| Research Article |
Open Access |
|
| Shetye Suhas1*, Mohan Rahul1, Shukla Sunil Kumar1, Maruthadu Sudhakar1,2 and Sahinagazi1 |
| 1National Centre for Antarctic & Ocean Research, Headland Sada, Vasco-Da-Gama, Goa 403 804, India |
| 2Goa university, Taleigao Plateau, Goa-403206, India |
| *Corresponding author: |
Shetye Suhas
National Centre for Antarctic & Ocean Research
Headland Sada, Vasco-Da-Gama
Goa 403 804, India
E-mail: suhassht@gmail.com |
|
| Â |
| Received June 25, 2012; Published October 30, 2012 |
| Â |
| Citation: Suhas S, Rahul M, Kumar SS, Sudhakar M, Sahinagazi (2012) Zirconium on Benthic Foraminiferal Tests in the Arctic: A Plausible Shield towards Ocean Acidification. 1:462. doi:10.4172/scientificreports.462 |
| Â |
| Copyright: © 2012 Suhas S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Foraminiferal samples from Kongsfjorden (Arctic Ocean) were analysed for elemental ratios using Energy Dispersive spectrometry (EDS), along with sediment organic carbon, grain size and trace metals (Zr and Hf). EDS results on benthic foraminifera N. labradorica tests surface indicated presence of zirconium (Zr), whereas other foraminiferal species like Elphidium excavatum, Cassidulina reniforme and Quinqueloculina stalkeri were devoid of any Zr traces. Both foraminiferal analysis (EDS) as well as sediment analysis (ICP-MS) indicated highest Zr percentage at the inner fjord station 4. Concentration of Zr showed opposite trend to salinity, temperature and organic carbon indicating that the source of Zr is through weathering of continental zircon. Zr showed close association with Hafnium (Hf) and the average Zr/Hf ratio within Kongsfjorden was 43. Benthic foraminiferal shells did not show any signs of dissolution. As Zr is resistant to any acid corrosion; it could possibly be protecting the foraminifera from dissolution. Hence, in the present study we conducted lab experiment on the benthic foraminifera from outer/ inner fjord by pH manipulation, to identify the possible effects on benthic foraminiferal tests in absence/presence of zirconium coating towards dissolution. Interestingly, at pH 6 foraminifera from inner fjord having Zr coating (as evidenced by EDS) did not show any dissolution, whereas outer fjord ones which are devoid of Zr shielding showed dissolution. These preliminary results indicate that with the continuing increase in atmospheric CO2 it may be interesting to study the test structure of Zr encrusted foraminiferal tests in Kongsfjorden. |
| Â |
| Keywords |
| Â |
| Kongsfjorden; Zirconium; Acidification |
| Â |
| Introduction |
| Â |
| Industrial activities have largely contributed to the widespread pollution of marine ecosystems. Contaminants include, trace metals and polycyclic aromatic hydrocarbons (PAHs). Some local processes may enrich the trace element composition of sea water dramatically [1]. Also changes in redox conditions Morford et al. [2] or anthropogenic pollution sources may influence heavy metal distribution in sea water. Some metals are essential to biota, but all have the potential to be biologically toxic if their concentrations exceed certain thresholds [3]. Changes in redox conditions or anthropogenic inputs influence their distribution. Such changes will be potentially recorded by foraminifera. Thus, analyses of foraminifera calcite trace metal incorporation potentially allow a more realistic monitoring of marine pollution. |
| Â |
