| Research Article |
Open Access |
|
| R. Devika1, S. Elumalai1*, E. Manikandan2* and D. Eswaramoorthy1 |
| 1P.G. and Research Dept. of Plant Biology & Plant Biotechnology, Presidency College, Chennai, India |
| 2Polymer Nanotechnology Centre (PNTC) & Dept. of Chemistry, B.S. Abdul Rahman University, Chennai, India |
| *Corresponding authors: |
S. Elumalai
P.G. and Research Dept. of Plant Biology & Plant Biotechnology
Presidency College, Chennai-600005, India
E-mail: anananadal@gmail.com |
| Â |
E. Manikandan
Polymer Nanotechnology Centre (PNTC) & Dept. of Chemistry
B.S. Abdul Rahman University, Chennai-600048, India
E-mail: maniphysics@ gmail.com |
|
| Â |
| Received October 03, 2012; Published November 30, 2012 |
| Â |
| Citation: Devika R, Elumalai S, Manikandan E, Eswaramoorthy D (2012) Biosynthesis of Silver Nanoparticles Using the Fungus Pleurotus ostreatus and their Antibacterial Activity. 1:557 doi:10.4172/scientificreports.557 |
| Â |
| Copyright: © 2012 Devika R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Metal Nanoparticles (m-NPs) are explored in recent years as an alternative approach to effectively kill drug resistant pathogenic microorganisms. Silver nanoparticles (Ag-NPs) are the metal of choice for antibiotic resistant microbes. Synthesis of silver nanoparticles through biological route is preferred over chemical route to promote green chemistry. In the present study, an eco-friendly process for the synthesis of nanomaterials, using a fungus (Pleurotus ostreatus) has been attempted. The fungus supernatant of seed culture was used for the biosynthesis of Ag-NPs. The aqueous silver ions (Ag+) were reduced to silver metal nanoparticles (Ag m-NPs), when treated with the fungal supernatant. After 72 h of treatment, silver nanoparticles (Ag-NPs) were obtained. These Ag-NPs were characterized by UV-Vis, Scanning Electron Microscopy (SEM) with Energy Dispersive Spectroscopy (EDS), X-ray Diffraction (XRD) and Transmission Electron Microscopes (TEM) were used to identify these NPs. The nanoparticles exhibited maximum absorbance peak at 440 nm in UV–Vis spectroscopy. From the XRD pattern of Ag-NPs exhibited 2θ~38.2° values, corresponding to the silver Ag (111) crystalline phase indexed. The NPs surface morphology revealed from SEM and TEM images shows formation of well-dispersed Ag-NPs of 50 nm, and the presence of silver was confirmed by EDX analysis. The microbes selected for the present study for the antibacterial activity were Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus and Vibrio cholera. This study conclude that the nanoparticles synthesized from the fungus open up the exiting possibility of rational strategy of biosynthesis of nanomaterials, and thus, silver nanoparticles has great potential as antimicrobial compound against pathogenic microorganisms studied, and that it can be used in the treatment of infectious diseases caused by bacteria. |
| Â |
| Keywords |
| Â |
| Silver nanoparticles; Pleurotus ostreatus; Antimicrobial activity; Spectroscopic studies |
| Â |
| Introduction |
| Â |
| The development of environmentally benign materials like fungi for the synthesis of Ag-NPs offers numerous benefits of eco-friendliness and compatibility for pharmaceutical and other biomedical applications, as they do not use toxic chemicals for the synthesis protocol. Several applications of nanoparticles are in the field of drug/gene delivery vehicles [1]. There is an increasing commercial demand for nanoparticles due to their wide applicability in various areas, such as electronics, catalysis, chemistry, energy and medicine. New application of nanoparticles and nanomaterials are emerging rapidly [2,3]. Nanocrystalline Ag-particles have found tremendous applications in the field of high sensitivity bio-molecular detection and diagnostics [4], antimicrobials and therapeutics [5,6]. One approach that shows immense potential is based