| Research Article |
Open Access |
|
| Tamane Wagaw and Chavan RB* |
| Institute of technology for textile, garment and fashion design, University of Bahir Dar, Bahir Dar, Ethiopia |
| *Corresponding author: |
Chavan RB
Institute of technology for textile
garment and fashion design
University of Bahir Dar, Bahir Dar, Ethiopia
Tel: 25182266246
Fax: 25182266246
E-mail: rbchavan@hotmail.com |
|
| Â |
| Received December 30, 2011; Published October 30, 2012 |
| Â |
| Citation: Wagaw T, Chavan RB (2012) Optimization of Caustic Soda Concentration for Causticization of Cotton. 1:448. doi:10.4172/scientificreports.448 |
| Â |
| Copyright: © 2012 Wagaw T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| The scientific basis of the mercerization of cotton with caustic soda is well documented in the literature. There is always a brief mention of the process of causticization for improvement in color yield in the chapter on mercerization in any standard book on chemical processing of cotton. In the literature the concentration of caustic soda recommended for causticization varies from 11-19% (w/v). Obviously there must be scientific basis for such recommendations. However, no experimental details are available in the literature for the choice of caustic soda concentration to be used for cauaticization of cotton. In the present investigation an attempt has been made to provide this missing link. For the authenticity of the results the experiments were designed on the basis of single factor completely randomized design using Design Expert Software. The experimental evidences indicated that optimum benefits of causticization for improved color yield can be obtained at 14-16% caustic soda concentration which is in agreement with the literature values. Wet-on-wet dyeing gave better color yield compared to dyeing after drying. In view of this it is suggested to carry out causticization on padding mangle followed by washing and dyeing on jigger. The option of wet on wet dyeing can be used for exhaust dyeing whereas for pad dyeing this option will not be suitable because of exchange of water from wet fabric resulting in dilution of pad liquor. Since the causticization is carried out without tension, fabric shrinkage is unavoidable. |
| Â |
| Keywords |
| Â |
| Causticization; Caustic soda Concentration; Wet-on-wet dyeing; Dyeing after drying; Color yield; Average shrinkage |
| Â |
| Introduction |
| Â |
| The mercerization consists in treatment of cotton with concentrated solutions of caustic soda (20-30%) at a temperature of 15 to 18°C. Cellulose undergoes chemical, physico-chemical and structural modifications on treatment with caustic soda solution of mercerizing strength. As a result of this there is improvement in various physical and chemical properties of cotton such as Swelling and shrinkage of cellulose [1-9]. Structural modification [10-15] increased luster [2,16-18] gain in strength [19-26] increased moisture absorption, dye absorption and reactivity, removal of immature cotton [7,27-34]. When cotton is immersed in a solution of caustic soda, swelling of cotton takes place due to diffusion of water and alkali. The degree of swelling depends on the concentration of caustic soda. At low concentration NaOH molecules are hydrated by large number of water molecules and the diameter of the hydrated ions is too large to penetrate into the macromolecular structure of cotton. As the concentration of caustic soda increases, the number of water molecules available for the formation of hydrates decreases and thus hydrated ion pairs are formed which are capable of penetrating into the fiber structure of cotton by breaking hydrogen bonds and weak van der Waal forces between cellulose chains resulting in fiber swelling and the corresponding fiber shrinkage. Swelling and shrinkage are more when there is no tension in the fiber [1,32,33]. Maximum swelling concentrations of different alkalis depend on the degree of hydration of the alkali ion In case of sodium hydroxide it is 18% [4,7]. This is the theoretical concentration of caustic soda for mercerization although practically 20-30% concentration of caustic soda is recommended for mercerization. |
