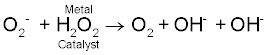| Research Article |
Open Access |
|
| Shafaq Noori* |
| Muhammad Bin Qasim Medical & Dental College, Karachi, Pakistan |
| *Corresponding author: |
Shafaq Noori
Muhammad Bin Qasim Medical & Dental College
Karachi, Pakistan
E-mail: shafaqnoori@hotmail.com |
|
| |
| Received August 25, 2012; Published November 03, 2012 |
| |
| Citation: Noori S (2012) An Overview of Oxidative Stress and Antioxidant Defensive System. 1:413. doi:10.4172/scientificreports.413 |
| |
| Copyright: © 2012 Noori S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Free radicals play an important role in cell's life and death. These are unstable/unpaired electrons in their outermost shell and may become highly reactive. Reactive oxygen species (ROS) are generated from molecular oxygen/nitrogen through Electron Transport Chain (ETC), cytochrome P450, and other cellular and sub-cellular functions. They affect beneficial metabolic and cellular processes and also play key role in pathological conditions of the body. It is normally balanced by endogenous antioxidant system. Imbalances in redox status may develop cellular oxidative stress. If the endogenous antioxidants fail to overcome the reactive metabolites production, then exogenous antioxidants would be necessary to balance redox status. Dietary sources, including plants, herbs, spices, vitamins and herbal extracts, play an important role in this regard. This review article summarizes the functional role of Reactive Oxygen Species (ROS), reactive nitrogen species, reactive halogen species and their pathological importance. It also highlights the significant role of endogenous, as well as exogenous antioxidant system in reactive generation of intermediates. |
| |
| Keywords |
| |
| Oxidative stress; Antioxidants; Reactive intermediates |
| |
| Introduction |
| |
| Oxidative process that is regularly going on in cell is essential for life and death of a cell. The following are the important key points taken into consideration: |
| |
| • Molecular oxygen has ability to un-pair and leave free radicals which are unstable; |
| |
| • This unstable radical is highly reactive and causes formation of reactive oxygen species; |
| |
| • Beneficial biological functions such as apoptosis, necrosis, phagocytosis are mediated by reactive oxygen species; |
| |
| • These reactive metabolites are selectively neutralized by body's defensive mechanism; |
| |
| • Principal defensive agents are antioxidant enzymes and endogenous antioxidants; |
| |
| • Balance is created between pro-oxidant and antioxidant in a cell, and any impairment in equilibrium causes deleterious effects on cell's life; |
| |
| • Increased level of antioxidants may interfere the normal oxidative process; |
| |
| • Decreased level of antioxidants generates reactive metabolites. |
| |
| It is known that unpaired electron of molecular oxygen react to form highly reactive species, which are known as reactive oxygen species. Reactive oxygen species are generated from enzymatic and non-enzymatic sources [1,2]. |
| |
| Non enzymatic sources |
| |
| Fenton's & Haber's reactions: Fridovick [3], Halliwell and Gutteridge [4] explain the reduction of molecular oxygen to form superoxide anions. These superoxide anions have ability to form more and highly reactive oxygen species. The dismutation of superoxides forms hydrogen peroxide. |
| |
O-2. + O-2. + 2H  H2O2 + O2 H2O2 + O2 |
| |
| H2O2, hydrogen peroxide, is more stable than O2- superoxide. It is permeable to plasma membrane and plays two important roles in the body. Either it is scavenged by catalase/GSH (glutathione peroxidase), or it helps in the formation of highly reactive oxygen species [4]. |
| |
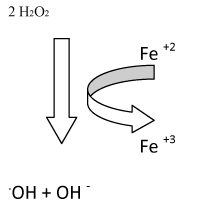
| Through the Fenton's reaction, these hydrogen peroxides react with metal iron or copper to form more highly reactive hydroxyl ions, OH-. |
| |
Fe2+ + H2O2  Fe3+ + OH- + OH- Fe3+ + OH- + OH- |
| |
| Through Haber-Weiss reaction |
| |
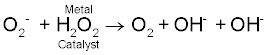 |
| |
| H2O2 reacts with Cl-, Br-, I- and is utilized by myloperoxidase to form more reactive hypochloric acid/hyperchlorite. This is important for protein aggregation and fermentation [5]. |
| |
H2O2 + Cl-  HOCl + OH- HOCl + OH- |
| |
| Formation of peroxynitrite is the primary reaction [6]. |
| |
O2- + NO  ONOO- ONOO- |
| |
| Enzymatic sources |
| |
| ROS are generated by oxygen metabolism, and have a single unpaired electron in their outer orbit that becomes highly reactive. It is produced in all aerobic organisms to perform cellular metabolisms. The enzymatic sources of ROS under subcellular levels are xanthine oxidases, cyclo-oxygenases (COX) and lipoxygenases (LOX), NO synthases (nitric oxide synthase) and mitochondrial oxidases [7]. |
| |
| Monoamine oxidase: In mitochondrial membrane under the physiological condition, Electron transport chain produces ATP and O 2-. A heme containing enzyme monoamine oxidase present in outer mitochondrial membrane catalyzes oxidative deamination of amines, and thus produces H2O2 in matrix and cytosol [8]. |
| |
| NADPH oxidase/Respiratory Burst Oxidase: Phagocyte NADPH oxidase plays an important role in host defenses against invading microbes by generating superoxides. It is present in neutrophils and produces O2-. It is multicomplex enzyme located in plasma membrane of activated cell. It contains several components including cytochrome b 558 (it is composed of gp91 phox, gp22 phox, phox phagocyte oxidase), p47, p67, p40 and rac 1 (monocytes)/rac 2 (neutrophils). The gp91 is bound to the cellular membrane. Upon stimulation, cytoplasmic subunits activate gp91 and cause respiratory bursts that activates superoxides, and releases them into the phagosomes [9,10]. |
| |
| In the same manner, endothelia, fibroblast, mesangial, osteoclast, chondrocytes and smooth muscles also generate superoxides, called NADPH-like oxidase which are activated by hormones and cytokines. Besides being host defense, it also helps in signaling [11]. |