on the biosynthesis of nanoparticles, using biological organisms such as bacteria. In this work, we have concentrated on the use of fungi in the extracellular production of metal nanoparticles. As part of our work, we have observed that aqueous silver ions, when exposed to the fungus Pleurotus ostreatus, are reduced in solution, thereby leading to the formation of an extremely stable silver particle. The Ag-NPs are in the range of 30 nm in dimensions, and are stabilized in the solution by proteins secreted by the fungus. It is believed that the reduction of the metal ions occurs by an enzymatic process, thus creating the possibility of developing a fungus-based method for the synthesis of nanoparticles. It has been well established that microorganisms are a virtually unlimited source, and have potential therapeutic applications. |
| Â |
| Materials and Methods |
| Â |
| Sample preparation |
| Â |
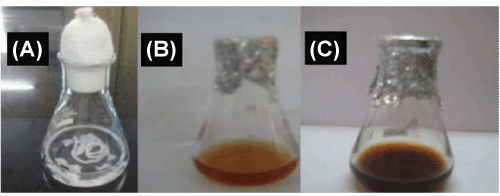
| All chemicals used were of analytical grade. P. ostreatus was grown in seed medium containing glucose 20 g/l, glycerol 30 g/l, peptone 8 g/l, NaNO3 2 g/l, MgSO4 1 g/l and soyabean meal 20 g/l, the pH was adjusted to 6.5 ± 0.2. The flasks were incubated in the incubator shaker at 1800 rpm at 25°C. After 3 days of incubation, the mycelia were separated by centrifugation (5000 rpm) and washed thrice with deionized water. The washed mycelia were suspended in deionized water, the aqueous extract obtained after separation of mycelia suspension was challenged with 100 ml of 1 mM silver nitrate [Ag(NO3)2]. It is well known that Ag-NPs exhibit yellowish brown color in aqueous solution due to excitation of surface plasmon vibrations in Ag-NPs [7]. A bottle of the fungal cells after removal from the culture medium, and before immersion in 1 mM Ag(NO3)2 solution. The fungal cells provided a pale (yellow) color, and the bottle containing the fungal cells after immersion in 1 mM Ag(NO3)2 solution for 72 hours can clearly be observed that the previous pale color of the reaction mixture has changed to a darker shade after 72 hours of reaction. The appearance of the yellowishbrown color in solution containing the biomass was a clear indication of the formation of Ag-NPs in the reaction mixture. The brown color of the solution is due to the excitation of surface plasmon vibrations, which is essentially the vibration of the group conduction electrons in the silver nanoparticles. |
| Â |
| Characterization techniques |
| Â |
| UV-Vis spectroscopy analysis: UV-Vis spectral analysis was done by using Elico UV-Vis spectrophotometer. The reduction of pure Ag+ ions was monitored by measuring the UV-Vis spectrum of the reaction medium at 72 hrs, after diluting a small aliquot of the sample into distilled water. |
| Â |
| FTIR analysis of Ag-NPs: FTIR analysis was done using Perkin Elemer Spectrum-1, and was used to identify the chemical constituents in the Mid Infrared (MIR) region of 400-4000 cm-1 of the Ag-NPs from fungus extracted samples. |
| Â |
| XRD measurement: XRD measurements of film of the biologically synthesized and Ag-NPs were cast into glass slides were done by Phillips PW 1830 instrument. The operating voltage of 40 kV and current of 30 mA with Cu kα radiation of 0.1541 nm wavelength, in the 2θ range 10- 80º, step size 0.02/θ. |
| Â |
| SEM analysis of Ag-NPs: Scanning Electron Microscopic (SEM) analysis was done by FEI QUANTA 200 FEG HR-SEM model, operated at 30 kV with the working distance 8 mm. Thin films of the sample were prepared on a carbon coated, a very small amount of the specimen on the sample holder, extra solution was removed using a blotting paper, and then the film on the SEM allowed to dry by putting it under a mercury lamp for 5 min. |
| Â |