| Â |
| Thus when cotton is treated with caustic soda solution of mercerization strength, the expanded, freed chains rearrange and reorient and when the caustic soda is removed, the chains form new bonds in the reorganized state. Because of the distortion of polymer network and changes in crystalline structure, the process of mercerization is irreversible [10-13,30]. |
| Â |
| It is also well known that mercerization decreases the amount of crystalline part or increases the amorphous content of the fiber and it is estimated that the number of available hydroxyl groups are increased by about 25%. This increase in the proportion of amorphous part and increased availability of hydroxyl groups is directly related to the increase moisture sorption, reactivity and dye absorption of mercerized cotton [7,32-34]. The same explanation is valid for the effect of causticization for improvement in dye uptake. However, the extent of improvement in dye uptake is less due to lower concentration compared to caustic soda concentration used for mercerization. |
| Â |
| It is essential to point out another important phenomenon related to the process of mercerization. At high concentration of caustic soda, swelling of the outermost fibers forms a barrier, inhibiting the penetration of liquor into the yarn core. In case of fabrics, swelling is further restricted by the yarn crossing points. In addition at high concentration the viscosity of caustic soda solution is also high. Both these factors result in poor mercerizing of the core and lack of uniformity as the reaction is restricted mainly to the surface of the yarn or fabric. This was the basis for the development of the process of hot mercerization [31]. |
| Â |
| From the above limited literature citation it is clear that the process of mercerization is very well documented scientifically. On the contrary the information on causticization is scanty. There are wide variations in recommended caustic soda concentration for causticization as seen from the following literature values. |
| Â |
| 30-35°Tw (16-18.5% w/v), [32] |
| Â |
| 25-30°Tw (13-16% w/v), [33] |
| Â |
| 23-36°Tw (11-19% w/v), [34] |
| Â |
| The main focus of the present paper is limited to systematically establish the most appropriate choice of caustic soda concentration for causticization for increased dye uptake, experimental details of which are missing in the literature. |
| Â |
| Materials |
| Â |
| Commercially bleached cotton fabric supplied by Bahir Dar Textile share company, Ethiopia was used. The fabric had following specifications |
| Â |
| 63 Ends/in, 43 picks/in, 134 GSM |
| Â |
| Commercial caustic soda in flake form |
| Â |
| Tubantin Blue FF2GL 200 (direct dye) supplied by Bezema, Switzerland. |
| Â |
| Experimental methods |
| Â |
| Treatment with caustic soda solution: The strength of the commercial caustic soda flakes was determined by titration with standard sulfuric acid and caustic soda solutions were prepared by converting the commercial caustic soda strength to 100% purity. The caustic soda strength is expressed in % (w/v) meaning g/100 ml. |
| Â |
| Commercially bleached cotton fabric was treated with caustic soda solution of concentrations varying from 5-30% containing 1 g/l wetting agent by two dip two nip padding technique. The % wet pick up was noted in each case. The padded fabric samples were rolled on glass rods, covered with plastic sheet and batched for 30 minutes. The padding and batching were done at room temperature (20-25°C). After the batching period the samples were repeatedly rinsed in cold water until the samples showed neutral pH as indicated by universal indicator test. |
| Â |