| |
| ROS is produced by monovalent oxygen reduction. |
| |
| 2O2 + NADPH → NADPH Oxidase → 2O2- + NADP+ + H+ |
| |
| Xanthine oxidoreductase: This enzyme catalyzes hypoxanthine into xanthine, and then into uric acid. Xanthine Oxidoreductase (XOR) is present in the form of Xanthine Dehydrogenase (XD); these two forms of xanthine are transformed. XD is transformed into XO, irreversibly by proteolysis and reversibly by oxidation of sulfhydryls, and produce large amount of H2O2 and O2-. It is also found that XOR can transform nitrates into nitrites and NO. It also catalyzes the NO with O2- and form highly reactive peroxynitrites [12-15]. |
| |
| ROS generation By arachidonic acid: During the metabolism of arachidonic acid, ROS is generated intracellularly in which cyclooxygenase, lipooxygenase,cytochrome P450 oxidase enzyme system are involved [16]. |
| |
| Cytochrome P450 oxidase: This is heme-containing enzyme; it is present in mitochondria and participates in metabolism of cholesterol, steroids, hormones, catabolism of bile acids, arachidonic acid and eicosanoids, hydroxylation of vitamin D3, retinoid acid by catalyzing intramolecular transfer of oxygen. It transfers 2e-; one is bound to oxygen and the second is reduced to water. A part of oxygen is reduced to superoxides, inevitably. |
| |
| Myeloperoxidase: This is heme-containing enzyme, present in neutrophils and eosinophils and catalyzes the H2O2 with various substrates to form highly reactive hypochloric acids [17,18]. |
| |
| Function of ROS in Cells |
| |
| ROS performs beneficial functions in our body. Redox level should be maintained. It is the mediator of phagocytosis, apoptosis, detoxification reactions, executioner of precancerous cells and infections, etc. It is beneficially involved in signaling pathways to maintain cellular homeostasis in body. The ROS regulates many metabolic and cellular processes including proliferation, migration, gene expression, immunity and wound healing [19]. Biochemical reactions are involved in the synthesis of prostaglandins, hydroxylation of proline and lysine, oxidation of xanthine and other oxidative processes [20]. |
| |
| Generation of ROS in Cells |
| |
| 1-Mitochondria |
| |
| 2-Endoplasmic reticulum |
| |
| 3-Phagocytosis |
| |
| 4-Other sources |
| |
| Production of ROS in mitochondria |
| |
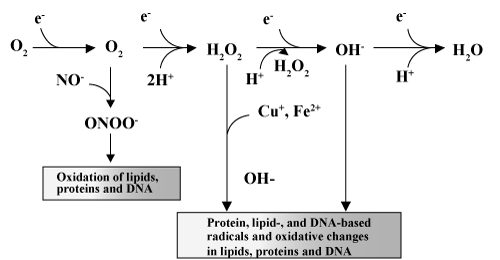
| Electron transport chain produces superoxide anion in mitochondria by the reduction of molecular oxygen. ROS are generated by mitochondria, via the release of electrons from the electron transport chain and the reduction of oxygen molecules into superoxides (O2-). Superoxides, through the reaction catalyzed by superoxide dismutase (SOD), are transformed into the much less reactive hydrogen peroxide moiety (H2O2). However, when hydrogen peroxide interacts with ions of transition metals such as iron and copper, the most reactive ROS, hydroxyl radicals (OH-) are formed (Fenton's reaction) [21]. |
| |
2O2 + 2 H2O  O2 + H2O2 + 2OH- O2 + H2O2 + 2OH- |
| |
2H2O2  2H2O + O2- 2H2O + O2- |
| |
|
|
| |
| Production of ROS in endoplasmic reticulum |
| |
| Cytochrome P450 complexes are used to detoxify the toxic hydrophobic chemical compounds from the body; as a result, superoxide anions are formed. Enzyme cytochrome P450 reductase is used to detoxify into hydrophilic compounds. NADPH and NADH provide electrons for reduction of cytochrome b5 and cytochrome P450 [ 2]. |
| |
| Phagocytosis |
| |
| In phagocytosis, the bacteria when engulfed by the phagocytic cell results in the production of ROS. NADPH supplies electron and gives NADP +, hexose monophosphate shunt supplies energy to the NADP+ by NADPH oxidase through cytochrome b245 and form ROS. Bacteria and toxic cells are destroyed by respiratory burst produced by ROS [ 22]. |
| |
| Other sources |
| |
| Apoptosis: Apoptosis is a process of programmed cell death. It activates the Bcl-2; a group of protein that stimulates the Bax, which causes leakage of Cytochrome c. This Cyt c binds to Apaf-1 and form apoptosomes. This activates the caspase 9 and finally, causes protein denaturation and phagocytosis of the cell [23,24]. |
| |
| Autooxidation of small molecules: Small molecules like dopamine, epinephrine, flavins and hydroquinones involve direct production of O 2- [25]. |
| |
| Peroxisomes generating hydrogen peroxide: Peroxisomescontaining enzymes; glycolate oxidase, d- amino acid oxidase, urate oxidase, 1-α-hydroxyacid oxidase, fatty acyl CoA oxidase are involved in generating H2O2. The catalases involve in varieties of peroxidative reactions [26]. |
| |
| ROS Generation by Lysosomes: It carries electron transport chain which involves pumping of proton. This system promotes 3e- reduction to oxygen and form highly reactive OH- [18]. |
| |
| ROS Generation During The Intercellular Membrane |
| |
| Catalytic reactions: Xanthine oxidase, aldehyde oxidase, dihydroorotate dehydrogenase, flavoprotein dehydrogenase, tryptophan dioxygenase can generate ROS during catalytic cycling. Xanthine oxidase is formed from xanthine dehydrogenase, in hypoxic tissues and thus, generates O2- [27]. |
| |
| ROS production in non-phagocytic cells |
| |
| 1- Phospholipid metabolism: membrane phospholipase A2 (PLA2) hydrolyzes the phospholipids to generate the Arachidonic acid. Arachidonic acid forms the 4 major classes of eicosanoids, which includes: prostaglandins, prostacyclins, thromboxanes and leukotrienes through the cyclooxygenase and lipoxygenase dependant synthesis. Their synthesizing steps involve the reactive intermediates. |
| |
| |
| 2- Leukotriens derived oxygen species implicated redox status signaling by angiotensin II, IL-1 and epidermal growth factor. Angiotensin II induced O2- production in smooth muscle cell is also dependant on phospholipid pathway [28]. |
| |
| Receptors mediated ROS generation |
| |
