|
|
| Magnani HN1* and Wester JPJ2 |
| 1Clinical consultant, Oss, The Netherlands |
| 2Department of Intensive Care Medicine, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands |
| *Corresponding author: |
Dr H.N. Magnani, MB, BS, MSc, PhD
Schoutstraat 54, 5345 MT Oss
The Netherlands
Tel: +031 412 639512
E-mail:- harry.magnani@hetnet.nl |
|
| Â |
| Received February 02, 2012; Published October 24, 2012 |
| Â |
| Citation: Magnani HN, Wester JPJ (2012) Is Danaparoid Anticoagulation Suitable for Patients with HIT and ARF Requiring CVVRT? An Analysis of Case Reports. 1:423. doi:10.4172/scientificreports.423 |
| Â |
| Copyright: © 2012 Magnani HN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| Purpose: To assess the efficacy and safety outcomes of case reports of danaparoid anticoagulation in critically ill ICU patients suffering from HIT who require CRRT. |
| Â |
| Method: A retrospective analysis of 103 cases. |
| Â |
| Results: Based on clinical signs, pre-treatment 4T scores and serological testing, HIT was reasonably ‘confirmed’ in 67.0% of the cases and ‘suspected’ in the remainder. The patients, with HIT and ARF received the danaparoid treatment regimen for CRRT for 1–39 (median 7) days. Dose adaptation was necessary in 40.2% of the patients to overcome thrombosis or bleeding (risk). Satisfactory CRRT anticoagulation was provided for 93.6% of 94 cases with information, 53.9% of the patients survived. The hospital mortality of 46.1% was mainly attributed to concomitant morbidity, but 5 deaths were related to thrombosis (n=2) or bleeding (n=3). Maintenance plasma anti-Xa activity correlated poorly with bleeding or thrombotic complications but positively with the minor bleeding frequency, hence advice to restrict the upper limit to 0.8 U/mL or preferably to use evidence of bleeding and systemic thrombosis/ circuit clotting to judge the need for dose adaptation. The benefit (no thrombosis)/harm (bleeding induction) ratio and combined inefficacy and major haemorrhagic frequency were 10.5 and 16.6% respectively, showing a generally favourable response to danaparoid. |
| Â |
| Conclusions: This case report analysis shows that danaparoid, despite its renal elimination, long half-life and lack of antidote, appears to provide efficacious and safe anticoagulation for the majority of critically ill patients with HIT and ARF who are undergoing CRRT. |
| Â |
| Keywords |
| Â |
| Acute renal failure; Continuous renal replacement Therapy; Danaparoid sodium; Heparin-induced thrombocytopenia; Intensive care unit; Major bleeding; Thrombo-embolic complication |
| Â |
| Abbreviations |
| Â |
| AMI: Acute Myocardial Infarction; ARF: Acute Renal Failure; MB: Major Bleeding; CRRT: Continuous Renal Replacement Therapy; CVVRT: Continuous Veno-Venous (Renal) Replacement Therapy; DIC: Disseminated Intravascular Coagulation; ECC: Extracorporeal Circulation; ICU: Intensive Care Unit; LMWH: Low Molecular Weight Heparin; MODS: Multiple Organ Dysfunction Syndrome; PCR: Platelet Count Reduction; TEC: Thrombo-Embolic Complication; UFH: Unfractionated Heparin |
| Â |
| Introduction |
| Â |
| Acute renal failure and heparin-induced thrombocytopenia |
| Â |
| Intensive care unit (ICU) patients who develop acute renal failure (ARF) requiring continuous renal replacement therapy (CRRT) have a high risk of further morbidity and mortality [1]. Up to 50% will develop platelet count reduction (PCR) for various reasons or suffer thromboembolic complications (TECs). In those patients recently or currently receiving unfractionated heparin (UFH) or a low molecular weight heparin (LMWH) these events should trigger suspicion of heparininduced thrombocytopenia (HIT), especially if they occur together, if the PCR worsens whilst continuing heparin or recovers promptly after heparin discontinuation. In this setting clinical confirmation of HIT is provided by: PCR recurrence after heparin re-challenge, multiple TECs especially affecting both venous and arterial circulations, heparin resistance, skin necrosis, white clot syndrome or an acute systemic reaction [2]. Their absence and variations in the timing or extent of the PCR have placed increasing reliance on serological detection of HIT antibodies to heparin:PF-4 complexes to confirm or exclude the diagnosis by functional methods that detect platelet activation – the PAT (platelet aggregation test), HIPA (heparin-induced platelet activation assay) and SRA (serotonin release assay) or the direct antibody assays by ELISA. The commonest of these, the ELISA and PAT, are insufficiently specific and sensitive respectively [3,4], hence a simple pre-test 4T clinical scoring system [5] was developed to assess HIT probability and improve interpretation of serological testing. |
| Â |
| Danaparoid |
| Â |
| Danaparoid (danaparoid sodium/Orgaran®, MSD (also known as Merck & Co. Inc.), Oss, The Netherlands) is a low molecular weight, heparin-free, natural mixture of mainly heparan and dermatan sulphates. It inhibits thrombin generation (TGI) [6-8], with a half-life of elimination (predominantly renal) of 7 hours that is prolonged about 50% in renal failure [9], but its effect on plasma anti-Xa activity is used for monitoring purposes. It is well tolerated for either acute or chronic use after intravenous (I.V) or subcutaneous (S.C.) administration [10]. |