| Causticization: The treatment given was similar to that mentioned above except the caustic soda concentration varied from 8-20%. |
| Â |
| Dyeing: The cotton samples treated with varying concentrations of caustic soda including those used for causticization were dyed with a direct dye in wet-on wet (without drying) and after drying. For wet on wet dyeing the samples after final rinsing were squeezed, and for dyeing after drying, the samples were allowed to air dry. The dyeing were carried out in high temperature dyeing machine under following conditions |
| Â |
| Tubantin Blue FF2GL 200 (Direct dye) ------2% (o.w.f) |
| Â |
| Sodium chloride------10g/L |
| Â |
| MLR--------------------1:30 |
| Â |
| Temperature---------80°C |
| Â |
| Â |
| Time-----------------45 minutes. |
| Â |
| The dyeing was commenced at room temperature. The temperature was raised to 80°C and dyeing continued at this temperature for 45 minutes. After dyeing the fabrics were rinsed three times with cold water, squeezed, air dried, pressed with hot iron and conditioned before K/S measurement. |
| Â |
| Shrinkage measurement: An area of 7 cm*7 cm was marked at the center of each sample by stitching with white thread. The changes in the dimensions of the marked area were recorded in terms of warp and weft shrinkages from which average shrinkage was calculated by taking the average of warp and weft shrinkage. |
| Â |
| Determination of color yield (K/S): The color yield or dye-uptake was determined by measurement of the K/S value of the dyed samples on Gretag Macbeth color-eye 3100 spectrophotometer by following the standard procedure. |
| Â |
| Experimental Results: The effect of causticization on improvement of color yield is well known. Measurements of color in dyed fabrics are analyzed in various ways, but for the purpose of comparing color yield changes due to mercerization as well as causticization most workers have used the Kubelkaâ€Munk function K/S where K is an absorption coefficient and S is a scattering coefficient [35]. The ratio K/S increases with increasing depth of shade. The measurement of K/S gives the comparative color yield or dye uptake and not the absolute quantity of dye present on dyed sample. |
| Â |
| The caustic soda concentration for causticization as reported in the literature [32-34] varies from 11-19% (W/V). Obviously there must be scientific basis, but no experimental details are available in recommending this wide concentration range. The main objective of present work is to establish experimentally the most appropriate concentration of caustic soda to be used for causticization. |
| Â |
| Effect of caustic soda concentration on color yield |
| Â |
| Wet-on-wet dyeing: Commercially bleached cotton fabric samples were treated with varying concentrations of caustic soda (5-30% w/v) and then wet-on-wet (without drying) dyed with the direct dye as explained in the experimental section. For the authenticity of the results the experiments were designed on the basis of single factor completely randomized design using Design Expert Software. The sample without treatment with caustic soda (0% caustic soda) was used as control. The % increase in K/S values indicated the increase in color yield on samples treated with varying concentrations of caustic soda compared to control (0% caustic soda). Initial trials were carried out with five replications which were then reduced to three replications after observing good consistency of the results. The completely randomized experimental design data for 35 experiments is given in table 1. |
| Â |
|
|
Table 1: Effect of caustic soda concentration on color yield for wet-on-wet dyeing. |
|
| Â |
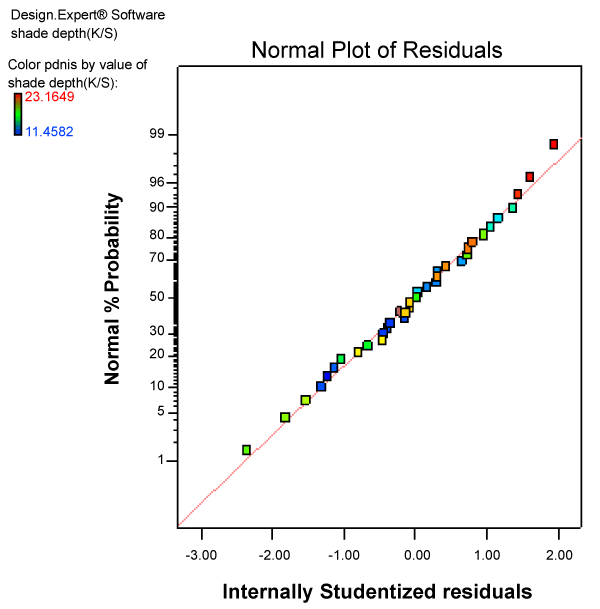