| TEM analysis of Ag-NPs: Sample for TEM analysis was prepared, as mentioned in IR sample preparations. The sample was first sonicated (Vibronics VS 80) for 5 minutes. Ag-NPs were loaded on carboncoated copper grids, and solvent was allowed to evaporate under Infra light for 30 minutes. TEM measurements were performed on Phillips modle CM 20 instrument, operated at an accelerating voltage at 200 kV. |
| Â |
| Antimicrobial assay |
| Â |
| Collection of microorganisms: The microbes selected for the present study were Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus and Vibrio cholerae. |
| Â |
| Preparation of inoculums: Each organism was recovered for testing by sub-culturing on fresh media. A loopful inoculum of each bacterium was suspended in 5 ml of nutrient broth and incubated overnight at 37°C. These overnight cultures were used as inoculums in all our experiments. |
| Â |
| Preparation of media: The growth media employed in the present study included nutrient agar and nutrient broth. The medium was adjusted to pH 7 and sterilized by autoclaving at 15 lbs pressure (121°C) for 15 minutes for sterilization. |
| Â |
| Sub-culturing of microorganism: The pure cultures of microorganism were maintained on nutrient agar slant by frequent sub culturing. These cultures were stored at 4ËšC for further experiments. |
| Â |
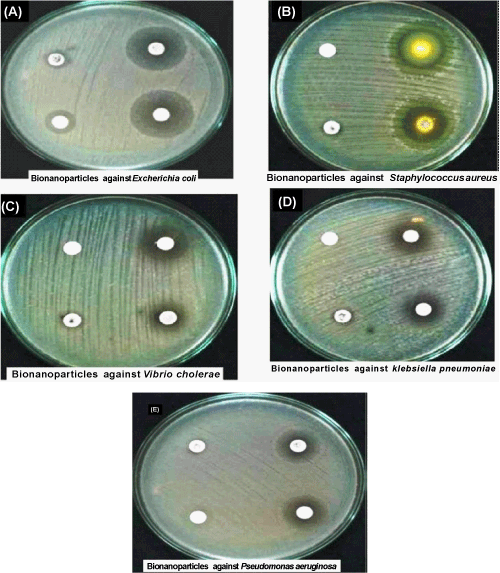
| Antibacterial activity: Bactericidal effects of Ag-NPs were studied against Gram-positive and Gram-negative bacteria. Antimicrobial activity was demonstrated by modified method described by Langfield et al. [8]. Then 0.1 ml of the diluted microbial cultures was spread on sterile nutrient agar plate. The soaked and dried discs of 6 mm diameter of Whatman filter paper No: 1 were then placed on the seeded plates and gently pressed down to ensure contact [9]. Four replicates were placed for control, Ag-NPs, antibiotics and Ag-NPs combined with antibiotics (Chloramphenicol, Rifampicin, Tetracyclin, Erythromycin, Amoxicillin), in each disc to confirm the inhibition a zone, and the plates were incubated at 37°C for 24 hours. After incubation period, the inhibition zone around the discs were measured and recorded, as the difference in diameter between the disc (6 mm) and growth free zone of Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus and Vibrio cholera were measured. |
| Â |
| Results and Discussion |
| Â |
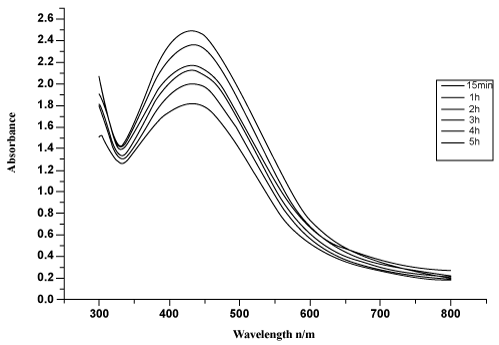
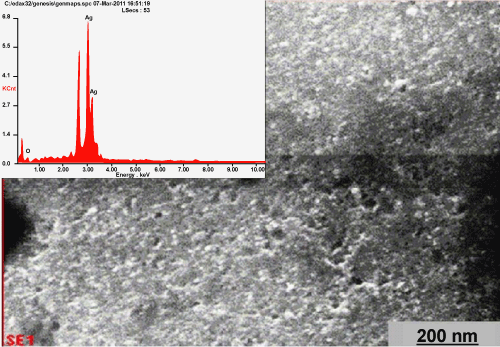
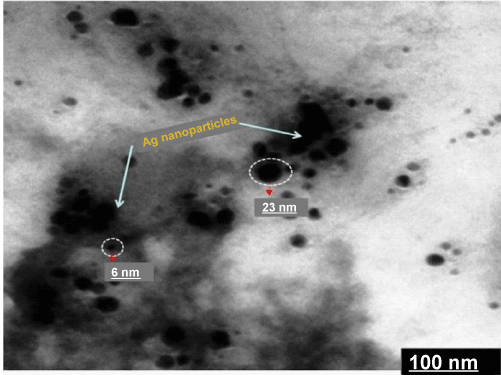
| The fungal cell filtrate after addition of aqueous Ag(NO3)2 (1 mM) was subjected to optical measurements by UV-Vis spectrophotometer; this analysis showed an absorbance shape peak at 440 nm (Figure 1), which belongs to the specific for the Ag-NPs. The exact mechanism of the synthesis of Ag-NPs was not known, but later it was hypothesized that the silver ions required the NADH-dependent nitrate reductase enzyme for their reduction [10,11], which was secreted by the fungus in its extracellular environment. The presence of NADH-dependent nitrate reductase enzyme in extracellular cell filtrate of the fungus used for the synthesis of nanoparticles has been confirmed, and the mechanism has been studied [12,13]. It is generally recognized that UV-Vis spectroscopy could be used to examine size and shapecontrolled nanoparticles in aqueous suspensions [14]. The UV-Vis spectra recorded from the P. ostreatus reaction vessel at different reaction times are shown in figure 2. The time at which the aliquots were removed for measurement is indicated next to the respective curves in figure 2. The strong surface plasmon resonance centered at about 420-440 nm is characteristic of colloidal silver and this peak shifted (red) from 440 to 455 nm as the reaction proceeded. The spectra also clearly show the increase in intensity of silver solution with time, indicating the formation of an increased number of Ag-NPs in the solution. From the figure 1, there was no appreciable change in the net magnitude of UV-Vis absorbance of the reaction product after 