotor activity
Spontaneous locomotor activity was measured in open-field chambers (26 x 26 cm). Mice were examined in a quiet room under normal fluorescent room light (500 lux). Mice were handled and weighed daily for 1 week prior to activity testing. On the test day, mice were weighed and placed immediately in the activity chambers. Horizontal distance traveled (cm) was recorded for 1 h using a Video tracking system (ViewPoint Life Sciences Inc., Montreal, Canada). For assessment of activity in the center of the field, the chamber floor was divided post hoc into a central zone (13 x 13 cm; center equidistant from all four walls of the chamber) and a peripheral zone (the remaining area of the floor).Time spent in each area was calculated from the locomotor activity data. After each test session, the equipment was cleaned with 70% ethanol to remove animal odors.
Marble-burying test
To assess perseverant behavior [24], we used the marble burying test as described [25]. Briefly, each mouse was placed in a cage containing 24 marbles, evenly spaced (4 cm apart) on top of bedding at a depth of 5 cm. After 30 minutes, the number of marbles buried up to 2/3 their depth in bedding was recorded for each animal.
Alcohol self-administration
Oral alcohol self-administration and preference were examined using a two-bottle choice experiment [10]. Mice were given one week to acclimate to individual housing conditions and handling. Mice were then given 24 h access to two bottles, one containing plain tap water and the other containing an ethanol solution. The concentration of ethanol was raised every fourth day, increasing from 3 to 5 to 10% (v/v) ethanol in tap water. The positions of the bottles were changed every 2 days to control for position preference. Throughout the experiments, fluid intake and body weight were measured every 2 days using an analytical balance (Denver Instrument, Arvada, CO) with a precision of 0.01 g. Leakage and evaporation were controlled for by placing 2 bottles, one containing the ethanol solution and one containing tap water, in 2 empty cages, weighing bottles as described, and subtracting the average leakage/evaporation for ethanol or water from the drinking data for each mouse. During the alcohol self-administration experiment, average ethanol consumption per day was obtained for each ethanol concentration. To obtain an accurate measure of ethanol consumption that corrected for individual physical differences in mouse weight, grams of ethanol consumed per kilogram of body weight per day were calculated for each mouse. A measure of relative ethanol preference (%) was calculated at each ethanol concentration by dividing the total ethanol solution consumption by the total fluid (ethanol plus water) consumption.
Statistical analysis
All data are presented as mean + SEM. Immobility time in the forced swim test for each sex and body weight in female mice were analyzed by two-tailed t-tests. Number of marbles buried and ethanol drinking data were analyzed using a two-way analysis of variance (ANOVA) with factors of sex and genotype. For open-field locomotor testing, data were analyzed by three-way ANOVA with betweensubject factors of genotype, sex, and test day. Where significant interactions Between subject were found, these analyses were followed by a Tukey’s post hoc test for individual comparisons. In all cases, results were considered significantly different where P < 0.05.
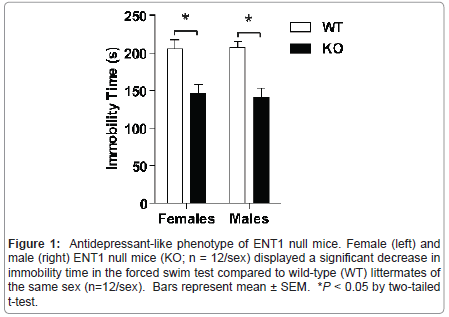
Figure 1:Antidepressant-like phenotype of ENT1 null mice. Female (left) and male (right) ENT1 null mice (KO; n = 12/sex) displayed a significant decrease in immobility time in the forced swim test compared to wild-type (WT) littermates of the same sex (n=12/sex). Bars represent mean �?± SEM. *P < 0.05 by two-tailed t-test.