| The consistency of data for K/S was confirmed from normal probability plot of residuals, plotted by design expert software. As shown in figure 1, the points are fairly close to a straight line indicating the validity the experimental data. Residuals mean difference in the observed value (experimental value) and the predicted value or fitted value (by the software). In order to check that the residuals are normally distributed (in other words to check the validity of experimental data), the mostly used technique is to plot normal probability plot of residuals. If the residuals fall approximately along a straight line, the residuals are considered to be normally distributed. In contrast, if the residuals do not fall fairly close to a straight line, the residuals are not normally distributed and hence the data do not come from a normal population i.e. the data is not valid. |
| Â |
|
|
Figure 1: Normal probability plot of residuals for K/S values. |
|
| Â |
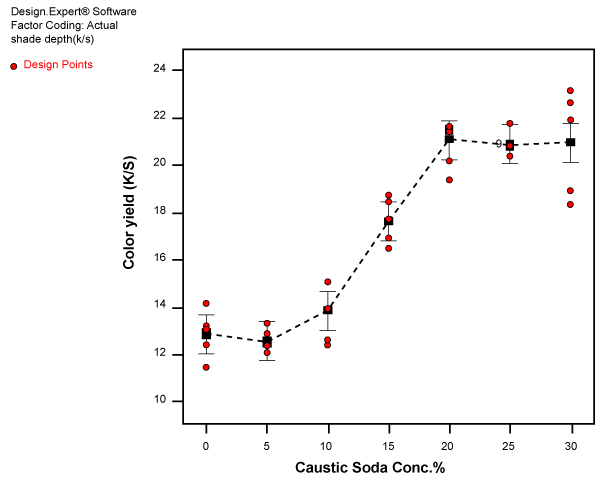
| The results of effect of caustic soda concentration on color yield for wet-on-wet dyeing measured as K/S for five replications are shown in figure 2. The small circular points indicate the individual values where as the square points indicate the averages of the five individual points at that particular concentration of caustic soda. The broken line is drawn in such a manner that it connects the average points at each concentration of caustic soda used for treatment. |
| Â |
|
|
Figure 2: Effect of caustic soda concentration on color yield (K/S) for wet-on-wet dyeing. |
|
| |
| Â |
| It can be seen that the effect of caustic soda concentration on dye uptake can be divided into four distinct phases. |
| Â |
| Phase 1: from 0-5% marginal decrease in color yield. |
| Â |
| Phase 2: 5-10% slow increase in color yield. |
| Â |
| Phase 3: 10-20% rapid increase in color yield. |
| Â |
| Phase 4: 20-30% leveling off color yield. |
| Â |
| The results can be interpreted on the basis of hydration of sodium hydroxide at different concentrations and its effect on swelling. There is some evidence that the degree of hydration of alkali hydroxide ions affects their ability to enter and swell cellulose fibers. At low concentrations of sodium hydroxide, the diameters of the hydrated ions are too large for easy penetration into the fibers. As the concentration of caustic soda increases, the number of water molecules available for the formation of hydrates decreases and therefore their size decreases. Small hydrates can diffuse into the high order, or crystalline regions, as well as into the pores and low-order regions of cellulose [1,32,33]. |
| Â |
| The results of the effect of caustic soda concentrations on color yield can be interpreted on the basis of the above facts. Up to 5% caustic soda concentration effect on color yield is marginally decreased indicating that there may not be further breaking of hydrogen bonds of already water swollen fiber due to high degree of hydration at low concentration caustic soda. From 5%-10% caustic soda concentration, the fiber swelling tends to increase as indicated by the slow increase in color yield. From 10%-20% caustic soda concentration, the increase in color yield can be related to the high rate of swelling along with possibility of hydrogen bond breaking in the inter crystalline regions. Beyond 20% caustic soda concentration, there may be excessive swelling resulting in the jamming of fiber structure with the result, the increase in dye uptake is leveled off. This is the reason for the development of the concept of hot mercerization [31]. |
| Â |
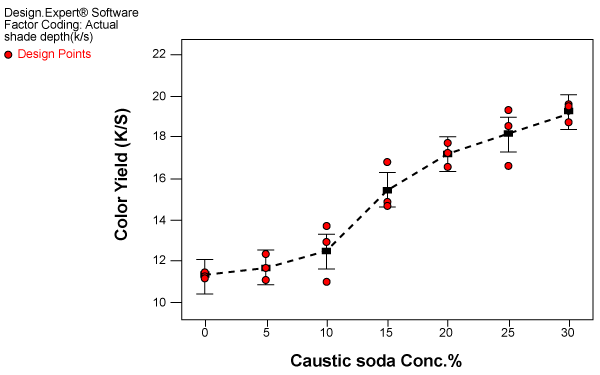
| Dyeing after drying: The experiments were carried out in triplicate and the authenticity of the experimental design data was tested by plotting residuals plot as in case of wet-on wet dyeing. The results of effect of caustic soda concentration on color yield for dyeing after drying are shown in figure 3. |
| Â |
|
|
Figure 3: Effect of caustic soda concentration on color yield for dyeing after drying. |
|
| Â |