| Ligand induced ROS is generated in nonphagocytic cells [29]. NADPH oxidase is activated in vascular smooth muscle cells by Angiotensin II and 5-HT neurotransmitter. This in turn, generates hydrogen peroxides and superoxide anions. ROS activated by Ang-II stimulate the p38 Mitogen Activated Protein Kinase (MAPK) pathway and cause cell hypertrophy. ROS activated by 5-HT stimulate ERK (extra cellular signal regulated kinase), MAPK pathway and cause cell proliferation. |
| |
| So, ultimately 3 important sources of ROS generation is depicted: |
| |
| a) Endogenous source |
| |
| b) Exogenous source |
| |
| c) Pathological source |
| |
| a) Endogenous Sources: These are the products of important metabolic processes that are continuously going on in our body, e.g. detoxification reaction involves the P450 enzyme system and electron transport chain in mitochondria. |
| |
| b) Exogenous Sources: Cigarette smoking, industrial waste products, ionization from radiations, ozone, asbestoses fibers, viral & bacterial infections. |
| |
| c) Pathological Sources: Radiation, immune cell activation, inflammation, ischemia, infections cancer, metabolism of environmental pollutants & certain drugs. |
| |
| Viral infections associated with reactive oxygen species |
| |
| Many of the viral infections are associated with ROS generation, with total decreased level of antioxidants in intracellular and extracellular. Reactive oxygen species and reactive nitrogen intermediates possess antimicrobial and anti tumor activities, as well as they also participates in spreading of many pathological infections. It is known that viral infections interfere with the metabolic and physiologic mechanism of host cell [30]. Sendai and influenza viruses causes respiratory burst in phagocytic cells and thus, elevates the ROS/RNS concentration of the cell. Herpes viruses activate the host cell in early infections. Semiconfluent and proliferating cell are best for viral growth, while quiescent cell and confluent are not. Influenza virus exhibits ROS generation by using xanthine oxidase pathway and produce superoxides O2-, and host posses severe systemic symptoms like fever, myalgia, headache, anorexia. Virus bind with protease inhibitor present on cell membrane of lung surfactant and enter into the cell, where it is synthesizes HA0 (Haemagglutinin) as a precursor which is non-infectious. This protein exhibits the proteolytic cleavage into HA1 and HA2; these are infectious strains. ROS increases in the lungs during infection, but inactivates the antiproteolytic activity of lung, therefore, causes inflammation and ultimately infection [31,32]. |
| |
| Similarly HIV (human immunodeficiency virus) increases the hydroperoxides and malonyldialdehyde (MDA) in human cells. HIV increases oxidative stress by stimulating transcription factor NFĸB (Nuclear Factor ĸB), cytokines and TNF-α, which may result in release of H2O2 from T-Cells. The HIV virus and HIV gp120 attributes enhance production of NO in monocytes and causes neurotoxic effects [33,34]. |
| |
| Hepatitis virus directly affects the host genome and results in the production of RNS and ROS. It is characterized by increased cell proliferation, which ultimately becomes cancer [35]. |
| |
| Rabies, Lymphocytic choriomeningitis virus [36], bovine viral diarrhea viruses [37], experimental allergic encephalomyelitis [38], all exhibits increase production of NO. It causes immunosuppressive effect during the course of infections. Similarly, lentivirus and mycoplasma exhibits the NO mediated toxicity. |
| |
| Pathways Involved in ROS Generation |
| |
| ROS are generated through different cellular pathways including calcium dependant pathway, protein tyrosine kinase, protein tyrosine phosphatase, serine threonine kinase, phospholipase, mitogen activated protein kinase, NFĸB, cytokines receptors, growth receptor, G–Protein coupled receptor, ion channel receptor and epidermal growth factor. These are briefly discussed. |
| |
| Cytokines receptors |
| |
| IL-1, TNF-α, NK-ĸB are among the first receptors to generate ROS in non-phagocytic cells. TNF-α and oxidants may synergistically activate NK-ĸB by ROS independent mechanism. TNF-α generates mitochondrial ROS implicated in apoptotic cell death. TNF-α activate IL-8, chemokines production, induction of cardiac myocytes hypertrophy by ROS dependant mechanism [39]. Three different cells specific pathways of NK-kB have been evaluated, which is activated by IL-1β. ROS generated by 5-LOX in lymphoid cells, 5-LOX independent pathway in epithelial cells and NADPH oxidase dependent ROS production in monocytes [40]. IFN-γ stimulates cyclooxygenase dependent peroxides in liver cell and exhibits antibacterial activity [41]. |
| |
| Protein kinase receptors |
| |
| For mitogenic signaling, a number of growth factors bind with protein kinase receptor for intra cellular ROS production. Fratti et al. [ 42], has demonstrated that platelet derived growth factor stimulates intracellular H2O2, required to induce tyrosine phosphorylation, DNA synthesis, chemotaxis, and MAPK activation [43]. Epidermal growth factor (EGF) induces H2O2 formation by inhibition of protein tyrosine phosphatase activity. It is found that EGF stimulates intracellular O2- by using LOX pathway. Heparin binding EGF stimulates ROS in vascular smooth muscle cells. |
| |
| EFG-induced peroxides production enhanced tumorigenicity, metastatic capacity. FGF-2 (Fibroblast growth factor-2) stimulates intracellular H2O2 by induction of c-Fos in chondrocytes, using flavoprotein oxidase [44]. |
| |
| Serine/Threonine kinase |
| |
| These receptors are belonging to the TGF-β1 superfamily. Shibanuma et al. [45], found growth inhibitory effect on H2O2 from mouse osteoblastic cell. TGF-β1 inhibits the expression of SOD; catalase enzymes activity thus stimulates the oxidative stress. It also lowers the intracellular concentration of GSH glutathione in lung cell, primarily endothelial and epithelial cells. The prooxidants effect of TGF-β1 is growth inhibitory effect, apoptosis, TGF-β1 autoinduction, activation of latent TGF-β, cellular transformation, collagen synthesis, myofibroblast differenciation, etc [45]. |