72 hrs, which is indicative of the fact that the reaction came to equilibrium at about 72 hrs. It should be pointed out that the reaction was allowed to proceed for about one month. The solution was extremely stable even after a month of reaction, with no evidence of aggregation of particles. The amide bands were identified (the FTIR spectrum is showed in the range of 1200 to 1800 cm−1 in the figure 3). The spectrum shows the presence of two bands. The bands at 1650-(1) and 1450-(2) cm are due to —C=O and N—H stretch vibrations present in the amide linkages of the proteins, respectively. The molecular vibrational positions of these bands are close to earlier reported literature for native proteins by Labrenz et al. [15]. Thus, the FTIR measurement indicates that the secondary structures of proteins in the P. ostreatus fungi are not affected because of their interaction with Ag+ ions or nanoparticles, as well as a number of aggregates. The morphology of the nanoparticles is highly variable. Under observation The XRD pattern, thus, clearly shows that the Ag-NPs were essentially crystalline. The intensity of the diffraction was much stronger than those of the other diffractions. The XRDdiffraction measured in this case resulted in four intense peaks shown in figure 4. Thus, it agrees the Bragg’s reflection of silver nanocrystals. This further confirms that the Ag-NPs formed in the extracellular filtrate are present in the form silver nanocrystals. Scanning Electron Microscope (SEM) surface morphology image showed relatively spherical shape nanoparticles formed with diameter range 40–50 nm in figure 5. Referring to figure 6, a representative TEM picture recorded from the Ag-NPs film deposited on a carbon coated copper TEM grid is shown, which displays individual silver particles of such images, these assemblies were found to be aggregates of Ag-NPs, in the size range of about 8-50 nm. The nanoparticles were not in direct contact even within the aggregates, indicating stabilization of the nanoparticles by a capping agent. The separation between the Ag-NPs seen in the TEM image could be due to capping by proteins and would explain the UV-Vis spectroscopy measurements, which is characteristic of welldispersed silver nanoparticles. The silver particles are crystalline, as can be seen from the selected area diffraction pattern recorded from one of the nanoparticles in the aggregates in figure 5. TEM imaging confirmed this (Figure 6). This image show agglomerates of small grains and some dispersed nanoparticles. The particle size histograms of silver particles (Figure 6) show that the particles range in size from 8 to 50 nm with mean diameter. |
| Â |
|
|
Figure 1: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
|
|
Figure 2: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
|
|
Figure 3: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
|
|
Figure 4: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
|
|
Figure 5: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
|
|
Figure 6: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
| Antibacterial activity |
| Â |
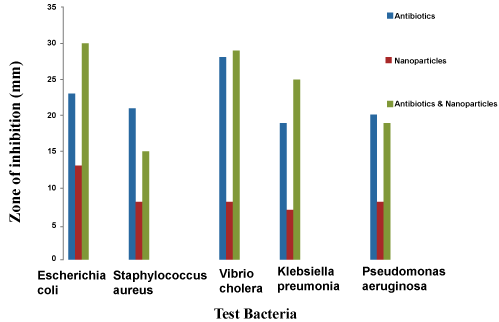
| Regarding antibacterial activity, the Ag-NPs showed activity against five bacteria (Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus and Vibrio cholera), and the highest antibacterial effect on Eschericia coli was found with zone of inhibition (13 mm) and lowest antibacterial effect in Ag-NPs on Klebsiella pneumonia (7 mm). The Ag-NPs also showed activity against Klebsiella pneumonia, Pseudomonas aeruginosa and Vibrio cholera, with zone of inhibition ranging from (7-8 mm). Results were summarized in figure 7. Among antibiotic, the strongest antibacterial effect on Vibrio cholerae at (28 mm) and the weakest activity was found in Klebsiella pneumonia with zone of inhibition (19 mm). Results were shown in table 1. Comparison of the Ag-NPs