Results
Genetic deletion of ENT1 results in an antidepressant-like phenotype in mice
We examined depression-like behavior in male and female ENT1 null (n=12/sex) and wild-type mice (n=12/sex) using a version of the forced swim test modified for mice (Figure 1). Using two-tailed t-tests, we showed that male and female ENT1 null mice display reduced immobility time compared to wild-types of the same sex ( t = 4.520, P < 0.001 for males; t = 3.668, P = 0.001 for females).
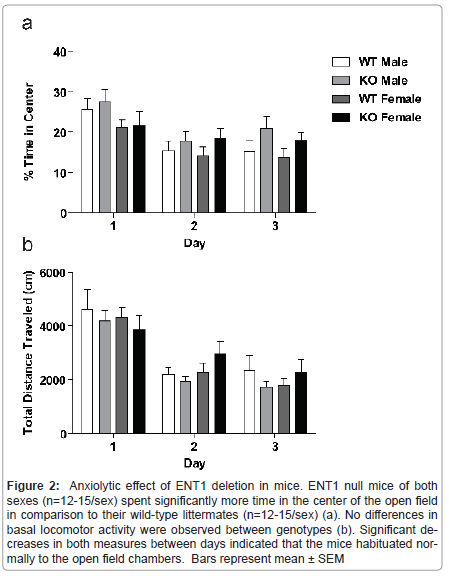
Anxiety-like behavior is reduced by genetic deletion of ENT1 in male and female mice
Because mice have a natural aversion to the brightly lit center of an open field, comparison of activity in the center compared to the periphery of the field gives an indication of anxiety-like behavior. We analyzed data for locomotor activity in the center of the open field for male and female ENT1 null mice (n=12-15/sex) and wild-type mice (n=12-15/sex) by three-way ANOVA (Figure 2a), which showed a main effect of genotype (F1,159=4.561, P = 0.034) and day (F2,159 = 10.954, P < 0.001), with a trend for difference in sex (F1,159=3.053, P = 0.083). There were no interactions between genotype and sex (F1,159 = 0.007, P = 0.933), genotype and day (F2,159 = 0.552, P = 0.557), sex and day (F2,159 =0.942, P = 0.392), or genotype, sex, and day (F2,159 = 0.138, P = 0.871).
Basal locomotor activity is differentially regulated in male and female mice by ENT1
Figure 2:Anxiolytic effect of ENT1 deletion in mice. ENT1 null mice of both sexes (n=12-15/sex) spent significantly more time in the center of the open field in comparison to their wild-type littermates (n=12-15/sex) (a). No differences in basal locomotor activity were observed between genotypes (b). Significant decreases in both measures between days indicated that the mice habituated normally to the open field chambers. Bars represent mean �?± SEM.
We examined spontaneous locomotor activity of male and female ENT1 null (n=12-15/sex) and wild-type mice (n=12-15/sex) in openfield chambers for 1 h per day over 3 consecutive days. A three-way ANOVA showed a significant effect of day (F2, 161 = 30.518, P < 0.001 ), indicating that the mice habituated to the open field chambers (Figure 2b). There was no effect of genotype (F1, 161 =0.142, P = 0.707), or sex (F1, 161 =0.110, P=0.741) Additionally, there were no interactions between genotype and sex (F1, 159 = 1.726, P = 0.191), genotype and day (F2, 159 = 0.618, P = 0.541), sex and day (F2, 159 = 1.053, P = 0.352) or genotype, sex, and day (F2, 159 = 0.545, P = 0.581).
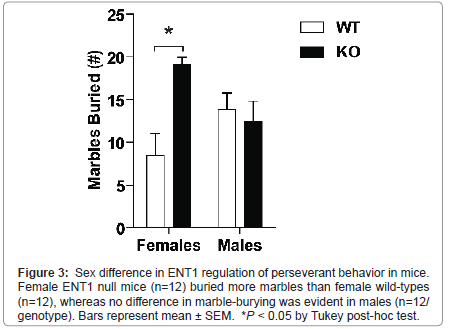
Female, but not male, ENT1 null mice display increased perseverant behavior
We investigated repetitive, perseverant behavior in male and female ENT1 null (n=12/sex) and wild-type mice (n=12/sex) using the marble-burying test (Figure 3). A two-way ANOVA showed a significant difference in genotype (F1, 47 = 7.776, P = 0.008), but not sex (F1, 47 < 0.001, P = 0.983), with a significant interaction between genotype and sex (F1, 47= 7.776, P = 0.008). Post hoc analysis revealed a significant difference between female ENT1 null and wild-type mice (P < 0.001), but not males (P = 1.0). A trend was evident for differences in sex within wild-types (P = 0.053) and knockouts (P = 0.057).