| |
| G-Protein receptors |
| |
| Different ligands include angiotensin II, serotonin, 5-HT (5 Hydroxytryptamine), bradykinin, thrombin and endothelin generate ROS [46]. Ang II stimulates the ROS production in vascular smooth muscles cells [47]. It is found that tyrosine phosphorylation of GTPase activating protein is induced by intracellular 5-HT, which generates superoxides by using mitogenic pathway through NADPH dependent oxidase activity [48]. Bradykinin is vasodilator mediated by NO. ROS generated in endothelial cells is more likely through arachidonic acid metabolism, using cyclooxygenase pathway [49]. Thrombin generates ROS in smooth muscle cell and endothelial cell using NADPH oxidase activity for mitogenesis [50]. |
| |
| Ion channel receptors |
| |
| Neurotransmitters like 5-HT, acetylcholine, glutamate, glycine, γ-aminobutyric acid are mediated by signaling between the electrically excitable cells through ion channels [51]. |
| |
| Pathophysiological Role of ROS |
| |
| Oxidative stress has been linked to the pathogenesis of numerous diseases including asthma (mitochondrial dysfunction) [52], atherosclerosis (oxidative modification of LDL) [53], endothelial cardiovascular disease, which is more prompt to inactivation of NO and ROS, thus predispose of these reactive molecules. The disease is characterized by altered anticoagulant and anti inflammatory properties [54], a cancer in which cells present mitochondria alterations at the level of mitochondrial DNA, oxidative phosphorylation and energy metabolism, all these activates the prooxidants and causes mitochondrial injury [55], inflammatory skin diseases in which Rho GTPases regulate the ROS production under the control of Rac protein in fibroblast, psoriasis and vitiligo [56]. Infertility to male due to impairment of spermatogenesis, as a result retention of cytoplasm in sperm midpiece causing increased activities of cytoplasmic enzymes like Glucose-6-Phosphate dehydrogenase, which in turn produce more NADPH and finally increases the production of O2- (ROS) [57]. |
| |
| Peroxynitrite reacts with body fluids and form nitrotyrosine in glial cell, which causes neurodegenerative diseases like Alzheimer's disease, in which Cu (I/II) involve the aggregation of amyloidogenic peptide with the production of Reactive Oxygen Species. Parkinson's disease is actually the neural degeneration due to the loss of pigmented neurons in substantia nigra, produces MPTP (Mitochondrial Permeability Transition Pore) which inhibits the complex I in Electron transport chain, thus results in decrease in the production of ATP. |
| |
| Iron changes is seen in multiple sclerosis, spastic paraplegia in which iron causes secondary changes and accumulation of iron is related to gliosis, which produces ROS [58]. ROS is regulator of AIDS [ 59], and cataract [60]. |
| |
| ROS is also generated in type I pneumocytes on alveolar epithelium and causes destruction to cells. It is found that during inflammation, phagocytes are the main source of ROS generation [61]. High glucose level in Diabetes and palmitic acid stimulate ROS through Protein kinase C dependant NADPH oxidase in smooth muscle cells and endothelial cells [62]. A study showed the biology of ROS in mitochondria specifies the aging process in mutant stains of Caenorhabditis elegans [63]. Siegfried and Leonard [62] found the gene SIR2 that exhibits the rate of aging. Arthiritis severity is regulated by gene Ncf1 which reduced oxidative burst response and NADPH oxidase complex [64]. |
| |
| Carbohydrates oxidation by ROS |
| |
| Free carbon and hydrogen of deoxy sugars are attributed to the oxidation of carbohydrates, e.g. mannitol and glucose. The free radicals binds with these carbohydrates and forms carbon centered radicals. These carbon centered radicals interacts with other carbohydrates, and thus series of autocatalytic chain reaction commence resulting in the destruction of the cells. Ketoamines and ketoaldehydes are the most common oxidative products of carbohydrates [65]. |
| |
| Protein oxidation by ROS |
| |
| Reactive oxygen species interacts on protein molecules at the specific amino acid side chain and form the modification in protein structure results fragmentation of the peptide chain, alteration in electrical charges; peroxynitrite nitrate protein is accumulated and thus, increases the proteolysis. Garrison [65] has found that active oxygen has potential to react with amino acid side groups and cleaving the polypeptide chain, thus resulting in the formation of reactive carbonyl groups [66,67]. Stadtman and Oliver [66] proposed the protein oxidation mechanism in which lys residue is converted to α amino-adipic semialdehyde. He found that ferrous ions formed by reduction through superoxide anion from ferric ion bind to cationic side of amino acid on protein molecule, in which one amino acid is lys. This bound metal react with hydrogen peroxide and form hydroxyl radical, which help in production of carbonyl radical. This radical in turn cleaves the polypeptide chain. Oxidative markers are protein carbonyl groups [68]. |
| |
| Lipid Peroxidation by ROS |
| |
| Oxidative stress causes damage to cellular macromolecules such as nucleic acids, proteins, and lipids. Among these targets, the peroxidation of lipids is particularly more damaging because the formation of lipid peroxidation products leads to a facile propagation of free radical reactions. Abstraction of a hydrogen atom from the Poly Unsaturated Fatty Acid (PUFA) moiety of membrane phospholipids initiates the process of lipid peroxidation. Alkyl radicals are formed which are ultimately rearranged to form conjugated diens, and stimulates the autocatalytic lipid peroxidation cascades. ROS directly attacks on phospholipid hydroperoxides and fatty acid hydroperoxides. The fatty acid carbon chain spontaneously was cleaved during lipid peroxidation process and yield highly toxic pentane, ethane, α, β unsaturated fatty acids aldehydes. Malonyldialdehyde (MDA), 4-HNE (4-hydroxy -2- nonenal) are the potent aldehydic lipid peroxidation products of ω3 and ω6 PUFA. The accepted markers for oxidative stress are aldehydic secondary products MDA and 4-HNE [69]. |
