Research Article Open Access
Semi-interpenetrated Hydrogels Composed of PVA and Hyaluronan or Chondroitin Sulphate: Chemico-Physical and Biological Characterization
Antonella D Agostino, Annalisa La Gatta, Teresa Busico, Mario De Rosa and Chiara Schiraldi*Department of Experimental Medicine, Section of Biotechnology and Molecular Biology, Second University of Naples, Via De Crecchio No 7, 80138 Naples, Italy
- Corresponding Author:
- Prof. Chiara Schiraldi
Department of Experimental Medicine
Section of Biotechnology and Molecular biology
via De Crecchio No 7, 80138 Naples, Italy
E-mail: chiara.schiraldi@unina2.it
Received date: April 26, 2012; Accepted date: June 23, 2012; Published date: June 25, 2012
Citation: Agostino AD, Gatta AL, Busico T, Rosa MD, Schiraldi C (2012) Semi-interpenetrated Hydrogels Composed of PVA and Hyaluronan or Chondroitin Sulphate: Chemico-Physical and Biological Characterization. J Biotechnol Biomater 2:140. doi:10.4172/2155-952X.1000140
Copyright: © 2012 Agostino AD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Physical hydrogels based on poly (vinyl alcohol) (PVA) were synthesized in the presence of natural polysaccharides, either hyaluronan or Chondroitin sulphate, in order to obtain versatile biomaterials with enhanced performances. The physical network is obtained by the freeze thawing technique, a simple method widely used for structuring PVA blends. The chemico-physical characterization of resulting materials consisted of swelling studies and mechanical analysis. Furthermore the release of embedded polysaccharides from the network was evaluated to improve understanding of the strength of hydrogen bonding between the different polymeric chains, and the effect of the sulphate groups on the interaction promoting network formation and stability. Biological response in terms of cytotoxicity, adhesion and cell vitality of murine fibroblast and human keratinocytes showed that the addition of glycosaminoglycans to a PVA polymer leads to a biomaterial with potential applications in biomedical fields.
Keywords
Hydrogels; Poly (vinyl alcohol); Chondroitin sulphate; Hyaluronic acid
Introduction
Hydrogels, highly hydrated polymer networks, are formed by chemical or physical crosslinking of the hydrophilic polymers. The characteristics of hydrogels, including sensitivity to the environment, tissue-like water content and elasticity, grant the potential for biomedical application.
Poly (vinyl alcohol) (PVA) is a semi-crystalline synthetic polymer with good mechanical properties, that proved biocompatible. These characteristics make this hydrogel particularly suitable for pharmaceutical, biomedical, and cosmetic applications [1]. Its structure presents some features that make it an excellent candidate for biomaterial synthesis. In fact it is non-toxic, non-carcinogenic, easy to be processed and bioadhesive. PVA is produced by the polymerization of vinyl acetate to poly (vinyl acetate), followed by hydrolysis of PVAc to PVA. The molecular weight distribution, the degree of deacetylation and the initial concentration of PVA are the main parameters that affect hydrogel properties such as crystallizability, adhesion, mechanical strength, and diffusivity [2]. Because of its high degree of swelling in water and in biological fluids and its rubbery and elastic nature, PVA is capable of simulating natural tissue and can be accepted into the body. PVA must be crosslinked in order to remain insoluble in solution, the crosslink may be accomplished by chemical agents (glutaraldehyde, acetaldehyde, formaldehyde), by the use of electron beam or γ-irradiation, under alkaline conditions and, by physical methods, such as repeated freeze-thawing cycles. The last method, avoiding the presence of crosslinking agents, overcomes toxicity issues. Moreover, such physical crosslinked materials exhibit higher mechanical strength than PVA gels structured by common techniques due to the presence of crystalline regions [3]. Different applications have been proposed for PVA hydrogels: they have been used as intervertebral disc nuclei [4] and artificial articular cartilage [5,6]; for drug delivery vehicles, alone as well as in combination with other polymers in order to obtain desirable release profiles (slow release) [7,8]. In particular Oka et al. [9] examined aspects such as lubrication, load bearing, biocompatibility, and attachment of the material to the bone to look at the overall biomechanics of the material. Microsphere of PVA interpenetrated with poly (acrylic acid) were used to deliver a model anti-inflammatory drug, diclofenac sodium, to the intestine [10]. PVA and methacrylate based interpenetrated polymer network (IPN) was synthesized from Darwis and co-worker, using formaldehyde as crosslinker, in order to obtain a hydrogel with physico-chemical properties resembling those of the artificial spinal disc [11]. A typical physical crosslinking involved repetitive freezing and thawing of PVA aqueous solution. This technique promotes the formation of ordered microcrystalline domains as a result of enhanced intra- and inter-polymer interaction in the unfrozen regions of the PVA-water system. Many studies have been carried out on the effect of addition of other components to PVA. PVA has been associated also with natural polysaccharide such as GAGs. Hydrogels made of Chondroitin sulphate and poly (vinyl alcohol) systems were introduced to obtain new bioartificial materials that have excellent mechanical properties, biocompatibility and enhanced rheological properties [12]. Furthermore Liu and collaborators, showed that PVA hydrogels are potential candidates for artificial blood vessels, when physically crosslinked with chitosan, gelatin and starch. In fact there was an improvement in cell adhesion without compromising the appropriate mechanical properties of PVA suitable for vascular system development [13]. Composites based on PVA and PLGA by freeze-thawing technique were proposed as drug delivery systems of dexamethasone. The latter is considered a model pharmaceutical active principle for its inflammatory capability, because exhibits an approximately zero-order controlled release profile over a period of one month [14].