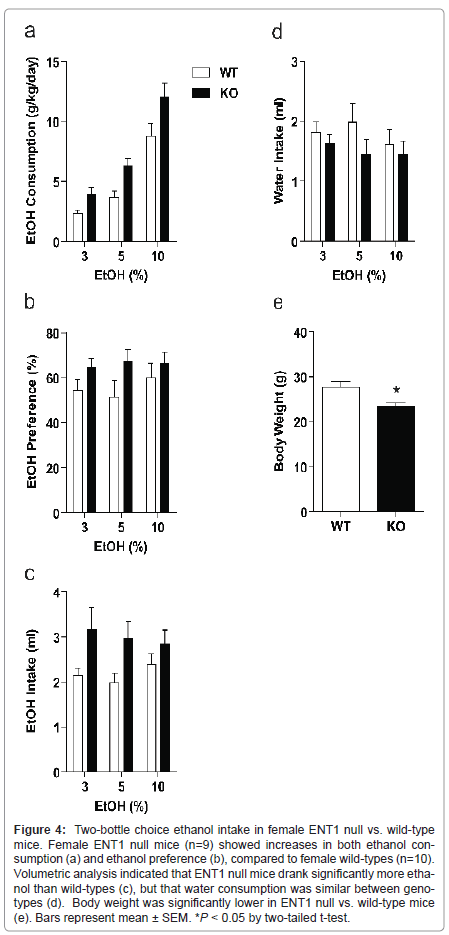
Female ENT1 null mice display higher ethanol consumption, and preference
In a separate group of female mice (n=9-10/genotype), we examined ethanol consumption and preference (Figure 4). Using a two-way ANOVA to investigate weight-corrected ethanol consumption, we showed a significant effect of genotype (F1,56=16.198, P < 0.001) and ethanol concentration (F2, 56=50.304, P < 0.001), with no interaction between factors (F2, 56 =0.588, P = 0.559). Likewise, ethanol preference differed significantly between ENT1 null and wild-type female mice (F1,56 = 5.498, P = 0.023). However, there was no significant effect of ethanol concentration (F2, 56 = 0.273, P = 0.763) or interaction between the factors (F2, 56=0.362, P = 0.698). We also examined daily ethanol (Figure 4c) and water intake volumes (Figure 4d) and body weight (Figure 4e). A two-way ANOVA revealed a significant effect of genotype in ethanol intake (F1,56 = 10.321, P = 0.002) but no effect of ethanol concentration (F2, 56 = 0.166, P = 0.847) or interaction between the two factors (F2, 56 = 0.517, P = 0.599). A two-way ANOVA revealed no differences in water consumption between genotypes (F1,56 = 2.248, P = 0.140), no effect of ethanol concentration (F2, 56 = 0.398, P = 0.674), and no interaction between the variables (F2, 56 = 0.389, P = 0.680). Finally, a two-tailed t-test showed a significant difference in body weight between ENT1 null mice and wild-types (t = -2.831, P = 0.012).
Figure 4:Two-bottle choice ethanol intake in female ENT1 null vs. wild-type mice. Female ENT1 null mice (n=9) showed increases in both ethanol consumption (a) and ethanol preference (b), compared to female wild-types (n=10). Volumetric analysis indicated that ENT1 null mice drank significantly more ethanol than wild-types (c), but that water consumption was similar between genotypes (d). Body weight was significantly lower in ENT1 null vs. wild-type mice (e). Bars represent mean �?± SEM. *P < 0.05 by two-tailed t-test.