| Â |
| Its advantages are: a favourable benefit (antithrombotic):harm (bleeding) ratio [10], no influence on plasma antithrombin, virtually no effect on platelets [6], a unique ability to disrupt interactions between UFH and platelet factor 4 (PF4) [11,12] or platelet receptors [13] and safe transition to vitamin K antagonists. Potential disadvantages for patients with ARF and HIT [9,14] are: clinical cross-reactivity with the HIT antibody, its long terminal half-life that could lead to accumulation and bleeding and lack of antidote (only plasmapheresis has successfully reduced blood levels and haemorrhage [15]. Nevertheless, it is highly recommended for HIT management [4,16]. |
| Â |
| Aim of the Study |
| Â |
| To assess the efficacy and safety outcomes of danaparoid anticoagulation in critically ill ICU patients suffering from both HIT and ARF requiring CRRT. |
| Â |
| Materials and Methods |
| Â |
| Case report search |
| Â |
| Case reports up to October 2011 of ICU patients with HIT and ARF, managed with CRRT and danaparoid anticoagulation, were indentified in the manufacturer’s in-house files and by Internet search engines (e.g. Medline, Medscape, Ovid, Scopus, Peer review, Embase, Google). Duplicates were screened to extract the most complete information. |
| Â |
| Diagnosis of HIT |
| Â |
| All reported diagnoses of HIT were accepted, but after retrospectively calculating pre-test 4T scores [17] cases were stratified into: |
| Â |
| Confirmed HIT: with a positive functional HIT test and/or clinical confirmation and/or a pre-danaparoid treatment 4T score of 6-8. |
| Â |
| Suspected HIT: all other cases. |
| Â |
| Laboratory data |
| Â |
| Results of routine blood biochemistry and haematology tests, and amidolytic anti-Xa activity were accepted as described in each report. |
| Â |
| Continuous renal replacement therapy |
| Â |
| Cases using veno-venous CRRT procedures for haemofiltration, haemodiafiltration, continuous haemodialysis or unspecified CRRT were included, but cases specifically using arterio-venous circuits were excluded. Circuit survival time after danaparoid anticoagulation was considered satisfactory if ³ 24 hours for at least the latter 50% of the procedures performed or stated to be ‘no problem/no clotting/supported’. |
| Â |
| Danaparoid dosing schedule |
| Â |
| The recommended danaparoid dosing schedule for CRRT was a loading i.v. bolus of 2250 U, followed by 400 U/h x4h, then 300 U/h × 4h and finally a maintenance infusion rate of 150-200 U/h for as long as necessary. However, dose adaptation [10] was possible for patients with initial circuit/systemic clotting problems or increased bleeding risk at least until the problem was overcome. |
| Â |
| Danaparoid treatment outcome events |
| Â |
| Clinical outcomes, reported for up to 32 days after danaparoid discontinuation, were stratified into those with: |
| Â |
| No or minor events: any event (including bleeding, systemic TE or circuit clotting) that responded favourably to transient danaparoid treatment interruption or dose adjustment/optimisation. |
| Â |
| Thrombotic events: systemic thrombo-embolism (fatal and non-fatal) and/or circuit clotting with survival times |
| Â |
| Composite inefficacy outcomes: includes thrombotic outcomes and unplanned amputations. |
| Â |
| Bleeding events: major bleeding - fatal or clinically relevant non-fatal events that led to transfusion of 2 or more units of blood product or re-operation or a serious consequence, e.g. haemorrhagic shock, heart failure etc., or required permanent danaparoid discontinuation. |
| Â |
| Minor bleeding - that did not meet the criterion of a major bleed. |
| Â |
| Composite adverse outcomes: include major bleeds and other fatal and non-fatal serious adverse events (excluding composite inefficacy outcomes) that resulted in permanent discontinuation of danaparoid therapy. |
| Â |
| Treatment was considered effective if the benefit (no thrombosis)/harm (bleeding induction) balance, i.e. (100%-composite inefficacy outcome)/major bleeding frequency; and the composite inefficacy outcome + major bleeding frequency values were >1 and <50% respectively. |
| Â |
| Statistics |
| Â |
| Fisher’s exact test is used for comparison between variables, the level of significance is |
| Â |
| Results |
| Â |
| One hundred and three unique case reports were identified: 47 in the manufacturer’s data base, 55 in 21 independent publications [3,15,18-35] and 1 in a marketing brochure. |
| Â |
| Presenting clinical status |
| Â |
| The male:female ratio was 2:5. The median platelet count nadir, available for 56 cases, was 30.7 Giga/L (range 4-118 Giga/L), with 16.1% Table 1) did not allow calculation of clinical severity scores in sufficient patients for analysis). |
| |
| Â |
|
|
Table 1: Co-morbid problems1 at danaparoid treatment initiation. |
|
| Â |
| HIT diagnosis |
| Â |