Concerning this topics, two well-known, glycosaminoglycan, Hyaluronic acid (HA) and Chondroitin sulphate (CS), both component of extracellular matrix, were employed for the synthesis of novel PVA based biomaterials.
CS plays a key role in biomaterials field: it is a widely distributed glycosaminoglycan in the human body, structurally present in cartilage and other tissues such as eye, aorta, skeletal muscle, lung and brain. In biomedical applications, CS has shown in vivo anti-inflammatory effect [15]. It also regulates metabolism in vitro, in fact CS can stimulate the production of Chondroitin sulphate proteoglycans over the entire period of culture in monolayer cultured chondrocytes [16]. It can be used for treating autoimmune and joint disease, for instance VISCOAT® (3% Chondroitin sulphate (aq) and 3% v/v sodium hyaluronate (aq), is used as a surgical aid in cataract extraction and lens implantation [17]. CS is also a component of the dermal layer of the FDA-approved skin substitute for treating burns.
However the most commonly exploited natural polysaccharide in scaffold assembly for tissue engineering and as component for implant materials is hyaluronic acid. HA, in fact, present a high capacity for lubrication, is very hydrophilic and influences several cellular functions such as migration, adhesion and proliferation [18,19]. It has a high molecular mass, up to millions of Daltons, and interesting viscoelastic properties influenced by its polymeric and polyelectrolyte characteristics. HA is ubiquitary in almost all biological fluids and tissues. In clinical practice, it is used as a diagnostic marker for many diseases including cancer, rheumatoid arthritis and liver pathologies, as well as for supplementation of impaired synovial fluid in arthritic patients by means of intra-articular injections. It is also used in certain ophthalmological and othological surgical techniques, in reconstruction of soft tissue, and in cosmaceutical regeneration protocols [20].
In presence of other synthetic polymers, HA is used for biological improvement. For example, in order to overcome the biological deficiencies of synthetic polymers, Cascone and co-workers blend in different ratios PVA and PAA with HA and collagens; the results showed that these biomaterials are new drug delivery systems for an important growth factor of bone and cartilage cells [21].
Starting from this background, the aim of this work is the synthesis of physical crosslinked PVA in presence of HA and CS and a chemicophysical and biological characterization of resulting network.
Materials
Polyvinyl alcohol (PVA) (molecular weight 78 kDa, (deacetylation degree (DD) 99.7%) was purchased from Polyscience. Hyaluronic acid sodium was purchased from BioPhyl (Milan, Italy), Chondroitin sulphate sodium salt, extracted from shark fin, was a kind gift from IBSA (Institute Biochimique SA, Lugano, Switzerland). Culture medium DMEM (Dulbecco Modified Minimum Essential Medium], 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Fetal bovine serum (FBS), Phosphate buffer solution (PBS), streptomycin, penicillin and fungizone were supplied by Life Technologies (Breda, The Netherlands). DAPI (diamino-2-phenyl-indol) and PKH26 and carbazole test reagents were purchased by SIGMA. 1,9-dimethyl dimethylene blu and glicine were purchased from SIGMA.