| Very little work has been carried out on the behaviour of Zr in the marine environment [4], although, in principle, it should provide important information on geochemical cycling. Zr concentration in ocean water is very low (2-30 ppt; Godfrey et al. [5] and the obvious sources for Zr in the ocean water are Zr-bearing minerals either derived from continental sources through sediments or from the basaltic ocean-floor rocks. Since seawater is very rich in halogens which can be available in the form of halides (F, Cl, Br), the halogens play an important role in the mobilization of zirconium into seawater or sediment pore water [6]. |
| Â |
| Ocean Acidification |
| Â |
| Global mean surface ocean pH (8.2) has decreased by about 0.1 units since the end of 18th century and, according to model projections, pH will decrease by another 0.3 unit by the end of the present century [7].There is great concern about the potential impact of the consequences of ocean acidification on high-latitude organisms and ecosystems. Models predict that the surface waters of the polar oceans will be the first to become under-saturated with respect to aragonite in 2050 in the Southern Ocean McNeil et al. [8] and as early as 2016 in the Arctic Ocean [9]. Shallow water systems such as Kongsfjorden are particularly interesting, because they are affected by environmental changes first and act as small-scale laboratories [10]. Foraminifera have been reported to build under calcified shells in acidifying conditions [11]. Comeau et al. [12] have seen impact of ocean acidification on a key Arctic pelagic mollusc Limacina helicina. We tried to investigate if there are any impacts of acidification on benthic foraminifera within Kongsfjorden. |
| Â |
| Study Area |
| Â |
| In Polar Regions, natural sources like weathering and erosion processes are expected to be more dominant sources of trace metals as compared to the anthropogenic ones. The fjords on the west coast of Spitsbergen balance Atlantic, Arctic, brine and freshwater inputs which are potentially sensitive indicators of environmental changes [13]. An example of a fjord influenced by a warm current is Kongsfjorden. In Kongsfjorden there is an active tidal glacier complex at the head of the fjord that generates steep environmental gradients in salinity, temperature, sedimentation rates and bottom sediment composition [14]. The catchment area has been estimated as 1260 km2, and approximately 80% of it is reported to be glaciated [15]. Kongsvegen and Kronebreen are tidewater glaciers in the inner fjord region of Kongsfjorden, and together drain an area of 1013 km2 within the Holtedahlfonna ice field [16]. In summer period, Kongsfjorden is strongly influenced by the freshwater run-off from these melting glaciers. Kongsfjorden system is particularly suitable for long term study of effects of climate change on ecosystem as it lies adjacent to both Arctic and Atlantic water masses [17]. The glacial input of fresh-water and sediments create steep environmental gradients in the fjord system which induces large changes in community composition and abundance from inner to outer fjord especially for benthos [18]. For paleoenvironmental reconstructions, planktic and benthic foraminifera have been earlier used from western and northern shelf of Svalbard [19]. |
| Â |
| Materials and Methods |
| Â |
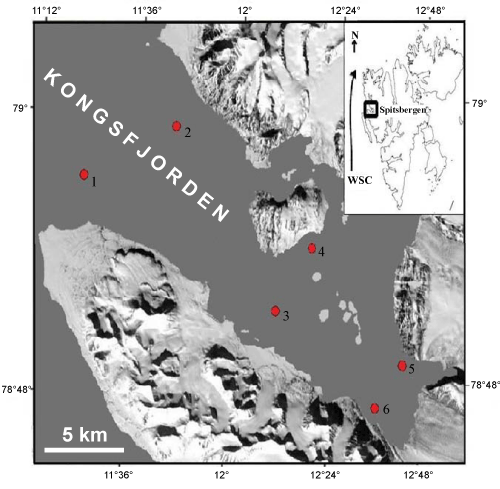