| The distribution of the 98 pre-test 4T scores for HIT probability that could be calculated was: low (1-3) 9.2%, intermediate (4-5) 50.0% and high (6-8) 40.8%. Serology for HIT, performed in 89 cases, was positive in 71.9% (43 by functional test ± ELISA, 13 by ELISA only and 8 methods unknown). Overall HIT could be reasonably confirmed in 67.0% (69/103) of the cases, the remainder have been designated suspected HIT. Cases having a functional or direct antibody test (some both) had median 4T scores of 6 and 6.5 respectively for positive test outcomes and 4 for either negative test outcome. |
| Â |
| Danaparoid dosing schedules and duration of use |
| Â |
| In all but 2 patients with information (88/90) danaparoid was infused continuously for a median of 7 days (range 1-39 days), usually by the recommended i.v. regimen. Filter clotting during early sessions was the usual reason for loading doses >2500 U (n=10) and/or infusion rates of 450-1000 U/h (n=10). An increased bleeding risk or an high UFH/LMWH dose immediately prior to the switch to danaparoid was the usual reason for omission (n=12) or reduction (n=8) of the recommended loading dose and/or maintenance infusion rate, but was associated with failure of PCR recovery (p<0.05) and a trend towards more frequent new clotting/TECs that in 6 reports was managed by increasing the danaparoid dosing intensity. Final maintenance infusions were then adjusted to the lowest rate that provided both circuit patency and adequate systemic anticoagulation (which for 17 patients was |
| Â |
| Plasma anti-Xa activity |
| Â |
| Plasma anti-Xa activity levels (available for 57 patients) during the maintenance infusions remained within the target range (0.4-0.8 U/mL) in 63.2% of the cases. Levels consistently above or below this range were reported for 14.0% and 22.8% of the cases respectively. |
| Â |
| Treatment outcomes |
| Â |
| The 102 available clinical outcomes (Table 2) show that 55 patients (53.9%) survived, of whom 85.5% developed no or minor problems. |
| Â |
|
|
Table 2: The frequency of clinical outcomes of danaparoid therapy (more than 1 event possible per patient). |
|
| Â |
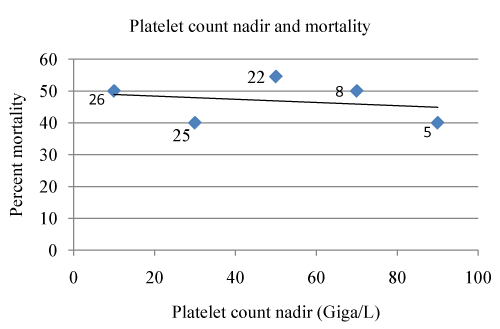
| The 47 deaths (46.1%), including 13 occurring a median 5 (range 3-30) days after danaparoid treatment cessation, were attributed to sepsis/MODS/DIC: 35 cases (77.8%); major bleeding 3 cases; systemic thrombosis 2 cases; cardiopulmonary arrest without thrombosis at autopsy 2 cases; cancer, heart-failure, encephalopathy 1 case each and 2 were unexplained. The mean ages of survivors and those who died were 61.3 and 60.4 years respectively and mortality was slightly higher in females: 51.7% vs. 42.5%. Figures 1 and 2 shows mortality was related to the number of major clinical problems (DIC/MODS, sepsis/septic shock, systemic thrombosis, active bleeding or recent major surgery, and any other severe clinical problems) at presentation (r=0.9721), but not to the heparin-induced platelet count nadir. The mean platelet counts for survivors (n=46) and non-survivors (n=40) were 39.78 and 38.12 Giga/L respectively. |
| Â |
| Eight cases (7.8%) developed an inefficacy end-point: persistent CRRT circuit/filter clotting (n=4) and/or systemic thrombosis (n=5, two fatal) including an unplanned amputation. |
| Â |
| Twelve major bleeding episodes were reported: 4 followed vascular injury (3 lacerated vessels, 1 leaking arterial suture) and were subsequently managed by surgery with perioperative danaparoid; one patient whose supply of danaparoid was used up died 7 days later from a coronary occlusion due to a bleed into an atherosclerotic plaque. In 6 of the 7 remaining episodes danaparoid treatment was discontinued prematurely: one of these patients was switched to lepirudin because of a new DVT and died 3 days later of a pulmonary haemorrhage; one patient in hepatic failure suffered bleeding episodes both before and after danaparoid discontinuation; 3 patients with cancer complicated by fungaemia, DIC and MODS respectively had repeated bleeding at vascular access sites, tracheostomy site and into the urinary tract respectively, that stopped after danaparoid discontinuation; one patient with DIC and hepatic failure had severe bleeding episodes before danaparoid use and subsequently died. The final patient developed tamponade after a re-do cardiac by-pass operation using danaparoid that recovered after aspiration and danaparoid discontinuation but died 8 days later in heart failure. Thus 3 patients died as a direct effect of their major bleeding. Fifty-two cases (51.0%) developed an adverse end-point. |
| Â |
| Fourteen minor bleeds (13.7%) were reported: 4 from tracheotomy sites, 3 post-operative, one each due to gastritis, haemothorax aspiration, herpes zoster lesions, haemorrhoids, urinary bladder catheter, retroperitoneal and unknown. All responded to danaparoid dose reduction and/or transient (up to 24h) treatment interruption (4 episodes). Four, of 13 episodes with information on plasma anti-Xa monitoring, occurred at levels >0.8 U/mL. |
| Â |
| The benefit/harm balance and the sum of the composite inefficacy + major bleeding frequencies are at best (i.e. excluding surgical/procedural errors) 10.5 and 16.6% respectively and at worst (including all major bleeding and persistent thrombotic events) 5.9 and 23.5% respectively. |