NIH Swiss 3T3 fibroblasts were provided by ATCC - American Type Culture Collection - (LGC Milano, Italia). Human keratinocytes (HaCat) cells, a spontaneously transformed non tumorigenic human keratinocyte cell line, were provided by Istituto Zooprofilattico (Brescia, Italy).
Methods
Preparation of PVA-CS hydrogels
A PVA solution 18% w/v was prepared in deionized water and autoclaved at 120°C for 20 minutes HA aqueous solutions at 2% and 4% w/v and separately CS solution at 2%, 4% and 10% w/v were prepared. Finally, a CS aqueous solution at 10% w/v was also prepared. Each of the biopolymer solutions was then mixed with PVA 18%w/v in 1:1 volume ratio in order to obtain solutions at the following w/v concentrations: PVA 9%-CS 1%, PVA 9%-CS 2% and PVA 9%-CS 5%, PVA 9%- HA 1%, PVA 9%-HA 2% w/v. A control mixture was prepared using water in place of the glycosaminoglycan solutions (PVA 9% w/v in water).
After intimate mixing, the solutions were loaded between two glass plates separated from silicon rubber (thickness 2 mm) to obtain a uniform film.
The physical crosslinking was obtained through freeze-thawing cycles (at -20°C for 24 hours followed by 4 hours at room temperature repeated four times).
The resulting hydrogels were then immersed into PBS or distilled water to remove the un-reacted residue not incorporated into the network or directly dried according to the characterization needs.
Swelling studies
The water absorbance was determined by gravimetric measurements, using an analytical balance (Ohaus Explorer, USA). In particular dried PVA, PVA-CS and PVA-HA hydrogels were placed in closed glass containers, in deionized water or in phosphate buffer solution (PBS) and kept in thermostatic bath at 37°C. Specimens, at different time, were removed until reaching the equilibrium state. Withdrawn samples were always blotted with filter paper to remove surface water and then they were weighed. The swelling degree was calculated as follows:
Swelling degree = ((ws-wd))/wd ×100 (1)
Where:
ws = swollen sample weight
wd = dried initial sample weight.
Determinations were run in triplicate.
Mechanical analysis
Rheological measurements were performed on materials swollen at the equilibrium in PBS at 37°C. A Physica MCR301 oscillatory rheometer (Anton Paar, Germany) equipped with parallel plate geometry (plate diameter of 25 mm) and a Peltier temperature control were used. In particular, strain sweep tests were performed at a constant oscillatory frequency of 1.59s-1 over a strain amplitude (γ) range of 0.001-10%. The storage modulus (G’), the loss modulus (G”) and the value of critical strain, γcr, (strain value at which G’ and G’’ cross over) were evaluated for each material.
Estimating the amount of CS and HA released respectively by PVA-CS and PVA-HA hydrogels
Polysaccharide release studies were performed on hydrogels directly dried after preparation. In particular, the dried hydrogels were incubated in water at 37°C for 24 h (equilibrium swelling conditions). The amount of biopolymer released in such conditions was quantified by means of different spectrophotometric methods depending on its nature.
In particular, for the quantification of CS released from PVA-CS hydrogels, 100 μl of the swelling medium were added with 1mL of aqueous reagent solution containing 1,9-dimethyl dimethylene blu 16 mg/L, glycine 3.04 mg/L, NaCl 2.37 g/L and HCl 9.5 mM. A525 was read immediately after mixing. The reagent solution added with water in place of the swelling medium was used as blank. A calibration curve obtained performing the test on CS standard solutions at various concentrations (25, 40, 80, 100, 120, 160 mg/L; A525 = 0.0036CS (mg/ mL)-0.0207 R2 = 0.99) was used. Measurements were performed using a spectrophometer DU 800 (Beckman Coulter) [22].
The amount of released HA from PVA-HA gels was estimated by carbazole assay [23]. In order to avoid interference due to the possible concomitant release of PVA from the hydrogels, the swelling medium was filtered on membranes with a cut-off 100 kDa and the assay was performed on the retentate containing only HA.
In both the quantification the amount of released biopolymer was calculated as:
Released biopolymer (%)=[(biopolymer mass in the medium (g)/ biopolymer total mass in the analysed specimen (g)]*100
Cell culture
Murine 3T3 fibroblasts and HaCat were routinely cultured in the medium containing DMEM supplemented with FBS (10% v/v), penicillin (100 units/ml), streptomycin (100 μg/ml). In the case of 3T3, fungizone (2.5 μg/mL) and 1% nonessential aminoacids were added to the medium. Cells were maintained in a humidified atmosphere at 37°C in 5% CO2.