| As part of Indian Arctic Programme, surface sediment samples were collected at six stations using grab during August 2008 using a Teisten boat in the Kongsfjorden fjord with depths ranging from 40 m and 294 m (Figure 1) by one of the participants (SKS). Approximately 20 gm of sediment samples were oven dried at 60°C and weighed. These dried sediment samples were used for grain size and organic carbon analysis. Textural analysis was carried out following standard techniques [20]. Total organic carbon (TOC) content was determined on decarbonated samples by TOC Analyser (Shimadzu TOC-V series SSM-5000A) using High temperature catalytic oxidation (HTCO) by platinum catalyst, with a precision of less than 5%. Glucose standards were used for calibration. One aliquot of sample was soaked for 24 hours in hydrogen peroxide and sodium hexa-metaphosphate. It was sieved with a 63 μm sieve and the coarse fraction was recovered and dried. Aliquots of samples were obtained using a dry microsplitter and counted under a stereozoom binocular microscope. Around 300 tests were hand-picked and mounted onto faunal chambered slides and identified. Planktic foraminifera were rare and the numbers available were too low for a meaningful quantitative analysis. As benthic foraminifera were abundant, these were separated, for species level identification. For microscopic imaging and qualitative elemental analyses, Nonionellina labradorica, Elphidium excavatum, Cassidulina reniforme and Quinqueloculina stalkeri were placed on an aluminium stub, and then coated with a 3 nm platinum coating and analyzed using a Scanning Electron Microscope (JEOL 6360LV SEM) equipped with Oxford Instruments Energy Dispersive Spectrometry (EDS) and INCA software. Images were collected at 3 kV and a working distance of 4 mm, whilefor EDS analysis a working distance of 8 mm and an accelerating voltage to 15 kV were used. |
| Â |
|
|
Figure 1: Map of Kongsfjorden with sediment sampling stations (modified after Svendsen et al., 2002). |
|
| Â |
| For Zr determination in sediment, powdered sediments were weighed accurately to ~30 mg, transferred to clean Teflon beakers and open acid digestion was carried out. The sediments were repeatedly digested by treating with a mixture of HCl, HNO3, HClO4 and HF under controlled conditions. Finally, 2% of HNO3 was added to the digested samples and the entire solution was brought to a standard volume. Analysis was carried out using ICP-MS (X-series with CCT) in NCAOR using Standard international standards (USGS Inorganic in Marine sediment). Triplicate analyses of randomly selected samples were performed to determine the precision of the analysis. The precision of measurements was about ±5%. |
| Â |
| We planned pH manipulation lab experiments and used N. labradorica, from outer fjord stn # 1 and inner fjord stn #4. Three different pH ranges of 3 (highly acidic), 6 (weakly acidic) and pH 7.8 (moderately alkaline and the predicted pH during 2100) were maintained. Seawater was maintained at the average temperature (4ºC) and salinity (32 psu). After 48 hrs the benthic foraminifera were picked and mounted on stub and analysed over SEM-EDS to see the plausible dissolution effects. |
| Â |
| Results and Discussion |
| Â |
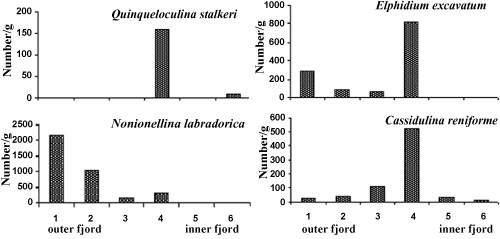
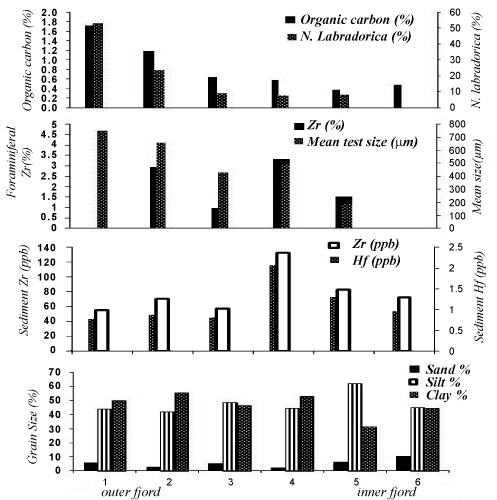
| N. labradorica is the dominant epibenthic foraminifera in Kongsfjorden and its percentage decreases from outer towards the inner fjord as it is known to be associated with Atlantic waters [21] and abundant organic matter [22]. N. labradorica was not encountered at inner fjord station 6. In the inner fjord the abundance of glaciomarine species such as Cassidulina reniforme, Elphidium excavatum and Quinquiloculina stalkeri increases (Figure 2). |
| Â |
|
|
Figure 2: Spatial distribution of benthic foraminiferal species in Kongsfjorden. |
|
| Â |
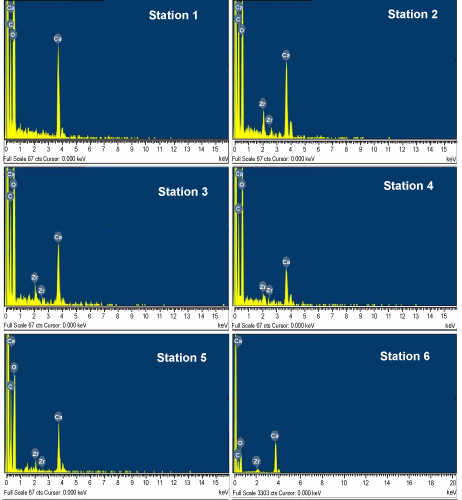
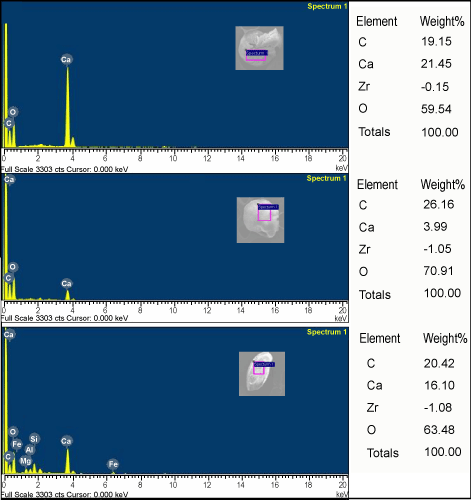
| We found Zr on the test surface of N. labradorica (max 3.2%) whereas no Zr was encounterd on Cassidulina reniforme, Elphidium excavatum and Quinquiloculina stalkeri (Figures 3 and 4). The station on the northern side of the fjord (2, 4 and 5) had higher Zr as compared to the southern stations (1, 3 and 6). Maximum Zr concentrations (3.2%) were found at Station #4 (Figure 3). This indicates that the source of Zr could be on the northern side of the fjord. |