| Â |
| During danaparoid administration 32 patients (31.4%) developed new/persistent thrombocytopenia. Twelve of these (including 4 with a diagnosis of sepsis and 1 with an artificial heart) received concomitant platelet transfusions (n=9) or UFH (n=2) or both as possible causes,16 others had DIC, sepsis, or MODS and the remaining 4 cases had no obvious cause. Four of these 32 patients developed new TECs (12.5%) and 21 died (65.6%). Comparative figures for patients whose PCR recovered are 4/70 (5.7%) and 26/70 (37.1%, p <0.0101) respectively. Mortality in patients with persistent/new PCR who received platelet transfusions was 44.4% compared with 75% for those who did not. |
| Â |
| Four patients received concomitant UFH: 3 developed new/persistent PCR and died, (one of widespread thrombosis following 42,000 IU UFH daily up to and including the day treatment was switched to danaparoid just before the patient’s demise). The survivor also received platelet transfusions, as did a further 11 patients because of low platelet counts (nadir |
| Â |
| CRRT outcomes |
| Â |
| The patients underwent 1-23 CRRT sessions (median 3) and circuit survival was satisfactory in 88 of 94 (93.6%) reports with information (Table 2). New/persistent PCR and/or resistant circuit clotting in the remaining 6 cases (in 3 associated with a positive cross-reactivity test (see below) led to premature danaparoid discontinuation and replacement by eprostanol, bivalirudin, argatroban (2) or lepirudin (2). For 9 cases no CRRT outcome data was reported despite danaparoid use for a median of 5 (range 1–27) days. |
| Â |
| Serological danaparoid cross-reactivity and/or premature treatment discontinuation |
| Â |
| Forty-five patients with a positive functional test for HIT were also tested for danaparoid cross-reactivity. Six (13.3%) were positive (Table 3), 5 before and one within 24h of treatment initiation, leading to premature danaparoid discontinuation in 3 cases. Four of the 6 had satisfactory CRRT patency on danaparoid (despite persistent PCR in 1) and 2 showed early circuit clotting (in one accompanied by systemic thromboses). Two patients died of sepsis and 1 with lung cancer developed fatal pulmonary bleeding 3 days after being switched to lepirudin. |
| Â |
|
|
Table 3: Patients with premature danaparoid discontinuation. |
|
| Â |
| Table 3 also includes 7 other premature danaparoid discontinuations after 2-18 (median 5) days of treatment because persistent PCR and/or circuit clotting led to suspected cross-reactivity. Circuit patency was inadequate in 3, satisfactory in 1 and there was no information for 3 cases. Two of these 7 patients died of sepsis (1 after switching to bivalirudin), 2 recovered on argatroban and for 3, information is lacking. |
| Â |
| Discussion |
| Â |
| Despite limitations (lack of a control group; the variable quality of the information supplied) this case report analysis, showing that 93% of the patients underwent successful CRRT and 53% survived beyond the 30 day follow-up period, provides evidence that danaparoid therapy was beneficial in the majority of these critically ill ICU patients with HIT and ARF. The 46.1% hospital mortality rate compares favourably with rates up to 80% reported for similar patients [1,3,36,37], but this still high mortality appears to be directly related to the number of presenting clinical insults (Figure 1) and not related to the platelet count nadir (Figure 2). The lowest rate of 33.3% in figure 1 compared with 19% and 0% for danaparoid-treated ICU patients with renal failure managed with intermittent haemodialysis and who had concomitant HIT or no HIT respectively [38,39] suggests that the combination of HIT and ARF requiring CRRT seriously reduces survival. The difference may also be related to the frequency of sepsis/DIC and MODS that are more likely to be present in patients requiring CRRT than intermittent haemodialysis and was more frequently cited as the cause of death - 88.4% vs. 24.1% [39] respectively (p<0.0001), but it also suggests that to avoid the risk of HIT development critically ill patients who require CRRT should not be given a heparin. |
| Â |
|
|
Figure 1: Mortality rates in relation to the presenting clinical burden. |
|
| Â |
|
|
Figure 2: Mortality rates in relation to the platelet count nadir. |
|
| Â |
| HIT diagnosis in critically ill patients |
| Â |
| Despite a clear difference in median scores between serology positive and negative patients in the current analysis, the severity and/or unusual timing of the thrombocytopenia and/or co-morbidity can undermine the predictive value of the pre-test 4T scoring system in critically ill patients. Coupled with over-diagnosis of HIT by the global ELISA tests [17,40,41], the analysis suggests that use of these diagnostic techniques requires further refinement in this patient setting to minimise errors or delays in HIT diagnosis. |
| Â |
| Danaparoid dosing, plasma anti-Xa activity response and safety |
| Â |