| |
| Oxidation of nucleic Acid/DNA by ROS |
| |
| ROS break the DNA strands, forms DNA adduct which is characterized by deletion, mutation and causes genetic effects. Sugars and base moieties are degraded by ROS and causes oxidation of bases and cross linking to protein. 8-hydroxyguanine, hydroxyl methyl urea, urea, thymine, and saturated products which are the oxidation of bases; polyadenosine diphosphate ribose synthesis in nuclei resulting in extensive depletion of cellular NADH pools is the DNA oxidation products. DNA-MDA adducts is the most characteristic feature of nucleic acid oxidation. The measuring oxidative marker is 4-hydroxyl,2-deoxyguonosine, which is the oxidative marker of DNA oxidation [70]. |
| |
| Thus, oxidative stress is ultimately defined as "the imbalances in the equilibrium between pro-oxidants/antioxidants status in cellular systems, which results in damaging the cells." Cells have an intact oxidation process to detoxify the cellular environment from oxidants, and thus create the equilibrium in oxidants and antioxidants from aerobic metabolism. The formation of pro-oxidants is readily balanced by antioxidants by a similar rate. The failure in the neutralization events of oxidative status result in oxidative stress which leads to the cell death by lipid peroxidation, carbohydrates oxidation, protein oxidation and nucleic acid oxidation. |
| |
| Principal Defense System |
| |
| The principal defense system against ROS/RNS is Superoxide dismutase, Glutathione reductase, Catalase, thioredoxins, antioxidant nutrients, etc. |
| |
| Cellular antioxidant system |
| |
| Antioxidants are chemical compounds which contain monohydroxy/polyhydroxy phenol; they just work to slow down the lipid peroxidation [71]. These compounds have low activation energy to donate hydrogen atom and therefore, cannot initiate the second free radicals. The free radical electrons are stable and thus, slow down the oxidation. Cells contain many antioxidant systems to prevent injury. Prevention of excessive ROS and repair of cellular damage is essential for cell's life [72]. |
| |
| Mechanism of Antioxidants [73] |
| |
| R + AH →RH + A |
| |
| RO + AH → ROH + A |
| |
| ROO + AH → ROOH + A |
| |
| R+ A → RA |
| |
| RO + A → ROA |
| |
| ROO + A → ROOH |
| |
| Antioxidant + O2 → Oxidized antioxidant |
| |
| Classification of antioxidants: Antioxidants are classified into 3 categories, as described by Gutteridge and Halliwell [74] |
| |
| 1. Primary antioxidants: It is involved in the prevention of oxidants formation |
| |
| 2. Secondary antioxidants: exhibits scavenger of ROS. |
| |
| 3. Tertiary antioxidants: repairs the oxidized molecules through sources like dietary or consecutive antioxidants. |
| |
| Antioxidants may be enzymatic or non-enzymatic. Enzymatic system directly/indirectly contributes to defense against the ROS. Catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, thioredoxin exhibits biological value. |
| |
| The non-enzymatic antioxidants are actually the scavangers of ROS and RNS; these involve glutathione, vitamin E and C (inhibits oxidation of membrane lipid), uric acid is the scavenger of peroxynitrite in plasma, albumin, bilirubin, N-Acetylcysteine (NAC), melatonin which directly reacts with ROS and form disulfides [75]. |
| |
| Mode of action of antioxidants: Buettner [75] and Vertuani et al. [ 76] explained that antioxidants may act on 2 folds: |
| |
| 1- Primary or chain breaking antioxidants: break chain reaction and resulting radical is less reactive |
| |
| ROO + AH→ ROOH + A |
| |
| ROOH + A→ ROOA |
| |
| 2- Secondary or Preventive antioxidants |
| |
| They may act either by |
| |
| • Chelators/Deactivate metals |
| |
| • Scavenge singlet oxygen (highly toxic) |
| |
| • Remove ROS |
| |
| Factors affecting the efficiency of antioxidants: |
| |
| Activation energy of antioxidants |
| |
| Oxidation / reduction potential |
| |
| Solubility |
| |
| pH Stability |
| |
| Antioxidants are broadly divided into two; depends on its solubility [ 77]. |
| |
| 1) Hydrophilic antioxidants |
| |
| 2) Hydrophobic antioxidants |
| |
| Hydrophilic antioxidants: Antioxidants react with oxidants in the cell cytoplasm and the blood plasma. For example: Ascorbic acid, Glutathione and Uric acid. |
| |
| Hydrophobic antioxidants: Protect cell membranes from lipid peroxidation. For example: Carotenes, α-tocopherol and Ubiquinol. These compounds may be synthesized in the body or obtained from the diet [78]. |
| |
| Important antioxidants & endogenous antioxidant enzymes |
| |
| ANTIOXIDANT DEFENSIVE AGENTS |
| |
| ROS Scavengers |
ROS Protective Enzymes |
Sequestration Of Transition Metal Ions |
| Glutathione |
Superoxide dismutase |
Transferrin |
| Uric acid |
Catalase |
Ferritin |
| Ascorbic acid |
Glutathione peroxidas |
Metallothionein |
| Albumin |
Glutathione reductase |
Ceruloplasmin |
|
| |
| PUFA resists the biological membrane from lipid peroxidation, with the help of metal ions and prooxidants [73]. Lipid peroxidation is the introduction of a functional group containing two catenated oxygen atoms O-O into unsaturated fatty acids, in a free radical mediated chain reaction [79]. Free radicals are constantly being generated in the body through various mechanisms, and are also being removed by endogenous antioxidant defensive mechanisms that act either by scavenging free radicals, decomposing peroxides, and/or binding with pro-oxidant metal ions [80], so the balance between the pro-oxidant and antioxidants is very important for the cell survival. All antioxidants generally influence the redox status, thereby protecting cells against Reactive Oxygen Species (ROS) under certain circumstances, while promoting ROS generation in others [81]. The cell damage through free radical mediated reactions can be protected by enzymatic and nonenzymatic defence mechanisms. |