In vitro cytotoxicity tests
The in vitro cytotoxicity was evaluated by the elution test method (ISO 10993-5) exposing 3T3 cell cultures grown to near confluence to fluid extracts from the materials under investigation. Fluid extracts were obtained placing the materials in cell culture medium, without FBS, at 37°C for 24 hours (1.5 mL medium/ cm2). Murine fibroblasts 3T3 cells (25×103; cells/ cm2) were cultured in the medium. Fluid extracts as such (100%) were then applied to murine fibroblasts 3T3 monolayers grown to confluence. After incubation for 24 h and 48 h at 37°C, cell viability was evaluated by means of the MTT assay.(3- [4,5 dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide). 1 ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide, 5 mg/ ml in PBS, was added to each well and incubated for 4 h at 37°C with 5% CO2. After this period, 1 ml of acidified isopropanol (0.1 M HCl in isopropanol) was added to each well and optical density was measured at 570 nm on a spectrophotometer DU 800 (Beckman Coulter).
Cell viability was expressed as percentage of viability with respect to the control (cells incubated with fresh nutrient medium).
Cell viability and morphology
PKH26 and DAPI fluorescent labeling: The cell adhesion and proliferation of HaCat on TCP (Tissue Colture Polistyrene) and on opaque hydrogels were accomplished by using two different fluorescent labeling: DAPI that binds to DNA, revealing the cell nucleus, and PKH26 that stains the cytoskeleton.
In the case of DAPI the staining was performed after 24h post seeding with an incubation time of 15min at T = 37°C. On the contrary the fluorescent dye PKH26 is a lipophilic fluorescent dye that binds irreversibly to the cell membrane and is not transferable to other cells. The staining of cells with this dye was performed prior the seeding according to the manufacturer (Sigma). Briefly, the cells were resuspended in a concentration of 107 cells/ml, followed by the addition of fluorescent dye PKH26 to a final concentrations of 2x10-6 molar. Two minutes later the reaction was stopped by addition of fetal bovine serum. Further, cells were washed three times with the medium and finally re-suspended in the medium. The cells were seeded immediately onto control and on the scaffolds.
The number of cells seeded for both the dyes is 12x103 cell/cm2.
The cellular response of HaCat cells on TCP and on PVA based hydrogels was followed by using a time lapse video microscopy in fluorescence, an innovative technique described in a previous article (art funz J Mater Sci: Mater Med). Briefly we select different fields of view for the control and for each sample (PVA, PVA-HA and PVACS) put in a 12-multiwell with the cells; only after a good visualization of selecting areas (absence of condense and T = 37°C), the time lapse experiment allowed us to monitor cell-materials interaction for 72-96 h. In particular the images in bright field and in fluorescence were recorded respectively every 1 h and every 6h throughout the experiments.
Cells morphology: All the novel biomaterials used as scaffolds, after 24 h incubation with cells, were rinsed in PBS, immersed in glutaraldehyde 2.5% v/v in PBS at room temperature for 1 h and then post-fixed with 1% w/v osmium tetroxide/PBS solution for 1 h at 4°C. The samples were then dried by treatment with ethanol/water solutions at increasing alcohol concentration (namely 30, 50, 70, 80, 95% v/v) for 5 mins, twice with 100% ethanol for 15min. The samples were then dehydrated with CO2 critical point drying. The gold-sputtered specimens were then observed by SEM (Zeiss Supra 40) in order to evaluate the morphology of adherent fibroblasts.
Results
Swelling studies
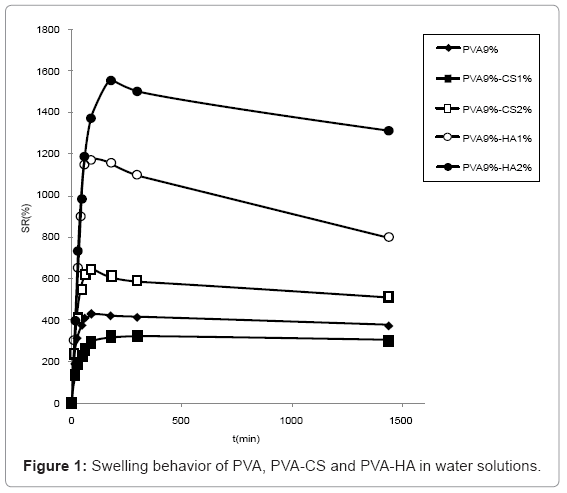
Swelling studies in water (Figure 1) showed that HA containing materials presented a higher water uptake than the ones containing CS, the most swollen being the one containing 2% w/v of HA. PVA 9%-CS 2% absorbed more water compared to PVA 9%-CS 1% that presented lower swelling degree respect to sole PVA.