| The recommended guideline for danaparoid dosing for CRRT was most often followed but 40.2% of the cases required dose adaptation at some time to overcome early circuit and/or systemic clotting [20,21,30] or bleeding (risk) [3]. However, omission of the loading i.v. bolus usually led to new circuit clotting so, unless bleeding is life-threatening, it is better to merely reduce the bolus to minimise delaying antithrombotic protection and to retain danaparoid’s unique ability to reduce HIT-induced platelet activation [11-13]. In several reports [3,33], sometimes because of an increased bleeding risk, adequate circuit patency was provided by lower than recommended maintenance rates. |
| Â |
| The 12 (11.8%) major bleeds are acceptable for this high risk patient population taking into account that 4 followed surgical errors. It appears that a specific danaparoid antidote would not have mitigated the severity of the bleeding events nor reduced the 2.9% fatality rate (especially since 2 fatal bleeds occurred after danaparoid discontinuation when the influence of danaparoid on plasma anti-Xa levels would have been nil. |
| Â |
| Despite poor correlation between plasma anti-Xa levels and major bleeding or thrombosis the current analysis (showing a trend towards increased minor bleeding) and other studies [42-44] suggest that the upper level of the target plasma anti-Xa activity range should be limited to 0.8 U/mL. Nevertheless, the need for danaparoid dosage adaptations in these critically ill patients is better judged by clinical criteria (bleeding/thrombosis risk balance) than by plasma anti-Xa activity monitoring. |
| Â |
| Danaparoid cross-reactivity and treatment discontinuation |
| Â |
| Table 3 lists cases of premature danaparoid discontinuation due to suspicion of clinical cross-reactivity. Only one case was serologically tested (and found positive). In the current analysis the frequency of positive serological cross-reactivity was greater than that reported for HIT patients without renal failure (6/45 versus 27/587) respectively (p=0.0257) as was failure of PCR recovery (31/102 vs. 57/609, p<0.0001) [10]. However, pre-treatment danaparoid cross-reactivity testing is considered unnecessary [14] because it has seldom been reported to cause clinical complications [4,13,45-47], especially at therapeutic dosing [48]. Thus the net clinical benefit of danaparoid with regard to PCR recovery [49] or efficacy [49-53] is better than or equal to comparative therapies (dextran, ancrod or lepirudin) regardless of possible cross-reactivity. Hence failed PCR recovery and/or thrombotic events, especially within the first 2-3 days of initiating danaparoid therapy, appear most likely to be due to: the natural progression of HIT especially if danaparoid dosing is suboptimal [51,54] (11/36 current cases), clinical manifestation of previously covert TECs (particularly in patients presenting with ‘isolated’ HIT) [55], co-morbidity [3,55] (26/36 current cases had MODS/DIC/sepsis) impending death [56], continued (LMW) heparin administration or use of heparin bonded catheters and circuits or, more contentiously (see below), platelet transfusions [57-60] (11/36 current cases). However if a new/persistent PCR or TEC cannot be reasonably explained by any of the above reasons then danaparoid should be withdrawn because of possible clinical cross-reactivity [2,12]. |
| Â |
| Clinical outcomes, platelet count nadirs and platelet transfusion |
| Â |
| Critically ill patients develop a high frequency of PCR and have a high mortality [59], but the current analysis shows no relation between mean platelet count nadirs and clinical outcomes. A similar lack of relationship was found for ICU patients with HIT and undergoing intermittent haemodialysis (Magnani, unpublished data). Thus the initial platelet count nadir does not appear to be a reliable marker of impending mortality, even for counts |
| Â |
| Most patients receiving platelet transfusions were severely ill with well documented alternative reasons for their very low platelet counts. The current data does not clarify the influence of these transfusions on the clinical outcomes of patients. In the 11 patients who received them new/persistent PCRs, TECs and deaths occurred more frequently, however, within the 32 patients with persistent/renewed PCR those who received platelet transfusions showed a mortality reduction of 41% compared with those who were not transfused. The current analysis tends to support a casual association between platelet transfusions and adverse outcomes, since the latter appear to be due to the critical clinical status that prompted their administrationin which case they may be life-saving [61,62], rather than a causal relationship (supporting avoidance of platelet transfusions unless absolutely necessary [14,60]). |
| Â |
| Conclusion |
| Â |
| Danaparoid can provide safe and effective anticoagulation for critically ill ICU patients with HIT and renal failure requiring CRRT, despite its renal elimination, long effect-half-life, potential clinical cross-reactivity with the HIT antibody and lack of antidote, but dosing may require adaptation to the individual patient’s thrombotic: bleeding risk balance. |
| Â |
| |
| References |
| Â |
- Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, et al. (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356: 26-30.