Discussion
Our findings suggest that ENT1 regulates mood-related and ethanol drinking behaviors in mice. The main finding of this study, and the one with the most potential importance, is the reducing effect of ENT1 deletion on behavioral despair in mice. Importantly, the reduced immobility time exhibited by ENT1 null mice in the forced swim test is not explained by genotype differences in spontaneous locomotor activity, which were absent. It is also intriguing that the ENT1 null mice in this study, as previously reported [26], showed reduced anxiety-like behavior. Since symptoms of depression and anxiety frequently occur together in psychiatric illness, our present results indicate that ENT1 inhibition may be useful in reducing both depressive and anxiety symptoms.
We have shown that ENT1 null mice have reduced levels of adenosine in the striatum as well as reduced A1 receptor activation compared to wild-types [10,27]. Because A2A receptors have similar affinity for adenosine as A1 receptors, it is likely that ENT1 null mice also have reduced A2A receptor-mediated signaling in the striatal region. This reduction of adenosine receptor-mediated signaling is consistent with the antidepressant effects of caffeine, a widely-used A1/A2A receptor antagonist [17-20]. Indeed, a recent longitudinal study reported a correlation between coffee drinking and reduction in depressive symptoms in women [17]. Based on these lines of evidence, it is reasonable to speculate that the genetic variant of ENT1 associated with depression [7] may result in increased release or decreased uptake of adenosine, resulting in over-activation of A1 and/or A2A receptors. An important caveat to this idea is that ENT1 appears to regulate adenosine differently in specific brain regions. For example, decreased adenosine levels in the striatum of ENT1 null mice indicate that ENT1 serves a release function in that brain region [10]. On the contrary, we have shown that ENT1 deletion or inhibition is related to increased A1 receptor activation and possibly increased adenosine levels in the amygdala, implying that ENT1 primarily clears adenosine in this area [26]. Thus, identifying the region or circuit responsible for the antidepressant effects of ENT1 deletion will be critical to future investigations.
It is also interesting that we found no sex difference in the antidepressant effect of ENT1 deletion given the substantial sex differences reported in the relation of adenosine signaling genes to depression, particularly in the case of ENT1, which was associated with depression only in women [7]. Since there were no differences in locomotor activity between genotypes or sexes, the reduction in immobility time appears to be a specific effect of ENT1 deletion, as mentioned above. Moreover, there was a striking similarity in immobility time between males and females (with respect to genotype differences). Because it is unlikely that all 24 female mice were in the same stage of estrous, these results suggest that the estrous cycle does not impact immobility time in such a way that it overrides ENT1 regulation of this behavior. Future studies to confirm normal estrous cycling in ENT1 null mice and examine whether the stages of estrous influence forced swim test performance in this strain will be important. Overall, the present results suggest that ENT1 may be a promising therapeutic target for depression in both sexes.
The significant reduction in anxiety-like behavior is consistent with our previous study indicating an anxiolytic effect of ENT1 deletion or inhibition [26]. These prior results indicate that ENT1 normally acts to reduce levels of extracellular adenosine in the amygdala and that absence or inhibition of ENT1 elevates extracellular adenosine in that brain region. Several other preclinical studies have implicated adenosine activation of the A1 receptor in the reduction of anxiety- like behavior [28-31]. Conversely, caffeine, which has A1/A2A receptor antagonist properties, is known to be anxiogenic at high doses, and at moderate doses in susceptible individuals [16]. Caffeine is also known to antagonize the intoxicating effects of acute ethanol [16], but may simultaneously increase its rewarding and reinforcing properties. Interestingly, clinical evidence points to genetic variants of the A2A, not the A1, receptor in susceptibility to anxiety in response to caffeine [32-34], amphetamine [35], and in several psychiatric conditions including autism spectrum disorders [36] and panic disorder [37,38]. The A2A receptor is enriched in the striatum, and mice lacking this receptor display increased anxiety [39]. Therefore, it is likely that the brain regions mediating the antidepressant and anxiolytic effects of ENT1 deletion are different, as adenosine receptor antagonism (e.g. by caffeine) has an antidepressant (and sometimes anxiogenic) effect, while adenosine receptor activation appears to relieve anxiety. Determining the role of ENT1 in different brain regions will be critical in the development of therapies.