| |
| Antioxidant system contains exogenous antioxidant (dietary sources) and endogenous antioxidants. |
| |
| Endogenous antioxidants |
| |
| It can be categorized into primary antioxidants and secondary antioxidants. SOD, Catalase and Glutathione peroxidase are the primary antioxidant enzymes which inactivate the ROS into intermediates [ 82]. Besides the antioxidant enzymes, primary antioxidants are water soluble and lipid soluble. Ascorbate, glutathione, uric acid etc. are water soluble, and lipids soluble are tocopherols, ubiquinols and carotenoids, etc. Secondary antioxidant enzymes are Glutathione reductase, Glucose-6-Phosphate dehydrogenase, glutathione-S-transferase and ubiquinone work directly to detoxify ROS by decreasing the peroxides level and continuously supplying the NADPH and glutathione for primary antioxidant enzymes to maintain their proper functioning. Copper, iron, manganese, zinc, selenium enhances the antioxidant enzyme activities [76,83]. |
| |
| Exogenous antioxidants |
| |
| These are mainly derived from food and other dietary sources. Several herbs, spices, vitamins, foods, vegetables etc exhibits antioxidant activities. Therefore, antioxidants based drugs for the treatment of various pathological diseases have gained attraction in clinical as well as research areas. |
| |
| Flavonoids, isoflavones, flavones, anthocyanins, coumarins, lignans, catechins, isocatechins, epicatechin, etc found in natural foods are called phytochemicals. Numerous types of bioactive compounds are being used in clinical and preclinical trials from plant sources. Plant derived drugs medicinally useful as it contains terpenoid, alkaloids, glycosides, polyphenolics, steroids which exhibits great importance in research area [84,85]. |
| |
| Role of dietary nutrients in defensive mechanism |
| |
| Protein and amino acids are responsible for the synthesis of antioxidant enzymes. GSH and Carnosine are the small peptides, nitrogenous metabolites like creatine and uric acid are the direct scavengers of reactive metabolites [86]. Taurine and taurine chloramines effect the iNOS expression and iNOS synthesis in various cells. Dietary deficiency of protein shows hazardous effect in antioxidant system of cell. Arginine and tetrahydrobiopterin deficiency directly affect c NOS which implicates the superoxide production and ultimately, oxidative stress in cells/tissues. Insufficient protein intake effect the availibilty of Zinc (cofactor of SOD). Plasma concentration of albumin (zinc transporter) and metallothionein (zinc carrier) is decreased. This results in increase in the iron level in protein malabsorption, as seen in Kwashiorkor [87]. |
| |
| The oxidative stress is due to increase level of tissue iron in protein deficient patient, in which iron-binding protein are deficient including transferrin, lactoferrin and ferritin. This iron overload exhibits the cardiovascular injury. Similarly, high protein diet exhibit oxidative stress. Homocysteine elevation exhibits endothelial suproxide anion in vasculature, increases inducible and constitutive NOS synthesis, and stimulate ROS generation in polymorphonuclear leukocytes and monocytic cells [88,89]. |
| |
| Lipids |
| |
| High intake of polyunsaturated fatty acids is prone to ROS generation, which is neutralized by Vitamin C, E and carotenoids etc. High PUFA intake may increase the risk of cardiovascular diseases. High saturated fat diet increases the risk of iNOS activity in liver and colon. Fish oil contain ω-3 PUFA which is the inhibitor of ROS, iNOS expression and NOS synthesis, decreases the cardiovascular risk by reducing triacylglycerol production in plasma [90]. |
| |
| Vitamins |
| |
| Vitamins exhibit anti atherogenic and anti-inflammatory role in the neurons. Vitamin A inhibits iNOS in endothelial cells, vascular muscles cells, cardiac myocytes, mesengial cells. Vitamin D3, K2 and niacin inhibit iNOS activity in brain cells (macrophage, microglia, and astrocytes) [89-91]. Vitamin E inhibits the ROS generation and thus, prevents the membrane from lipid peroxidation [92]. Irradiation decreases the vitamin C and folate concentration, thus prone to ROS generation. Vitamin B12 and folic acid reduces radical-induced radiation damage and improves leukocytes counts, Vitamin C and Choline prevents DNA damage and hepatocellular carcinoma. One carbon unit metabolism exhibited by vitamin B12, folic acid and choline, and is an essential participant in methylation of DNA and protein. Vitamin B12, B6 and folate serves as a cofactor for the synthesis of cystathionine synthase and cystothionase (B6), methionine synthase (B12) and also act as a substrate for methionine synthase. These vitamins help to reduce cardiovascular diseases in humans and rats. Similarly, NADP, NADH, FAD, nicotinamide and riboflavin prevent cells from ROS generation. Thiamin is a cofactor of NADPH. NADPH and FAD are required for glutathione reductase, an antioxidant enzyme. NADPH is essential for catalase activity [93,94]. |
| |
| Micronutrients and Minerals |
| |
| Selenium is a cofactor of glutathione transferase enzyme and other selenoproteins. It has potential antioxidant acitivity and also anticarcinogenic activity, as well. Copper, zinc and manganese are the cofactors of superoxide dismutase enzyme. Deficiency of copper or zinc increases the cytochrome P450 activity in microsomes of liver and lungs, and thus enhances the ROS generation and iNOS expression [95]. |
| |
| Phytochemicals |
| |