- Warkentin TE (2006) Think of HIT. Hematology Am Soc Hematol Educ Program 2006: 408-414.
- Lindhoff-Last E, Betz C, Bauersachs R (2001) Use of a low-molecular-weight heparinoid (danaparoid sodium) for continuous renal replacement therapy in intensive care patients. Clin Appl Thromb Hemost 7: 300-304.
- Selleng K, Warkentin TE, Greinacher A (2007) Heparin-induced thrombocytopenia in intensive care patients. Crit Care Med 35: 1165-1176.
- Warkentin TE, Heddle NM (2003) Laboratory diagnosis of immune heparin-induced thrombocytopenia. Curr Hematol Rep 2: 148-157.
- Meuleman DG (1992) Orgaran (Org 10172): its pharmacological profile in experimental models. Haemostasis 22: 58-65.
- Ofosu FA (1992) Anticoagulant mechanisms of Orgaran (Org 10172) and its fraction with high affinity to antithrombin III (Org 10849). Haemostasis 22: 66-72.
- Danhof M, de Boer A, Magnani HN, Stiekema JC (1992) Pharmacokinetic considerations on Orgaran (Org 10172) therapy. Haemostasis 22: 73-84.
- Frei U, Wilks MF, Boehmer S, Crisp-Lindgren N, Schwarzrock R, et al. (1988) Gastrointestinal blood loss in haemodialysis patients during use of a low-molecular-weight heparinoid anticoagulant. Nephrol Dial Transplant 3: 435-439.
- Magnani HN, Gallus A (2006) Heparin-induced thrombocytopenia (HIT). A report of 1,478 clinical outcomes of patients treated with danaparoid (Orgaran) from 1982 to mid-2004. Thromb Haemost 95: 967-981.
- Chong BH, Ismail F, Cade J, Gallus AS, Gordon S, et al. (1989) Heparin-induced thrombocytopenia: studies with a new low molecular weight heparinoid, Org 10172. Blood 73: 1592-1596.
- Greinacher A, Michels I, Mueller-Eckhardt C (1992) Heparin-associated thrombocytopenia: the antibody is not heparin specific. Thromb Haemost 67: 545-549.
- Krauel K, Fürll B, Warkentin TE, Weitschies W, Kohlmann T, et al. (2008) Heparin-induced thrombocytopenia--therapeutic concentrations of danaparoid, unlike fondaparinux and direct thrombin inhibitors, inhibit formation of platelet factor 4-heparin complexes. J Thromb Haemost 6: 2160-2167.
- Williamson DR, Boulanger I, Tardif M, Albert M, Grégoire G (2004) Argatroban dosing in intensive care patients with acute renal failure and liver dysfunction. Pharmacotherapy 24: 409-414.
- Schmahl KS, Ganjoo AK, Harloff MG (1997) Orgaran (Org 10172) for cardiopulmonary bypass in heparin-induced thrombocytopenia: role of adjunctive plasmapheresis. J Cardiothorac Vasc Anesth 11: 262-263.
- Warkentin TE, Greinacher A, Koster A, Lincoff AM (2008) Treatment and prevention of Heparin-induced thrombocytopenia: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133: 340S-380S.
- Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, et al. (2006) Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 4: 759-765.
- Bennett G, Hanley MR (2004) Multiple embolic events in a patient with heparin induced thrombocytopaenic thrombotic syndrome and mural thrombus. Anaesth Intensive Care 32: 821-824.
- Christiansen S, Jahn UR, Meyer J, Scheld HH, Van Aken H, et al. (2000) Anticoagulative management of patients requiring left ventricular assist device implantation and suffering from heparin-induced thrombocytopenia type II. Ann Thorac Surg 69: 774-777.