| Â |
|
|
Figure 3: EDS analysis of benthic foraminifera N. labradorica from outer to inner fjord stations. |
|
| Â |
|
|
Figure 4: EDS analysis of benthic foraminifera Elphidium excavatum, Cassidulina reniforme and Quinqueloculina stalkeri from station 4. |
|
| Â |
| Sediment Transport and Organic Carbon |
| |
| Â |
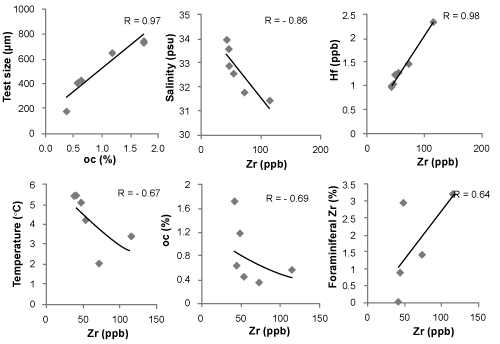
| Sediment grain size showed that the fjord is constituted of fine grained sediment. Coarser grained sediment fraction increases towards the mouth of the glaciers. The fjord sediment constituted of fined grained sediment (silt and clay). Coarser grained fraction (sand) increased from outer fjord towards inner fjord. Sedimentary organic carbon varied from 0.3-1.7%. Benthic foraminifera living at water depths greater than a few tens of meters are entirely dependent on the imported organic material supply for their energy requirements. This is reflected by the distribution pattern of N. labradorica as it is a potential indicator of highly productive conditions and it feeds on the buried organic matter or free phytodetritus [22]. The importance of phytodetritus as a food source for deep-sea foraminifera was noted by Gooday et al. [23], who found abundant foraminiferal populations in fresh algal aggregates. Organic matter also plays important role in preventing removal of trace metals like Zr from the seawater as this metal has high affinity towards organic colloids and macromolecules. Inner fjord stations having low organic carbon (0.3 %) thus could not adsorb significant Zr and hold it in the surface waters (Figure 5). Thus allowing the Zr to settle down and reach the surfacial sediment where the benthic foraminifera inhabit and could accumulate Zr on its surfacial layers. In the inner fjord sand % is high (10.1%), especially at Stn.#6 (Figure 5), which is very close to the Kongsvegen glacier the fresh water run-off from this glacier increases the turbidity and hence diminishes the vertical extent of the euphotic zone [24]. In Kongsfjorden the depth of the euphotic zone decreases from 30 m at the outer fjord to 0.3 m in the turbid inner fjord waters close to the glacier fronts [25]. Availability of food supply will have impact on the test size of foraminifera. The mean size measurements of N. labradorica varied from 733 μm at the outer fjord sample (Stn. #1) to 210 μm at the inner fjord station (Stn. #5) and shows direct relationship with organic carbon (r =0.97) (Figure 6). |
| Â |
|
|
Figure 5: Variation in trace metals, N. labradorica, organic carbon and grain size from outer fjord to inner fjord. |
|
| Â |
|
|
Figure 6: Correlation between Zr and various environmental parameters. |
|
| Â |
| Sources of Zirconium in Kongsfjorden |
| Â |
| Zirconium concentration in Kongsfjorden sediment varied from 41-114 ppb. Interestingly, Zirconium in sediment and on N. labradorica showed similar spatial variation within Kongsfjorden (Figure 6). Zirconium is the twentieth most abundant element in the earth’s crust, with an average concentration of 16.5 ppm [26]. Weathering and erosion of the continents is the major source of most metals in seawater and is the expected major source of seawater Zr [27]. The resistance of zircon, to physical and chemical weathering and adsorbtion onto particle surfaces results in very low levels of Zr in sea water [28,29]. The strong negative correlation between Zr and Salinity, temperature, organic carbon and depth (R= -0.86, -0.67, -0.69 and -0.52 