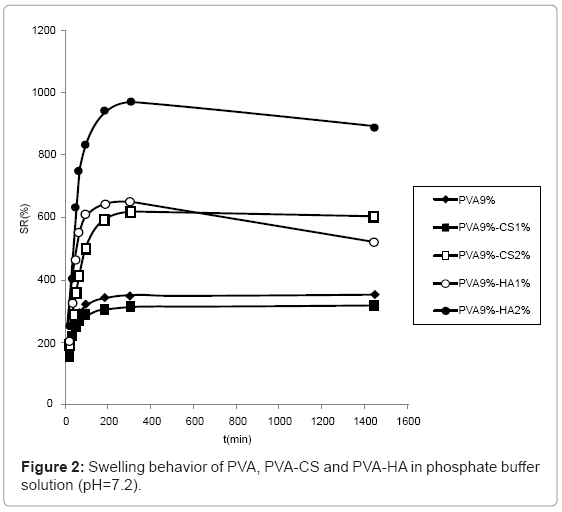
The swelling behaviour in PBS solution (Figure 2) was similar to the one observed in water although the differences are reduced due to the minor water uptake in saline solution. In particular, the swelling of hydrogels containing CS 2% and HA 1% became comparable.
The swelling of hydrogels containing 5% CS are not reported because the network broke and was dispersed in solution during water absorption.
Mechanical analysis
Results of the strain sweep tests are reported in table 1. As shown, all the gels showed an essential elastic behavior: as reported, the storage modulus G’ was far higher than the loss modulus G’’ measured at 1.59s-1 angular frequency for all the tested materials. HA containing hydrogels resulted more rigid than the other gels presenting higher G’ values (21.0 ± 1.1 and 30.4 ± 5.3 MPa for HA 1% and HA 2% hydrogels, respectively). The CS containing materials and the PVA hydrogel showed similar elasticity (G’ values around 15-16 MPa; p > 0.05). γcr values were found consistent with the elastic modulus values: materials exhibiting the lower G’ values behave as the more deformable hydrogels presenting a crossing over between G’ and G’’ at higher deformation values.
| Hydrogel | G’(MPa) | G’’(MPa) | γcr(%) |
|---|---|---|---|
| PVA 9% | 88 ± 8 | 3.3 ± 0.3 | 3.8 ± 0.4 |
| PVA 9%-CSA 1% | 88 ± 8 | 3.5 ± 0.1 | 3.4 ± 0.7 |
| PVA 9%-CSA 2% | 93 ± 9 | 4.4 ± 0.2 | 4.5 ± 0.8 |
| PVA 9%-HA 1% | 124 ± 8 | 4.9 ± 0.5 | 2.9 ± 0.4 |
| PVA 9%-HA 2% | 128 ± 40 | 8.2 ± 0.4 | 1.1 ± 0.1 |
Table 1: Mechanical Analysis of PVA based hydrogels. In particular the tables reported young’s modulus (storage and loss modulus G'e G'') and critical strains (γcr). Rheological parameters determined for PVA and CS containing hydrogels resulted not significantly different (p > 0.05).
Rheological measurements (Table 1) revealed a predominant elastic behaviour for all the tested materials thus proving them as crosslinked networks.
As shown in table 1, the introduction of CS into the PVA hydrogel at both concentrations did not significantly affect rheological properties of the PVA gel. On the other hand, the introduction of the same weight percentages of the other polysaccharide (HA) significantly increased rigidity of the material: G’ values for HA 1% and HA 2% containing hydrogels became about 1.4 and 2 fold higher with respect to PVA 9% elastic modulus. This finding could be related to the higher molecular weight of HA compared to CS.
Release of HA and CS from the hydrogels
CS and HA release was evaluated respectively by dimethylene blue and by carbazole test as describe in materials and methods.