Perseveration is a component of compulsive behavior and common to many psychiatric disorders, most notably obsessive- compulsive disorder (OCD) [40]. Although OCD is categorized as an anxiety disorder, others have conceptualized OCD as a disorder of behavioral addiction. This idea has been substantiated in a number of studies showing impairments in executive control over behavior in patients with OCD [41]. Recent evidence suggests that corticostriatal reward circuitry is compromised in OCD, implicating mechanisms in common with addiction and alcoholism, including dysregulation of glutamate signaling in the nucleus accumbens [41,42]. Interestingly, we have shown similar changes, increased striatal glutamate levels and impairments in NMDA-mediated intracellular response in the nucleus accumbens, in ENT1 null mice [27]. These changes, along with ethanol intake, are reversed with administration of an NMDA receptor antagonist [27]. Preclinical studies indicate that NMDA antagonism also reduces marble-burying behavior [40], and that marble-burying is increased during ethanol withdrawal in mice [43]. It is possible that the sex difference we observed indicates that ENT1 plays a role in sex-specific hormonal regulation, which has been shown to affect behavioral expression of OCD [44].
We have previously reported that male ENT1 null mice show decreased sensitivity to acute ethanol intoxication and increased alcohol consumption and preference in comparison to their wild-type littermates [10]. Female ENT1 null mice in this study also consumed more ethanol than wild-types. The average ethanol consumption in female mice tended to be higher than that previously reported for male mice with respect to genotype. Several other studies report similar sex differences in alcohol drinking, with females usually drinking more ethanol than males. This sex difference may be due to hormonal fluctuations in female mice, differences in body fat or body water content, or perhaps even differences in reward network wiring [45-49]. Interestingly, the high ethanol intake in female ENT1 null mice does not appear to be related to genotype differences in ethanol sensitivity among female mice, as preliminary evidence suggests that female ENT1 null mice show similar ethanol-induced ataxia as female wild-types (unpublished observations). Future studies to determine whether there are sex differences in ethanol sensitivity may be warranted. It is noteworthy that female ENT1 null mice also showed increased ethanol preference compared to wild-types, which rules out the concern that their reduced body weight may have artificially inflated the consumption calculations. From the volumetric analyses, it is clear that female ENT1 null mice consistently consume more of the ethanol solution than female wild-type mice. Importantly, the effect of ENT1 deletion was specific for ethanol rather than fluid intake in general, as water intake was similar between female ENT1 null and wild-type mice for the duration of the experiment.
Finally, it is interesting to consider the high alcohol drinking in ENT1 null mice, both females in this study and males in our other studies [10], in relation to their antidepressant phenotype. Depression is one of the most common psychiatric disorders associated with alcoholism, and this has been demonstrated in several rodent models. However, there are subtypes of alcoholism that are not associated with negative affect [50]. It is unknown whether alcoholics of these types are actually less depressed or anxious than non-alcoholics, but there is evidence that this population exhibits impaired impulse control. The signaling changes in the striatum of ENT1 null mice and the increase in marble-burying in female mice are consistent with the possibility of impaired inhibitory control over behavior, which may be related to the observed elevation in alcohol intake. Importantly, the present results suggest that the mechanisms underlying alcohol dependence can be dissociated from those involved in mood regulation, and that the adenosinergic system may represent a point of divergence. Future studies differentiating the potentially beneficial antidepressant effect of blocking ENT1 from the effects on alcohol consumption will be critical to the understanding of the role of ENT1 and adenosine in these psychiatric disorders.