respectively) indicate that the source of Zr is through weathering of continental zircon (Figure 6). We did not encounter Zr over benthic foraminiferal shells from outer fjord station #1 which also had the highest organic carbon percentage (1.7%). The association between Zr and DOC was previously found by Pokrovsky OS et al. [30]. Tosiani et al. [31] suggests that organic complexation sustains dissolved Zr concentrations through the zone of colloid flocculation. The industrial use of Zr suggests they could have a pollutant source to the environment Gobeil et al. [32] Godfrey et al. [29] but in Kongsfjorden, anthropogenic input is minimal. |
| Â |
| Zr and Hafnium Association |
| Â |
| Zr and Hf were closely associated in the fjord (Figure 5) and showed strong positive correlation (R=0.98). Hafnium concentrations were very low, ranging from 0.9-2.3 ppb, and we could not find any Hf over foraminiferal shells. The average Zr/Hf ratio was 43 in Kongsfjorden. The Zr/Hf ratio was higher in inner fjord stations 45.3, as compared to outer fjord ratio of 40. The Zr/Hf ratio in river water is 28, whereas in coastal seawater the ratio raises upto 32 and decreases upto 5 in open ocean waters [33]. Godfrey et al 2008 reported Zr/Hf ratio of 78 in surface estuarine waters, whereas ratio decreases up to 68 at deeper depths. Because of the lanthanide contraction, Zr and Hf chemistries are remarkably similar; and are intimately associated in geologic materials [29]; the two are almost in-variably found in close association in rock-terming minerals and one would expect this association to continue throughout the weathering and transport processes involved in the sedimentary cycle [29]. |
| Â |
| Acidification |
| Â |
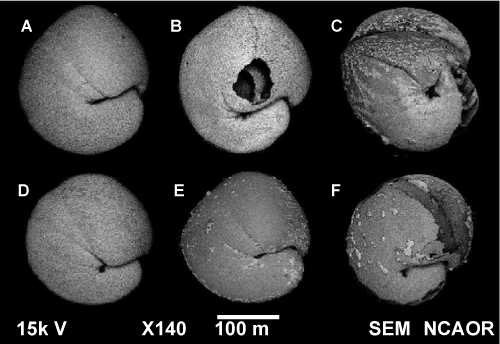
| Here we report some interesting results which could protect foraminifer’s dissolution despite having undercalcified shells. Our SEM-EDS results indicate Zr coating on the surface of the benthic foraminifer’s shells from Kongsfjorden (Figure 3). Zr coating has been used to protect metals from acid corrosion in industries and can tolerate even hot HCl and intermediate strength H2SO4 [34]. Surprisingly we found that foraminifera from Stn #4 overlaid with Zr coating were resistant towards dissolution even at moderately acidic pH 6, while foraminifera from Stn #1 having no Zr showed dissolution (Figure 7). |
| Â |
|
|
Figure 7: Results of lab experiment. A-C SEM images of N. labradorica specimens from station 1 which is devoid of Zr. D-F are SEM images of N. labradorica specimens from station 4 which has highest Zr in N. labradorica. A & D represent pH 7.8 (Predicted pH by 2100), B & E represents pH 6 (moderately acidic), C & F represents specimens in pH 3 (highly acidic conditions). |
|
| Â |
| This Zr coating that can protect benthic foraminifera at pH 6 would certainly prevent dissolution of the undercalcified benthic foraminifer’s shells despite the 0.3 units decrease in pH by the end of the present century (Caldeira and Wickett, 2003) [7]. At pH 7.8 benthic foraminifera from neither station showed any dissolution, whereas at pH 3 both the samples showed dissolution (Figure 7). Now whether this zirconium coating over N. labradorica is an adaptation towards climate change or how the Zr ended up being onto the test of N. labradorica needs to be answered and requires long term monitoring of Kongsfjorden with respect to bioaccumulation studies. |
| Â |
| Conclusions |
| Â |