| The observations of the effect of caustic soda concentration on dyeing after drying are different compared to wet-on-wet dyeing. The curve can be divided into three phases |
| Â |
| Phase 1: 0-10%, slow increase in color yield. |
| Â |
| Phase 2: 10-20%, rapid increase in color yield. |
| Â |
| Phase 3: 20-30%, slow increase in color yield with no tendency of leveling off. |
| Â |
| In this case also beyond 10% caustic soda concentration there is rapid increase in color yield up to 20% caustic soda concentration. However, unlike in wet on wet dyeing, beyond 20% caustic soda concentration no tendency of leveling off was observed. On the contrary the increase in dye uptake continued to rise. |
| Â |
| Comparison of color yield for wet-on-wet dyeing and dyeing after drying: The comparison of color yield expressed in terms of K/S for wet-on-wet dyeing and dyeing after drying is shown in figure 4. |
| Â |
|
|
Figure 4: Comparison of color yield (K/S) for wet-on-wet dyeing and dyeing after drying. |
|
| Â |
| It will be observed that at all concentrations of caustic soda the color yield for wet-on-wet dyeing was higher than that of dyeing after drying. Maximum difference in dye uptake was observed at 20% caustic soda concentration. |
| Â |
| This may be explained on the basis of difference in swollen state of cotton before drying and after drying. It is well known that due to evaporation of water during drying the hydrogen bonds broken during the wet state are reformed. This results in collapse of porous fiber structure with the formation of strong hydrogen bonds between cellulose molecules [1]. This means that the breaking of hydrogen bonds formed during drying will not be to the same extent when dry cotton is re-wetted. Thus the extent of swelling in case cotton in wet condition (not dried) is more than that of cotton which is dried and re-wetted. This phenomenon probably is responsible for higher color yield during wet-on wet dyeing. |
| Â |
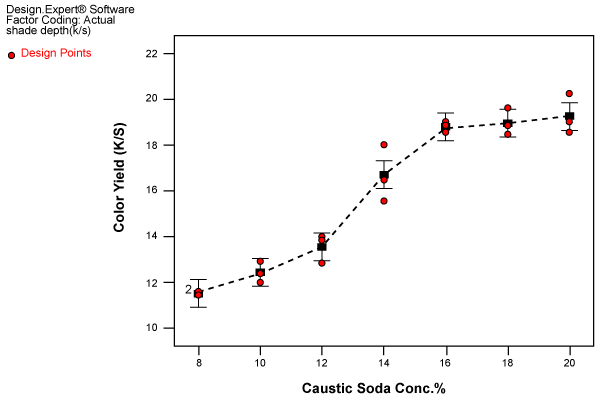
| Choice of caustic soda concentration for causticization: The aim of the present investigation was to find out the optimum concentration of caustic soda for causticization to derive advantage of increase in color yield. Therefore subsequent experiments (triplicate random experiment design) were carried out to study the effect of caustic soda concentration in the narrow range of 8-20% on dye uptake and fabric shrinkage. Wet-on-wet dyeing and dyeing after drying were carried out after each concentration of caustic soda treatment. The results for wet-on-wet dyeing are shown in figure 5. |
| Â |
|
|
Figure 5: Effect of caustic soda concentration on color yield for wet-on-wet dyeing. |
|
| Â |
| The observations on dye uptake were as follow |
| Â |
| Phase 1: 8-12 % caustic soda concentration, slow increase in color yield. |
| Â |
| Phase2: 12-16 % caustic soda concentration, rapid increase in color yield. |
| Â |
| Phase 3: 16-20 % caustic soda concentration, slow increase in color yield. |
| Â |
| In case of dyeing after drying the trend was similar to that of wet-on-wet dyeing except the color yield at each concentration was less as observed earlier. |
| Â |
| Effect of caustic soda concentration of average shrinkage: The experiments were carried out in triplicate and the authenticity of the experimental design data was tested by plotting residuals plot. Since the causticization treatment is carried out without tension, it is necessary to study the effect of caustic soda on fabric shrinkage and suggest an optimum concentration of caustic soda for causticization which will strike the balance between increase in color yield and fabric shrinkage. The effect of caustic soda concentration on % shrinkage of fabric is shown in figure 6. |
| Â |
|
|
Figure 6: Effect of caustic soda concentration on average % shrinkage of cotton fabric. |
|
| Â |