Acknowledgements
We thank O. Abulseoud for helpful discussions, S. Choi for mouse husbandry, and B. Boudreaux for assistance with manuscript preparation. This study was suported in part by grants from the National Institutes of Health (NIH) to D.-S. C. (AA015164, AA018779) and by the Samuel Johnson Foundation for genomics of drug addiction program at Mayo Clinic to D.-S. C.
References
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, et al. (2004) Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61: 807-816.
- Bagby RM, Ryder AG, Cristi C (2002) Psychosocial and clinical predictors of response to pharmacotherapy for depression. J Psychiatry Neurosci 27: 250-257.
- Hasin DS, Grant BF (2002) Major depression in 6050 former drinkers: association with past alcohol dependence. Arch Gen Psychiatry 59: 794-800.
- Dunwiddie TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24: 31-55.
- Ruby CL, Adams CA, Knight EJ, Nam HW, Choi DS (2010) An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev 3: 163-174.
- Asatryan L, Nam HW, Lee MR, Thakkar MM, Saeed Dar M, et al. (2011) Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res 35: 584-594.
- Gass N, Ollila HM, Utge S, Partonen T, Kronholm E, et al. (2010) Contribution of adenosine related genes to the risk of depression with disturbed sleep. J Affect Disord 126: 134-139.
- Peng L, Huang R, Yu AC, Fung KY, Rathbone MP, et al. (2005) Nucleoside transporter expression and function in cultured mouse astrocytes. Glia 52: 25-35.
- Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS (1990) Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem 265: 1946-1951.
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, et al. (2004) The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci 7: 855-861.
- Parkinson FE, Xiong W, Zamzow CR, Chestley T, Mizuno T, et al. (2009) Transgenic expression of human equilibrative nucleoside transporter 1 in mouse neurons. J Neurochem 109: 562-572.
- Short JL, Ledent C, Borrelli E, Drago J, Lawrence AJ (2006) Genetic interdependence of adenosine and dopamine receptors: evidence from receptor knockout mice. Neuroscience 139: 661-670.
- Bell RL, Kimpel MW, McClintick JN, Strother WN, Carr LG, et al. (2009) Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav 94: 131-147.
- Sharma R, Engemann S, Sahota P, Thakkar MM (2010) Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J Neurochem 115: 782-794.
- Kim JH, Karpyak VM, Biernacka JM, Nam HW, Lee MR, et al. (2011) Functional role of the polymorphic 647 T/C variant of ENT1 (SLC29A1) and its association with alcohol withdrawal seizures. PLoS One 6: e16331.
- Ruby CL, Adams CA, Mrazek DA, Choi DS (2011) Anxiety Disorders: Adenosine Signaling in Anxiety. InTech, Rijeka, Croatia. 51-68
- Lucas M, Mirzaei F, Pan A, Okereke OI, Willett WC, et al. (2011) Coffee, caffeine, and risk of depression among women. Arch Intern Med 171: 1571- 1578.
- Lara DR (2010) Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis 20 Suppl 1: 239-248.
- Ribeiro JA, Sebastiao AM (2010) Caffeine and adenosine. J Alzheimers Dis 20 Suppl 1: 3-15.
- El Yacoubi M, Costentin J, Vaugeois JM (2003) Adenosine A2A receptors and depression. Neurology 61: S82-S87.
- Lobato KR, Binfare RW, Budni J, Rosa AO, Santos AR, et al. (2008) Involvement of the adenosine A1 and A2A receptors in the antidepressantlike effect of zinc in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry 32: 994-999.
- Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, et al. (2004) Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett 355: 21-24.
- Lucki I (1997) The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8: 523-532.
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, et al. (2009) Marble burying reflects a repetitive and perseverative behavior more than noveltyinduced anxiety. Psychopharmacology (Berl) 204: 361-373.
- Deacon RM (2006) Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 1: 122-124.
- Chen J, Rinaldo L, Lim SJ, Young H, Messing RO, et al. (2007) The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice. Genes Brain Behav 6: 776-783.