| N. labradorica was the dominant foraminifera in the outer fjord, C. reniformis and Q. Stalkeri dominated the inner fjord glaciomarine environment. Benthic foraminifera showed no signs of dissolution despite Arctic Ocean being one of the first sites to show impacts of climate change. N. labradorica tests surface indicated presence of Zr, whereas other foraminiferal species like E. excavatum, C. reniforme and Q. stalkeri were devoid of any Zr traces. Both foraminiferal analysis as well as sediment analysis indicated highest Zr percentage at the inner fjord station 4. Concentration of Zr showed opposite trend to salinity, temperature and organic carbon indicating that the source of Zr is through weathering of continental zircon. Zr showed close association with Hafnium (Hf) and the average Zr/Hf ratio within Kongsfjorden was 43. |
| Â |
| Our lab experiment suggests that the inner fjord N. labradorica won’t get affected by the 0.3 units decrease in pH by the end of this century. A long term monitoring of Kongsfjorden is required to explain the exact processes involved in accumulation of Zirconium over N. labradorica and to reveal whether the Zr coating is responsible for N. labradorica being the dominant foraminifera in Kongsfjorden. |
| Â |
| Acknowledgements |
| Â |
| Authors would like to thank Secretary, Ministry of Earth Sciences for funding the project. Special thanks to Kings Bay, Arctic Research Base, Ny-Alesund and Captain, Teisten Boat for all their help and support in data collection. Thanks are to Dr. Thamban Meloth for providing the ICP-MS facility of Ice Core Lab., Mr. Prashant Redkar for running the samples for trace metals and Mr. Mahalinganathan for carrying out TOC analysis. This is NCAOR contribution No XXXX. |
| Â |
| |
| References |
| Â |
- German CR, von Damm KL (2004) Hydrothermal processes, in: The Oceans and Marine 30 Geochemistry, edited by: Elderfield H, Treatise on Geochemistry, volume 6, edited by: Holland HD,Turekian KK, Elsevier, Amsterdam, Heidelberg: 181-222.
- Morford JL, Emerson S (1999) The geochemistry of redox sensitive trace metals in sediments. Geochim et Cosmo Acta 63: 1735-1750.
- Richardson BJ (1995) The problem of chlorinated compounds in Australia's marine environment. In: Zann LP, Sutton DC, Editors, The State of the Marine Environment Report for Australia, Technical Annex: 2 Pollutants, Great Barrier Reef Marine Park Authority, Townsville 1995: 53-61.
- Bruland K (1983) Trace elements in sea-water. In: Riley JP, Chester R (Editors), Chemical Oceanography, Academic Press, London 8: 157-220.
- Godfrey LV, White WM, Salters VJM (1996) Dissolved zirconium and hafnium distributions across a shelf break in the northeastern Atlantic Ocean. Geochimica Cosmochima Acta60: 3995-4006.
- Nayak B, Das SK, Bhattacharyya KK (2011) Detrital and authigenic(?) baddeleyite (ZrO2) in ferromanganese nodules of Central Indian Ocean Basin Bibhuranjan Nayak, Swapan Kumar Das, Kalyan Kumar Bhattacharyya. Geosc Front 2: 571-576.
- Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425: 365.
- Mc Neil BI, Matear RJ (2007) Climate change feedbacks on future oceanic acidification. Tellus B 59: 191-198.
- Steinacher M, Joos F, Fr¨olicher TL, Plattner GK, Doney SC (2009) Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences 6: 515-533.
- Arntz WE, Gutt J, Klages M (1997) Antarctic marine biodiversity: an overview. In: B Battaglia, J Valencia, D Walton (eds) Antarctic communities. Proc 6th SCAR Biology Symposium, Venice 1994, Cambridge Univ Pr, Cambridge: 3-14.
- Moel1 H De, Ganssen1 GM, Peeters FJC, Jung SJA, Brummer GJA, et al. (2009) Planktic foraminiferal shell thinning in the Arabian Sea due to anthropogenic ocean acidification? Biogeosciences 6: 1811-1835.
- Comeau S, Gorsky G, Jeffree R, Teyssi JL, Gattuso JP (2009) Key Artic pelagic mollusc (Limacina helicina) threatened by ocean acidification. Biogeos Disc 6: 1-14.