The data, in table 2, reported CS release as percentage in the medium (deionized water) respect to dry weight of hydrogel. PVA 9%- CS 5% shows the highest percentage of release, while PVA 9%-CS 2% released only 0.3% of polysaccharide. The release of HA in materials based on PVA-HA is more consistent, in particular the hydrogel containing HA 2% releases a high percentage of GAG, probably due to a less interpenetrated system.
| Sample | CS or HA release (%) |
|---|---|
| PVA 9%-CSA 1% | 0.4 |
| PVA 9%-CSA 2% | 0.3 |
| PVA 9%-HA 1% | 1.5 |
| PVA 9%-HA 2% | 23 |
| PVA 9%-CSA 1% | 37.5 |
Table 2: Release of CS and HA respectively from PVA- CS and PVA-HA materials. The release is calculated as released biopolymer (%) = [(biopolymer mass in the medium (g)/biopolymer total mass in the analysed specimen (g) )] * 100.
Biological response
All the tested materials were found to be not toxic (data not shown); it is evident that both the presence of Chondroitin and hyaluronan make the materials more biocompatible than sole PVA.
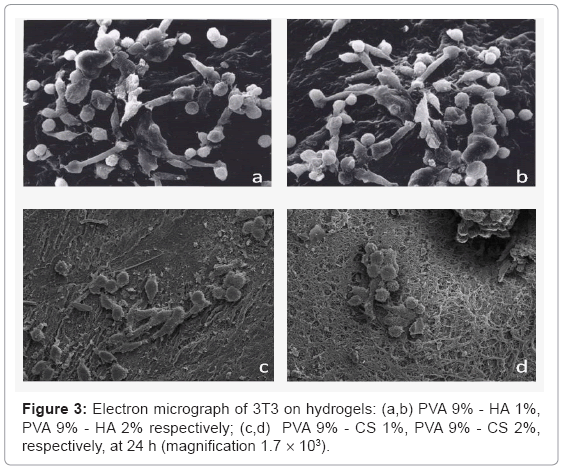
Concerning the adhesion and the morphology of cells by SEM analysis the figure 3, showed the absolute absence of 3T3 on PVA hydrogels. All the materials containing the GAGs favour cell adhesion, in particular the cells on hydrogel with CS 1% w/v have a better morphology respect to the ones containing CS 2%. The fibroblasts on hydrogels containing hyaluronan are adherent and there are not substantial differences by varying the percentage of polysaccharide.
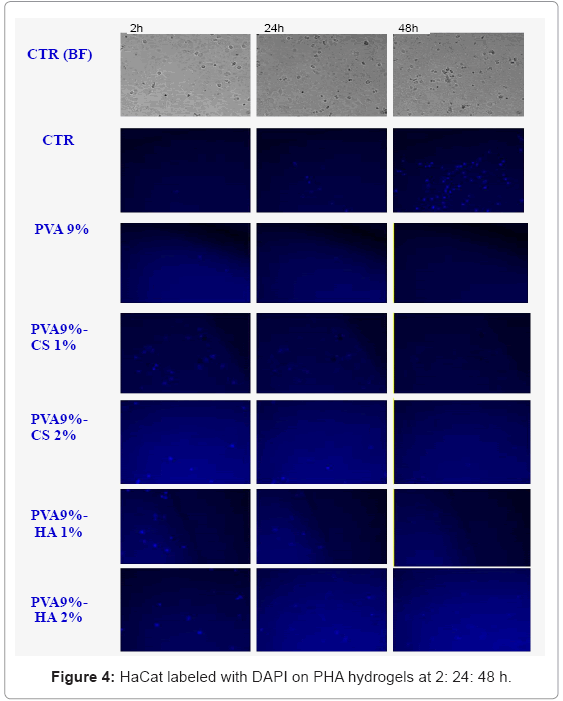
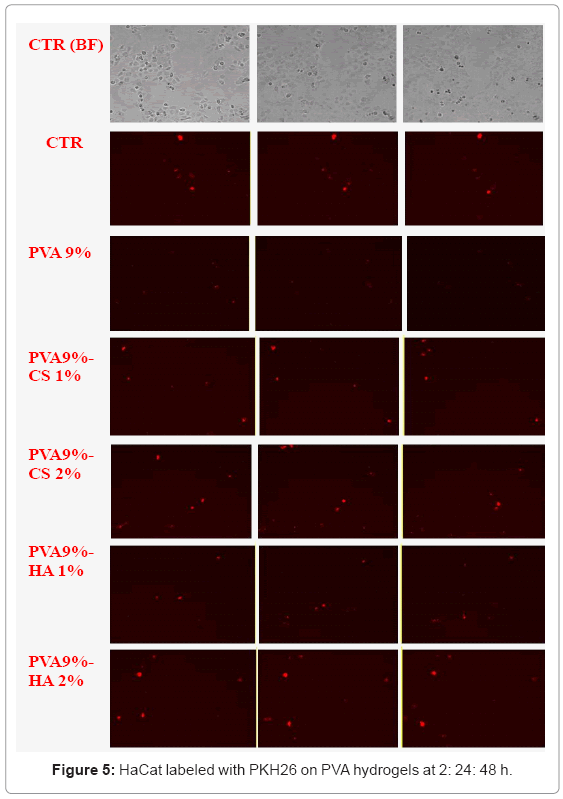
The results obtained using fluorescent labelling (Figure 4-5) show that the staining percentage is low so the blue and red visible cells are considerably minor respect to that found in the bright field acquisition. Nevertheless we observed that there are anchored cells on the surface of PVA-GAG based materials respect to the sole PVA; in particular the materials containing HA show more adherent cells after 24 h. The images at 48 h are not clear probably for oversaturation of the dye and release in the medium.