| Phenolic and polyphenolic compounds possess antioxidant activities. These are the natural antioxidants present in grapes, berry crops, tea, herbs, nutmeg, tea, etc. All the herbs and plants contain natural antioxidants compounds including flavonoids, isoflavones, flavones, anthocyanins, coumarins, lignans, catechin, isocatechin, gallic acid, esculatin, etc. [96]. Many medicinal plants are considered to have antioxidants activities and contain high content of phenolics like gallic acids and other active constituents. Terminalia chebula, T. bellerica, T. muelleri, and Phyllanthus emblica, Hemidesmus indicus, Cichorium Intybus, Withania somnifera, Ocimum sanctum, Mangifera indica and Punica granatum are known to have potential antioxidant activities [97]. |
| |
| Cellular oxidative stress |
| |
| It is speculated from the previous researches that cell life and death is regulated by reactive oxygen species. Reactive oxygen species are the derivatives of cellular oxygen, constantly produced in our body. ROS includes superoxides, hydrogen peroxides, singlet oxygen and hydroxyl ions. Oxidative stress is also produced by Reactive Nitrogen Species (RNS), which includes nitrate, nitrite, nitric dioxide, nitric oxide and peroxynitrite. It is formed by the reaction of molecular oxygen to nitric oxide, as shown in enzymatic and non-enzymatic reactions that are involved in ROS generation [4]. |
| |
| REACTIVE OXYGEN SPECIES |
| Superoxide anion O2- |
| Hydroxyl ion OH- |
| Singlet oxygen O- |
| Peroxyl ROO- |
| Alkoxy RO- |
| Hydrogen peroxide H2O2 |
| Hypochlorite HOCl |
| REACTIVE NITROGEN SPECIES |
| Peroxynitrite ONOO- |
| Nitrogen dioxide NO2- |
| Nitrate/ nitrite NO3-/NO2- |
|
| |
| It is known that molecular oxygen play an important role in cell's life and death. Oxidative phosphorylation and electron transport chain in mitochondria of the cell processes the O2 to ATP, which is high energy phosphate bond formed by oxidation/ reduction reactions. The reduction of O2 molecules give rise to superoxide anion, which is converted to H2O2, hydrogen peroxide by superoxide dismutase. This H 2O2 when binds with metal Fe+2/Cu+2, the highly reactive hydroxyl radicals (OH) are formed [97]. |
| |
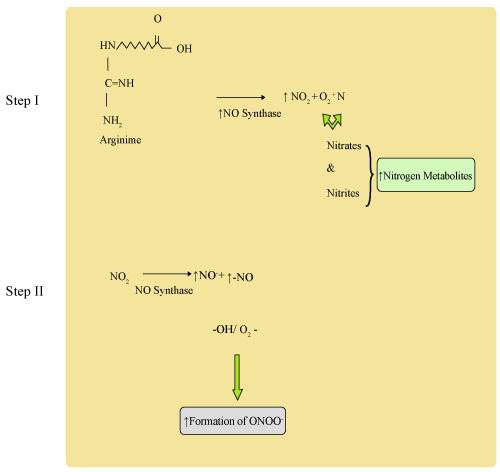
| Proposed synthesis of Nitric Oxide Synthase from Arginine: (Figure 1) |
| |
|
|
Figure 1: Synthesis of Nitric Oxide Synthase from Arginine. |
|
| |
| Reactive Metabolites (Figure 2) |
| |
|
|
Figure 2: Reactive Metabolites. |
|
| |
| Intracellular redox status |
| |
| It is observed from previous studies that glutathione oxidized and reduced state is in equilibrium, and is major cellular thiol. |
| |
2GSH + H2O2  GSSG + 2H2O GSSG + 2H2O |
| |
2GSH + ROOH  GSSG-ROH + H2O GSSG-ROH + H2O |
| |
| It is known that redox buffering capacity is regulated by thioredoxin and glutathione. Alteration in their equilibrium confers cell signaling mechanism. |
| |
| Conclusion |
| |
| It is found that generation of reactive metabolites play a central role in cell's life. These metabolites are continuously controlled by endogenous antioxidant enzyme systems, which may be enzymatic or non-enzymatic or transition metals. Thus, the balance is created between pro-oxidants and antioxidants. Reactive metabolites play important biological functions in cells in a controlled environment. The impairment of antioxidant status, either by exogenous or endogenous sources, may disturb the cellular redox balance and the pathological conditions would be the main characteristics and forms oxidative stress in cells or tissues. At this scenario, exogenous antioxidants which are of dietary source could be beneficial to level the redox balance status. It is worth important to note that increased antioxidants may interrupt the normal biological oxidant processes, and thus stop the reactive metabolites to perform their normal biological functions, which may activate the precancerous substances. |
| |
| |
| References |
| |
- Orient A, Donko A, Szabo A, Leto TL, Geiszt M (2007) Novel sources of reactive oxygen species in the human body. Nephrol Dial Transplant 22: 1281-1288.
- Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245-313.
- Fridovich I (1984) Overview: biological sources of O2-. Methods Enzymol 105: 59-61.
- Halliwell B, Gutteridge JMC (1989) Free Radicals in Biology and Medicine. (2nd ed.), Clarendon Press, Oxford, UK.
- Babior BM (2000) Phagocytes and oxidative stress. Am J Med 109: 33-44.
- Szabo C (2003) Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett 140: 105-112.
- Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181-189.
- Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222-230.
- Prata C, Maraldi T, Fiorentini D, Zambonin L, Hakim G, et al. (2006) Is Nox the source of ROS involved in Glut1 activity in B1647 cells? Acta Biologica Szegediensis 50: 79-82
- El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C (2005) Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 53: 199-206.
- Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, et al. (1999) Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension 33: 1353-1358.
- Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840-844.
- Vorbach C, Harrison R, Capecchi MR (2003) Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol 24: 512-517.
- Gomez-Cabrera MC, Pallardo FV, Sastre J, Vina J, Garcia-del-Moral L (2003) Allopurinol and markers of muscle damage among participants in the Tour de France. JAMA 289: 2503-2504.
- Judge AR, Dodd SL (2004) Xanthine oxidase and activated neutrophils cause oxidative damage to skeletal muscle after contractile claudication. Am J Physiol Heart Circ Physiol 286: H252-H256.
- Ivanov I, Saam J, Kuhn H, Holzhütter HG (2005) Dual role of oxygen during lipoxygenase reactions. FEBS J 272: 2523-2535.
- Omura T (1999) Forty years of cytochrome P450. Biochem Biophys Res Commun 266: 690-698.
- Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77: 598-625.
- Salganik RI (2001) The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 20: 464S-472S.
- Spooner R, Yilmaz O (2011) The Role of Reactive-Oxygen-Species in Microbial Persistence and Inflammation. Int J Mol Sci 12: 334-352.
- Kowaltowski AJ, Castilho RF, Vercesi AE (2001) Mitochondrial permeability transition and oxidative stress. FEBS Lett 495: 12-15.