- Nilsen F, Cottier F, Skogseth R, Mattsson S (2008) Fjord-shelf exchanges controlled by ice and brine production: The interannual variation of Atlantic Water in Isfjorden, Svalbard. Cont Shelf Res 28: 1838-1853.
- Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, et al. (2002) The physical environment of Kongsfjorden- Krossfjorden, an Arctic fjord system in Svalbard. Pol Res 21: 133-166.
- Ito H, Kudho S (1997) Characteristics of water in Kongsfjorden, Svalbard. Polar Meterology Glaciology, Tokyo: National Institute of Polar Research. Proceedings of the NIPR Symposium 11: 211-232.
- Bennett EM, Reed TL, Houser JN, Gabriel JR, Carpenter SR (1999) A phosphorus budget for the Lake Mendota watershed. Ecosystems 1: 69-75.
- Hop H, Falk-Petersen S, Svendsen H, Kwasniewski S, Pavlov V, et al. (2006) Physical and biological characteristics of the pelagic system across Fram Strait to Kongsfjorden. Prog Oceanogr 71: 182-231.
- Holte B, Gulliksen B (1998) Common macrofaunal dominant species in the sediments of some north Norwegian and Svalbard glacial fjords. Pol Biol19: 375-382.
- Slubowska-Woldengen M, Rasmussen TL, Koc N, Kristensen DK, Nilsen F, et al. (2007) Advection of Atlantic Water to the western and northern Svalbard shelf since 17,500 cal yr BP. Quat Sci Rev 26: 463-478.
- Folk RL (1968) Petrology of Sedimentry rocks:Austin, Texas, Hemphills 170.
- Hald M, Korsun S (1997) Distribution of modern benthic foraminifera from fjords of Svalbard, European Arctic. J Foram Res 27: 101-122.
- Corliss BH (1991) Morphology and Microhabital Preferences of Benthic Foraminifera from the Northwest Atlantic Ocean. Mar Micropaleontol 17: 195-236.
- Gooday AJ (1988) A response of benthic foraminifera to the deposition of phytodetritus in the deep sea. Nature 332: 70-73.
- Keck A, Wiktor J, Hapter R, Nilsen R (1999) Phytoplankton assemblages related to physical gradients in an Arctic glacier fed fjord in summer. ICES J Mar Sci 56: 203-214.
- Hop H, Pearson T, Hegseth EN, Kovacs KM, Wiencke C, et al. (2002) The marine ecosystem of Kongsfjorden, Svalbard. Pol Res 21: 167-208.
- Taylor SR (1964) Abundance of chemical elements in the continental crust: A new table. Geochim Cosmo Acta 28: 1273- 1285.
- Pettke T, Lee DC, Halliday AN, Rea DK (2002) Radiogenic Hf isotopic compositions of continental eolian dust from Asia, its variability and its implications for seawater Hf. Earth Plan Sci Lett 202: 453-464.
- Milnes AR, FitzPatrick RW (1989) Titanium and zirconium minerals. In: J.B. Dixon and S.B. Weed, Editors, Minerals in Soils Environments SSSA Book Series, No. I1131-1205.
- Godfrey LV, Field MP, Sherrell RM (2008) Estuarine distributions of Zr, Hf, and Ag in the Hudson River and the implications for their continental and anthropogenic sources to seawater. Geochem Geophys Geosyst 9: Q12007.
- Pokrovsky OS, Schott J (2002) Iron colloids/organic matter associated transport of major and trace elements in small boreal rivers and their estuaries (NW Russia). Chem Geol 190: 141-179.
- Tosiani T, Loubet M, Viers J, Valladon M, Tapia J, et al. (2004) Major and trace elements in river-borne materials from the Cuyuni basin (southern Venezuela): evidence for organo-colloidal control on the dissolved load and element redistribution between the suspended and dissolved. Chem Geol211: 305-334.
- Gobeil C, Rondeau B, Beaudin L (2005) Contribution of municipal effluents to metal fluxes in the St. Lawrence River. Environ Sci Technol 39: 456-464.
- Boswell SM, Elderfield H (1988) The Determination of Zirconium and Hafnium in Natural Waters by Isotope Dilution Mass Spectrometry. Mar Chem 25: 197-209.
- Atik M, Kha CR, Lima Neto P, Avaca LA, Aegerter MA, et al. (1995) Protection of 316L stainless steel by zirconia sol-gel coatings in 15% H2S04 solutions. J Mat Sci L 14: 178-181.
|
| Â |
| Â |