| The results indicated that between 8-12% caustic soda concentration increase in average shrinkage was low, rapid rise in shrinkage observed between 12-16 % caustic soda concentrations. Beyond 16 % caustic soda concentration the increase in shrinkage slowed down. The results of average % increase in dye uptake and average % shrinkage data is summarized in table 2. |
| Â |
|
|
Table 2: Effect of caustic soda concentration on % increase in K/S and shrinkage. |
|
| Â |
| The data shown in table 2 indicated that up to 12% caustic soda concentration the increase in dye up take is low. However there is substantial increase in dye uptake between 14-16% caustic soda concentrations. Therefore, the concentration of 14-16 % can be recommended for the causticization of cotton to get the advantage of increase of in color yield. This recommendation is in agreement with the literature values [33-35]. |
| Â |
| Since causticization is usually carried out without tension shrinkage of the fabric during causticiztion cannot be avoided. |
| Â |
| Effect of causticization on color yield: After establishing 14-16%, caustic soda concentration as the appropriate choice for causticization, the effect of causticization on % increase in color yield using 14% concentration on one direct and two reactive dyes was studied. The dyeing was carried out in triplicate and the average K/S values were recorded. The results for dyeing after drying are shown in table 3. |
| Â |
|
|
Table 3: Effect of causticization on increase in color yield. |
|
| Â |
| The above results clearly indicated that the causticization at 14% concentration of caustic soda resulted in increase in color yield both in case of direct dye and reactive dyes. The increase in color yield was higher for the reactive dyes compared to direct dye. This may be accounted to the difference in molecular weight of dyes. |
| Â |
| Conclusion |
| Â |
| 1. On the basis of single factor completely randomized experimental design data an optimized choice of caustic soda concentration for causticization suggested is 14-16%. |
| Â |
| 2. The wet-on-wet dyeing gave higher color yield compared to dyeing after drying. Since the basic aim of the causticization was to get advantage of improved color yield, the exhaust dyeing operation should be carried out without drying after causticization. For this reason the causticization may carried out on padding mangle followed by washing and dyeing on jigger. This option may not be available for pad dyeing due to exchange of liquor during padding resulting in dilution of pad liquor. |
| Â |
| 3. The increase in color yield after causticization was higher in case of reactive dyes compared to the direct dye. |
| Â |
| 4. Since the causticization is usually carried out without tension, fabric shrinkage is unavoidable. |
| Â |
| |
| References |
| Â |
- Warwicker JO (1966) A Review of the Literature on the Effect of Caustic Soda and Other Swelling Agents on the Fine Structure of Cotton. Cotton, Silk and Man-Made Fibres Research Association. Manchester, UK.
- CowardHF, SpencerL (1923) The Absorption of Caustic Soda Solutions by Cotton.J TestInst 14:14-32.
- Willows ES (1922) Changes in Section of Cotton Hairs on Mercerising. J Textile Inst 13: T237-T240.
- Sadov FI, Korchagin MV, Matetsky AI (1973) Chemical Technology of Fibrous Materials. Mir Pub, Moscow.
- BancroftWD, Calkin JB (1934) The Action of Sodium Hydroxide on Cellulose:Part 1, Section 1: Determination of Sodium Hydroxide taken up from Solution; Change-in-Titer Method; Compound Formation vs. Adsorption. Text Res J 4: 119-140.
- Neale SM (1929) The Absorption of caustic soda by cellulose. J Phys Chem 39: 1245-1246.
- Marsh JT (1951) Mercerizing. Chapman and Hall, London.
- AnsJ,Jager A (1925) Sodium hydroxide and pulp. Cellulosechemie6:137-151.
- Goldwait CF (1965) Shrinkage of Cotton Fibers in Sodium Hydroxide Solutions and Resulting Elastic Properties. Text Res J35:986-992.
- Lal G (1974) Phase Transformations in Cotton Cellulose During Mercerization. Text Res J 44: 313:314.