- Christin F, Theissen-Laval O, Loeb JP (1998) Danaparoid (orgaran) for continuous venovenous hemodiafiltration in a patient with heparin-induced thrombocytopenia. Ann Fr Anesth Reanim 17: 82-83.
- Durand M, Lecompte T, Hacquard M, Carteaux JP (2008) Heparin-induced thrombocytopenia and cardiopulmonary bypass: anticoagulation with unfractionated heparin and the GPIIb/IIIa inhibitor tirofiban and successful use of rFVIIa for post-protamine bleeding due to persistent platelet blockade. Eur J Cardiothorac Surg 34: 687-689.
- Dworschak M, Hiesmayr JM, Lassnigg A (2002) Lifesaving citrate anticoagulation to bridge ineffective danaparoid [correction of to bridge to danaparoid treatment. Ann Thorac Surg 73: 1626-1627.
- van Eps RS, Wester JPJ, de Ruiter FE, Girbes IRG (2000) Danaparoid sodium in contimuous venovenous hemofiltration in patients with multiple organ dysfunction syndrome. Neth J Med 56: A40-A41.
- Gindre L, de Maistre E, Perrin A, Lecompte T, Hoffman MA (1997) Thrombocytopenia due to heparin therapy. Use of danaparoid (Orgaran), 13 monocentric cases under authorization of temporary prescription (ATU). Therapie 52: 591-597.
- Hampton KA (2005) Case of HIT complicating cardiothoracic surgery. MIMs Advances 6: 10-12.
- John G, Shehabi Y, Mudaliar Y (1991) Use of a low molecular weight preparation of heparin--ORG 10172 (Lomoparan) in the critically ill. Anaesth Intensive Care 19: 588-591.
- Klein M, Tomer A, Swartz A, Koyffman L, Weksler N (2006) Bivalirudin for anticoagulation in mechanical aortic valve replacement and heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis 17: 331-333.
- Kollig E, Senkal M, Hentsch S, Mumme A, Zumtobel V (1997) Heparin-induced thrombocytopenia (HIT) during continuous veno-venous haemodiafiltration (abstract). Acta Anaesth Scand 41: 354,096.
- Lasocki S, Piednoir P, Ajzenberg N, Geffroy A, Benbara A, et al. (2008) Anti-PF4/heparin antibodies associated with repeated hemofiltration-filter clotting: a retrospective study. Crit Care 12: R84.
- Moonen M, Rorive G (1997) Low molecular weight heparinoid Org 10172 (Orgaran®) during continuous veno-venous haemofiltration in patients with heparin-induced thrombocytopenia. Nephrol Dial Transplant 12: A169.
- Newall F, Barnes C, Ignjatovic V, Monagle P (2003) Heparin-induced thrombocytopenia in children. J Paediatr Child Health 39: 289-292.
- Oliver M, de Pina J-J, Pollet L, Bohand X (2000) Thrombopenie induit par lÂ’Heparine: a propos dÂ’un cas. Revue Francaise des Laboratoires 323.
- de Pont AC, Hofstra JJ, Pik DR, Meijers JC, Schultz MJ (2007) Pharmacokinetics and pharmacodynamics of danaparoid during continuous venovenous hemofiltration: a pilot study. Crit Care 11: R102.
- Schneider T, Heuer B, Deller A, Boesken WH (2000) Continuous haemofiltration with r-hirudin (lepirudin) as anticoagulant in a patient with heparin induced thrombocytopenia (HIT II). Wien Klin Wochenschr 112: 552-555.
- Yoshika M, Komiyama Y, Hirakawa A, Nakatani T, Takahashi H (2011) A difficult diagnosis case of prolonged thrombocytopenia with sepsis and disseminated intravascular coagulation. Clin Appl Thromb Hemost 17: 410-413.
- Wester JPJ (2004) Guidelines for anticoagulation with danaparoid sodium or lepirudin in continuous venovenous hemofiltration. Neth J Crit Care 8: 293-301.
- Palazzo M (2005) Support for acute renal failure. Care of the Critically Ill 21: 105-113.
- Tardy-Poncet B, Tardy B, Reynaud J, Mahul P, Mismetti P, et al. (1999) Efficacy and safety of danaparoid sodium (ORG 10172) in critically ill patients with heparin-associated thrombocytopenia. Chest 115: 1616-1620.
- Magnani HN (2010) A review of 122 published outcomes of danaparoid anticoagulation for intermittent haemodialysis. Thromb Res 125: e171-176.
- Greinacher A (2007) Too many hits in HIT? Am J Hematol 82: 1035-1036.
- Baker J, De Loughery TG, Boshkov L (2004) Lack of value of clinical predictors of heparin-induced thrombocytopenia (HIT) in complex patients. Blood 104: 569a.