Discussion
The combination of natural polymers with synthetic ones through chemical and physical interaction is among the core topics recently investigated in biomaterial sciences.
PVA hydrogels represent very versatile synthetic materials used for biomedical applications.
In this work, the combination of PVA with glycosaminoglycans such as HA and CS was obtained in order to exploit the different features of these polymers towards biomaterials with improved performances. In the last years several studies focused on blending CS and HA with synthetic polymer like acrylic resins, polyester etc. The use of chondroitin and hyaluronan as natural polymer to blend with PVA hydrogels through solvent casting technique has been extensively exploited by Crivoi and collaborators that showed the higher biocompatibility in the presence of these polysaccharides [24]. Lee and collaborators chemically crosslinked PVA with CS obtaining a scaffold suitable for tissue engineering [22]. Although elastic modulus and biological response proved better generally the use of a chemical crosslinker, may lead to the release of toxic substances and thus requires additional purification steps. Bearing this in mind, in this project the freeze thawing technique is a simple and efficient method to obtain a physical crosslinked network conjugating the properties of natural polymers with the synthetic ones.
In particular in the framework of this experimental practice we synthesized different PVA based networks using different concentrations of HA e CS ranging from 1 to 5% in order to assess the concentration of the polysaccharides able to deliver better benefits.
The repetitive cycles of freeze thawing make all the materials more elastic and handy also when containing the GAGs.
The anionic nature and the chains relaxation due to repulsion between the negative charges are responsible for the major swelling capacity of polysaccharides containing materials with respect to pure PVA hydrogels. The higher water uptake of PVA 9%-CS 2% respect to the one containing CS1% was probably due to a major semiinterpenetrated structure of these materials; conversely the higher presence of sulphate (negative charge) in the PVA 9%-CS 2% makes the structure less tighten and consequently more swellable. The higher water uptake of hydrogels containing HA respect to the ones containing CS may be due to a major extent of hyaluronan that was unable to fill the pores of PVA network making it more porous and consequently more swellable. On the contrary CS, being smaller, fills the holes of the structure making it more compact and less porous.
Rheological measurements (Table 1) revealed a predominant elastic behaviour for all the tested materials thus proving them as crosslinked networks. As shown in table 1, the introduction of CS into the PVA hydrogel at both concentrations did not significantly affect rheological properties of the PVA gel. On the other hand, the introduction of the same weight percentages of HA significantly increased rigidity of the material: G’ values for HA 1% and HA 2% containing hydrogels became about 1.4 and 2 fold higher with respect to PVA 9% elastic modulus. This finding could be related to the higher molecular weight of HA compared to CS.
The biocompatibility assay of the tested materials revealed a better biological response in the presence of GAG as predictable by modern literature [24,25].
Conclusion
However, the materials containing hyaluronan seemed more cytocompatible respect the ones containing chondroitin sulfate. These behaviors are evident in the SEM micrographs (observations) where on PVA-HA there are many cells, with regular shape typical for a sound interaction/adhesion site. Preliminary experiment using fluorescent labeling confirmed the idea that chondroitin and hyaluronan improves the biocompatibility of PVA hydrogels. Also in this case HA seemed to elicit the best biological response respect to CS.
Acknowledgements
It is a great pleasure to thank Dr. A. Stellavato for time lapse support and collaboration.