- OkanoTSarko A (1984) Mercerization of Cellulose I: X-Ray Diffraction Evidence for Intermediate Structures.J Appl Polym Sci29: 4175-4182.
- Okano T,SarkoA (1985) Mercerization of cellulose II: Alkali-cellulose intermediates and a possible mercerization mechanism.J Appl Polym Sci 30: 325–332.
- Nishimura H,SarkoA (1987) Mercerization of cellulose. III. Changes in crystallite sizes. J Appl Polym Sci 33: 855-866.
- Lewin M (1984) Handbook of Fiber Science and Technology Volume 1: Chemical Processing of Fibers and Fabrics-Fundamentals and Preparation. Taylor & Francis, USA.
- Philipp HJ, Nelson ML, Ziifle HM (1947) Crystallinity of Cellulose Fibers as Determined by Acid Hydrolysis. Text Res J 17:585-596.
- Noddler CR (1922) A of Study of Flax and Kindred Fibres. Textile Institute.
- FourtL, Sookne AM (1951) The Improvement of Luster of Cotton:Part I: Measurement of Reflectance Characteristics Related to Luster. Text Res J 21:469-479.
- Fourt L, Elliot HJ (1955) The Improvement of Luster of Cotton Part X: Special Swelling Treatments and Nonthermosetting Additives. Sage: 25: 11-19.
- Rollin SO, Burgis AW, Andrews FR, Grant GN (1959) Physical Properties of Mercerized and Decrystallized Cottons:Part I: Effects of Swelling Solutions on Fibers and Yarns. TextResJ 29:349-355.
- WakehamH,SpicerN(1951) The strength and weakness of cotton fibres. TextResJ21:187-194.
- Wakeham H, Spicer N (1955) The Strength and Weakness of Cotton Fibers Part II: Reversal Distribution and Breaking Properties. Text Res J 25: 585.
- Shelat BR, Radhakrishnan T, Iyer BV (1960) The Relation between Crystallite Orientation and Mechanical Properties of Mercerized Cottons. Text Res J 30:836-842.
- ZeronianSH,AlgerKW,CabradillaKE(1976) Tensile Properties Of Cotton Treated With Alkali Metal Hydroxides.JApplPolymer Sci20:1689-1693.
- Herbert JJ, Muller LL, Schmidt RJ, Rollins ML (1973) The effect of sodium hydroxide on crystallite orientation measurements in cotton fibers. JApplPolymer Sci17:585-587.
- Hearle JWS, Sparrow JT (1979) Mechanics of the extension of cotton fibers. I: Experimental studies of the effect of convolutions. JApplPolymer Sci 24: 1465-1477.
- Kroschwitz JI (1990) Polymers: fibers and textiles : a compendium. Wiley, New York, USA.
- Cheeks L, Wilcock A, Hsu L (1987) Effects of Mercerization, Submercerization, and Liquid Ammonia on Fiber Morphology of Neps in Cotton Fabric. Text Res J 57:690-696.
- Heap SA (1976) The mercerizing process: Fundamental aspects. Colourage 28-34.
- Morton TH (1976) Apparent color yield in dyed textiles. J Soc Dyers Colour 92: 149-157.
- NishimuraH, SarkoA (1987) Mercerization of cellulose IV: Mechanism of mercerization and crystallite sizes.JApplPolym Sci33:867-874.
- De Boer JJ, Borsten H (1962) An Empirical Relation for the Degree of Penetration of a Mercerization Solution into a Cotton Yarn. Journal of the Textile Institute Transactions 53.
- Menachem L (2007) Hand book of fiber chemistry. (3rd edn), Taylor & Francis, London.
- Karmakar SR (1999) Chemical technology in the pre-treatment technology of textile processes. ELSEVIER, Amsterdam, Netherlands.
- Tomassino C (1992) Chemistry and technology of fabric preparation and finishing. Department of textile engineering, chemistry and science college of textiles, North Carolina State University.
- Billmeyer FW, Saltzman M (1981) Principles of color technology. (3rd edn)Wiley,New York.
|
| Â |
| Â |