- Massey EW, Biller J, Davis JN, Adams HP, Marler JR, et al. (1990) Large dose infusions of heparinoid Org 10172 in ischemic stroke. Stroke 21: 1289-1292
- Wester JP, Haas FJ, Biesma DH, Leusink JA, Veth G (2004) Thrombosis and hemorrhage in heparin-induced thrombocytopenia in seriously ill patients. Intensive Care Med 30: 1927-1934.
- Wester JP, de Valk HW, Nieuwenhuis HK, Brouwer CB, van der Graaf Y, et al. (1996) Risk factors for bleeding during treatment of acute venous thromboembolism. Thromb Haemost 76: 682-688.
- Newman PM, Swanson RL, Chong BH (1998) Heparin-induced thrombocytopenia: IgG binding to PF4-heparin complexes in the fluid phase and cross-reactivity with low molecular weight heparin and heparinoid. Thromb Haemost 80: 292-297.
- Magnani HN (1997) Orgaran (danaparoid sodium) use in the syndrome of heparin-induced thrombocytopenia. Platelets 8: 65-82.
- Tardy-Poncet B, Wolf B, Lasne M, Alhenc-Gelas M, Camarasa P, et al. (2007) Orgaran cross-reactivity with heparin induced thrombocytopenia antibodies: report of 12 cases. J Thromb Haemost 5(Suppl 2):P-W-338.
- Schenk JF, Pindur G, Stephan B, Mörsdorf S, Mertzlufft F, et al. (2003) On the prophylactic and therapeutic use of danaparoid sodium (Orgaran) in patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 9: 25-32.
- Chong BH, Gallus AS, Cade JF, Magnani H, Manoharan A, et al. (2001) Prospective randomised open-label comparison of danaparoid with dextran 70 in the treatment of heparin-induced thrombocytopaenia with thrombosis: a clinical outcome study. Thromb Haemost 86: 1170-1175.
- Warkentin TE (1996) Danaparoid sodium (Orgaran®) for the treatment of heparin-induced thrombocytopenia (HIT) and thrombosis: effects on in- vitro thrombin and cross-linked fibrin generation and evaluation if the clinical significance of in-vitro cross reactivity (XR) of danaparoid for HIT IgG. Blood 88: 626A.
- Lubenow N, Warkentin TE, Greinacher A, Wessel A, Sloane D-A, et al. (2006) Results of a systematic evaluation of treatment outcomes for heparin-induced thrombocytopenia in patients receiving danaparoid, ancrod and/or coumarin explain the rapid shift in clinical practice during the 1990s. Thromb Res 117: 507-515.
- Farner B, Eichler P, Kroll H, Greinacher A (2001) A comparison of danaparoid and lepirudin in heparin-induced thrombocytopenia. Thromb Haemost 85: 950-957.
- Curzio KM, Cheng-Lai A, Kheyfets V, Sinnet M, Billett HH (2009) A comparison of direct thrombin inhibitors in the treatment of Heparin-Induced Thrombocytopenia: a single institution experience. J Thromb Thrombolysis 28: 117-123.
- Elalamy I, Tardy-Poncet B, Mulot A, de Maistre E, Pouplard C, et al. (2009) Risk factors for unfavorable clinical outcome in patients with documented heparin-induced thrombocytopenia. Thromb Res 124: 554-559.
- Tardy B, Tardy-Poncet B, Fournel P, Venet C, Jospe R, et al. (1999) Lower limb veins should be systematically explored in patients with isolated heparin-induced thrombocytopenia. Thromb Haemost 82: 1199-1200.
- Kress DC, Aronson S, McDonald ML, Malik MI, Divgi AB, et al. (2007) Positive heparin-platelet factor 4 antibody complex and cardiac surgical outcomes. Ann Thorac Surg 83: 1737-1743.
- Hoffman U, Huhle G, Haseroth K, Heene DL, Harenberg J (1999) A fatal complication in a patient with heparin-induced thrombocytopenia type II re-exposed to heparin. Eur J Surg Traumatol 9: 205-207.
- Hopkins CK, Goldfinger D (2008) Platelet transfusions in heparin-induced thrombocytopenia: a report of four cases and review of the literature. Transfusion 48: 2128-2132.
- Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, et al. (2002) Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med 30: 1765-1771.
- Gettings EM, Brush KA, Van Cott EM, Hurford WE (2006) Outcome of postoperative critically ill patients with heparin-induced thrombocytopenia: an observational retrospective case-control study. Crit Care 10: R161.
- Refaai MA, Chuang C, Menegus M, Blumberg N, Francis CW (2010) Outcomes after platelet transfusion in patients with heparin-induced thrombocytopenia. J Thromb Haemost 8: 1419-1421.
- Thachil J (2010) The myths about platelet transfusions in immune-mediated thrombocytopenias. Br J Haematol 150: 494-495.
|
| Â |
| Â |