combined with antibiotics (Chloramphenicol, Rifampicin, Tetracyclin, Erythromycin, Amoxicillin) data obtained in this study, the maximum activity was observed in Ag-NPs combined with antibiotics against Eschericia coli, and minimum activity was observed in Ag-NPs combined with antibiotics against Staphylococcus aureus, at inhibition variation was observed in the range of 15 mm. Ag-NPs combined with antibiotics was also showed good zone of inhibition range between (19-29 mm), at activity against Klebsiella pneumonia, Vibrio cholerae and Pseudomonas aeruginosa were summarized in figure 8. |
| |
| Â |
|
|
Figure 7: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
|
|
Figure 8: Schematic diagram of fungal supernatant (A) culture medium (B) Ag (NO3)2 solution + samples (C) Formation of Ag NPs. |
|
| Â |
|
|
Table 1: Zone of inhibition of Ag-NPs against various pathogenic bacteria. |
|
| Â |
| Conclusion |
| Â |
| The present study conclude that the silver nanoparticles (Ag- NPs) have a great potential as antimicrobial compounds against pathogenic micro-organisms, and that they can be used in the treatment of infectious diseases caused by them. Thus, Ag-NPs along with antibiotics show maximum antibacterial activity, and so this can lead for the development of new pharmaceuticals that address hither to unmet therapeutic needs. Thus, it is proven from this study that the Ag-NPs synthesized from P. ostreatus species seems to be promising and effective antibacterial agent against the multidrug resistant strains of bacteria. |
| Â |
| Acknowledgments |
| Â |
| The authors (R. Devika and S. Elumalai) are grateful to the Principal and Head, Plant biotechnology department, Presidency College, Chennai, India, and SAIF (Sophisticated Analytical Instruments Facility), IITM, Chennai, India. |
| Â |
| |
| References |
| Â |
- Otsuka H, Nagasaki Y, Kataoka K (2003) PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev 55: 403-419.
- Nalwa HS (2000) Hand book of Nanostructured Materials and Nanotechnology: Electrical properties. Academic Press, Waltham, USA.
- Murphy CJ (2008) Sustainability as an emerging design criterion in nanoparticle synthesis and applications. J Mater Chem 18: 2173-2176.
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, et al. (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3: 95-101.
- Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27: 76-83.
- Elechiguerra JL, Morones JR, Burt JL, Camacho AB, Gao X, et al. (2005) The bactericidal effect of silver nanoparticles with HIV-1. J Nanobiotechnology 3: 6.
- Shankar SS, Ahmad A, Sastry M (2003) Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog 19: 1627-1631.
- Langfield RD, Scarano FJ, Heitzman ME, Kondo M, Hammond GB, et al. (2004) Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. J Ethnopharmacol 94: 279-281.
- Wiley BJ, Xiong Y, Li ZY, Yin Y, Xia Y (2006) Right bipyramids of silver: a new shape derived from single twinned seeds. Nano Lett 6: 765-768.
- Asres K (2006) Antibacterial activity of Swartzia extract. J Tradi comple and Alter Medi 3: 2.
- Oboh IE, Akerele JO, Obasuyi O (2007) Antimicrobial activity of the ethanol extract of the aerial parts of sida acuta burm.f,(malvaceae). Tropical Journal of Pharmaceutical Research 6: 809-813.
- Song HY, Ko KK, Oh IH, Lee BT (2006) Fabri`cation of silver nanoparticles and their antimicrobial mechanisms. Eur Cell Mater 11: 58.
- Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, et al. (2000) A mechanistic study of the anti-bacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52: 662-668.
- Yamanaka M, Hara K (2005) A review on the application of inorganic nano-structured materials in the modification of textiles. J Appl Environ Microbiol 71-11: 7589-7593.
- Labrenz M, Druschel GK, Thomsen-Ebert T, Gilbert B, Welch SA, et al. (2000) Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science 290: 1744-1747.
|
| Â |
| Â |