- Nam HW, Lee MR, Zhu Y, Wu J, Hinton DJ, et al. (2011) Type 1 equilibrative nucleoside transporter regulates ethanol drinking through accumbal N-methyl-D-aspartate receptor signaling. Biol Psychiatry 69: 1043-1051.
- Florio C, Prezioso A, Papaioannou A, Vertua R (1998) Adenosine A1 receptors modulate anxiety in CD1 mice. Psychopharmacology (Berl) 136: 311-319.
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, et al. (2001) Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A 98: 9407-9412.
- Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, et al. (2002) Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci 16: 547-550.
- Lang UE, Lang F, Richter K, Vallon V, Lipp HP, et al. (2003) Emotional instability but intact spatial cognition in adenosine receptor 1 knock out mice. Behav Brain Res 145: 179-188.
- Alsene K, Deckert J, Sand P, de Wit H (2003) Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 28: 1694-1702.
- Childs E, Hohoff C, Deckert J, Xu K, Badner J, et al. (2008) Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 33: 2791-2800.
- Rogers PJ, Hohoff C, Heatherley SV, Mullings EL, Maxfield PJ, et al. (2010) Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology 35: 1973-1983.
- Hohoff C, McDonald JM, Baune BT, Cook EH, Deckert J, et al. (2005) Interindividual variation in anxiety response to amphetamine: possible role for adenosine A2A receptor gene variants. Am J Med Genet B Neuropsychiatr Genet 139B: 42-44.
- Freitag CM, Agelopoulos K, Huy E, Rothermundt M, Krakowitzky P, et al. (2010) Adenosine A(2A) receptor gene (ADORA2A) variants may increase autistic symptoms and anxiety in autism spectrum disorder. Eur Child Adolesc Psychiatry 19: 67-74.
- Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, et al. (2004) Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology 29: 558-565.
- Hohoff C, Mullings EL, Heatherley SV, Freitag CM, Neumann LC, et al. (2010) Adenosine A(2A) receptor gene: evidence for association of risk variants with panic disorder and anxious personality. J Psychiatr Res 44: 930-937.
- Deckert J (1998) The adenosine A(2A) receptor knockout mouse: a model for anxiety? Int J Neuropsychopharmacol 1: 187-190.
- Egashira N, Okuno R, Harada S, Matsushita M, Mishima K, et al. (2008) Effects of glutamate-related drugs on marble-burying behavior in mice: implications for obsessive-compulsive disorder. Eur J Pharmacol 586: 164- 170.
- Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, et al. (2011) Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry 69: 867- 874.
- Rosenberg DR, MacMillan SN, Moore GJ (2001) Brain anatomy and chemistry may predict treatment response in paediatric obsessive--compulsive disorder. Int J Neuropsychopharmacol 4: 179-190.
- Umathe S, Bhutada P, Dixit P, Shende V (2008) Increased marble-burying behavior in ethanol-withdrawal state: modulation by gonadotropin-releasing hormone agonist. Eur J Pharmacol 587: 175-180.
- Alonso P, Gratacos M, Segalas C, Escaramis G, Real E, et al. (2010) Variants in estrogen receptor alpha gene are associated with phenotypical expression of obsessive-compulsive disorder. Psychoneuroendocrinology 36: 473-483.
- Becker JB (2009) Sexual differentiation of motivation: a novel mechanism? Horm Behav 55: 646-654.
- Forlano PM, Woolley CS (2010) Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518: 1330-1348.
- Galankin T, Shekunova E, Zvartau E (2010) Estradiol lowers intracranial selfstimulation thresholds and enhances cocaine facilitation of intracranial selfstimulation in rats. Horm Behav 58: 827-834.
- Cross CP, Copping LT, Campbell A (2011) Sex differences in impulsivity: a meta-analysis. Psychol Bull 137: 97-130.
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, et al. (2011) Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci 33: 1706- 1715.
- Moss HB, Chen CM, Yi HY (2007) Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend 91: 149-158.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14370
- [From(publication date):
specialissue-2011 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 9999
- PDF downloads : 4371