References
- Masci G, Husu I, Murtas S, Piozzi A, Crescenzi V (2003) Physical Hydrogels of Poly(vinyl alcohol) with Different Syndiotacticity Prepared in the Presence of Lactosilated Chitosan Derivatives. Macromol Biosci 3: 455–461.
- Lozinsky VI, Vainerman ES, Domotenko LV, Mamtsis AM, Titova EF, et al. (1986) Study of cryostructurization of polymer systems VII. Structure formation under freezing of poly(vinyl alcohol) aqueous solutions. Colloid Polym Sci 264: 19-24.
- Hassan CM, Peppas NA (2000) Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 33: 2472-2479.
- Qi-bin B, Paul AH (1991) Hydrogel intervertebral disc nucleus USP: 623/17.16
- Noguchi T, Yamamuro T, Oka M, Kumar P, Kotoura 1991) Poly(vinyl alcohol) hydrogel as an artificial articular cartilage: Evaluation of biocompatibility. J Appl Biomater 2: 101-107.
- Oka M, Ushio K, Kumar P, Ikeuchi K, Hyon SH, et al. (2000) Development of artificial articular cartilage. Journal of Engineering in Medicine 214: 59-68.
- Takamura A, Ishii F, Hidaka H (1992) Drug release from poly(vinyl alcohol) gel prepared by freeze-thaw procedure. J Control Release 20: 21-27.
- Morita R, Honda R, Takahashi Y (2000) Development of oral controlled release preparations, a PVA swelling controlled release system (SCRS). I. Design Of SCRS and its release controlling factor. J Control Release 63: 297–304.
- Oka M, Noguchi T, Kumar P, Ikeuchi K, Yamamuro T, et al. (1990) Development of an artificial articular cartilage. Clin Mater 6: 361–381
- Kurkuri MD, Aminabhavi TM (2004) Poly(vinyl alcohol) and poly(acrylic acid) sequential interpenetrating network pH-sensitive microspheres for the delivery of diclofenac sodium to the intestine. J Control Release 96: 9-20.
- Darwis D, Stasica P, Razzak MT, Rosiak JM (2002) Characterization of poly(vinyl alcohol) hydrogel for prosthetic intervertebral disc nucleus. Radiation Physics and Chemistry 63: 539-542.
- Kim SJ, Park SJ, Kim SI (2003) Swelling behavior of interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and chitosan. React Funct Polym 55: 53–59.
- Liu Y, Vrana NE, Cahill PA, McGuinness GB (2009) Physically crosslinked composite hydrogels of PVA with natural macromolecules: structure, mechanical properties, and endothelial cell compatibility.. J Biomed Mater Res B Appl Biomater. 90B: 492-502.
- Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, et al. (2005) Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J 7: E231-E240.
- Ronca F, Palmieri L, Panicucci P, Ronca G (1998) Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage 6: 14-21.
- Schwartz NB, Dorfman A (1975) Stimulation of chondroitin sulfate proteoglycan production by chondrocytes in monolayer. Connect Tissue Res 3: 115-122.
- Tomita N, Sando S, Sera T, Aoyama Y (2004) Macrocyclic proteoglycan mimics. Potent inhibition of cell adhesion by a bundle of chondroitin sulfate chains assembled on the calyx[4]resorcarene platform. Bioorgan Med Chem Lett 14: 2087-2090.
- Luo Y, Kirker KR, Prestwich GD (2000) Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release 69: 169-184.
- Greco RM, Iocono JA, Ehrlich HP (1998) Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J Cell Physiol 177: 465–473.
- Kogan G, Soltés L, Stern R, Gemeiner P (2007) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Letter 29: 17-25.
- Cascone MG, Sim B, Downes S (1995) Blends of synthetic and natural polymers as drug delivery systems for growth hormone. Biomaterials 16: 569-574.
- Lee CT, Kung PH, Lee YD (2005) Preparation of poly(vinyl alcohol)-chondroitin sulfate hydrogel as matrices in tissue engineering. Carbohydr Polym 61: 348–354.
- Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal biochem 4: 330-334.
- Crivoi F, Stefan L, Moldovan L, Vasile C (2009) New biocompatible materials by PVA/HC and biological polymers. Journal of optoelectronics and advanced materials 11: 356-365.
- Hartwell R, Leung V, Chavez-Munoz C, Yang H, Ko F, et al. (2011) A novel hydrogel-collagen composite improves functionality of an injectable extracellular matrix. Acta Biomaterialia 7: 3060-3069.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15430
- [From(publication date):
July-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10728
- PDF downloads : 4702