- Ghosh MK, Mukhopadhyay M, Chatterjee IB (1997) NADPH-initiated cytochrome P450-dependent free iron-independent microsomal lipid peroxidation: specific prevention by ascorbic acid. Mol Cell Biochem 166: 35-44.
- Kam PC, Ferch NI (2000) Apoptosis: mechanisms and clinical implications. Anaesthesia 55: 1081-1093.
- Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy. Cancer 73: 2013-2026.
- Freeman BA, Crapo JD (1982) Biology of disease: free radicals and tissue injury. Lab Invest 47: 412-426.
- Tolbert NE, Essner E (1981) Microbodies: peroxisomes and glyoxysomes. J Cell Biol 91: 271s-283s.
- Parks DA, Williams TK, Beckman JS (1988) Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol 254: G768-G774.
- Marumo T, Schini-Kerth VB, Brandes RP, Busse R (1998) Glucocorticoids inhibit superoxide anion production and p22 phox mRNA expression in human aortic smooth muscle cells. Hypertension 32: 1083-1088.
- Fanburg BL, Lee SL (1997) A new role for an old molecule: serotonin as a mitogen. Am J Physiol 272: L795-L806.
- Peterhans E (1997) Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr 127: 962S-965S.
- Rott R, Klenk HD, Nagai Y, Tashiro M (1995) Influenza viruses, cell enzymes, and pathogenicity. Am J Respir Crit Care Med 152: S16-S19.
- Kido H, Sakai K, Kishino Y, Tashiro M (1993) Pulmonary surfactant is a potential endogenous inhibitor of proteolytic activation of Sendai virus and influenza A virus. FEBS Lett 322: 115-119.
- Chochola J, Strosberg AD, Stanislawski M (1995) Release of hydrogen peroxide from human T cell lines and normal lymphocytes co-infected with HIV-1 and mycoplasma. Free Radic Res 23: 197-212.
- Westendorp MO, Shatrov VA, Schulze-Osthoff K, Frank R, Kraft M, et al. (1995) HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. EMBO J 14: 546-554.
- Ames BN, Gold LS, Willett WC (1995) The causes and prevention of cancer. Proc Natl Acad Sci U S A 92: 5258-5265.
- Butz EA, Hostager BS, Southern PJ (1994) Macrophages in mice acutely infected with lymphocytic choriomeningitis virus are primed for nitric oxide synthesis. Microb Pathog 16: 283-295.
- Adler H, Frech B, Meier P, Jungi TW, Peterhans E (1994) Noncytopathic strains of bovine viral diarrhea virus prime bovine bone marrow-derived macrophages for enhanced generation of nitric oxide. Biochem Biophys Res Commun 202: 1562-1568.
- Hooper DC, Ohnishi ST, Kean R, Numagami Y, Dietzschold B, et al. (1995) Local nitric oxide production in viral and autoimmune diseases of the central nervous system. Proc Natl Acad Sci U S A 92: 5312-5316.
- Meier B, Cross AR, Hancock JT, Kaup FJ, Jones OT (1991) Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem J 275: 241-245.
- Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, et al. (1999) Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol 19: 1950-1960.
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296-299.
- Fratti RA, Ghannoum MA, Edwards JE Jr, Filler SG (1996) Gamma interferon protects endothelial cells from damage by Candida albicans by inhibiting endothelial cell phagocytosis. Infect Immun 64: 4714-4718.
- Krieger-Brauer HI, Kather H (1995) Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem J 307: 549-556.
- Thannickal VJ, Fanburg BL (1995) Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334-30338.
- Shibanuma M, Kuroki T, Nose K (1991) Release of H2O2 and phosphorylation of 30 kilodalton proteins as early responses of cell cycle-dependent inhibition of DNA synthesis by transforming growth factor beta 1. Cell Growth Differ 2: 583-591.
- Touyz RM, Schiffrin EL (1999) Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 34: 976-982.
- Lee SL, Wang WW, Lanzillo J, Gillis CN, Fanburg BL (1998) Superoxide scavenging effect of Ginkgo biloba extract on serotonin-induced mitogenesis. Biochem Pharmacol 56: 527-533.
- Holland JA, Pritchard KA, Pappolla MA, Wolin MS, Rogers NJ, et al. (1990) Bradykinin induces superoxide anion release from human endothelial cells. J Cell Physiol 143: 21-25.
- Holland JA, Meyer JW, Chang MM, O'Donnell RW, Johnson DK, et al. (1998) Thrombin stimulated reactive oxygen species production in cultured human endothelial cells. Endothelium 6: 113-121.
- Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH (1999) Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A 96: 2414-2419.
- Reddy PH (2011) Mitochondrial dysfunction and oxidative stress in Asthma; implications for Mitochondria-targeted antioxidant therapeutics. Pharmaceuticals (Basel) 4: 429-456.
- Vogiatzi G, Tousoulis D, Stefanadis C (2009) The role of oxidative stress in atherosclerosis. Hellenic J Cardiol 50: 402-409.
- Chen AF, Chen DD, Daiber A, Faraci FM, Li H, et al. (2012) Free radical biology of the cardiovascular system. Clin Sci (Lond) 123: 73-91.
- Koevary SB (2012) Selective toxicity of rose bengal to ovarian cancer cells in vitro. Int J Physiol Pathophysiol Pharmacol 4: 99-107.
- Dell'Anna ML, Ottaviani M, Bellei B, Albanesi V, Cossarizza A, et al. (2010) Membrane lipid defects are responsible for the generation of reactive oxygen species in peripheral blood mononuclear cells from vitiligo patients. J Cell Physiol 223: 187-193.
- Chen H, Zhao HX, Huang XF, Chen GW, Yang ZX, et al. (2012) Does high load of oxidants in human semen contribute to male factor infertility? Antioxid Redox Signal 16: 754-759.
- Singh RP, Sharad S, Kapur S (2004) Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. JIACM 5: 218-225.
- Hegde KR, Kovtun S, Varma SD (2007) Induction of ultraviolet cataracts in vitro: prevention by pyruvate. J Ocul Pharmacol Ther 23: 492-502.
- Zhu DM, Shi J, Liu S, Liu Y, Zheng D (2011) HIV infection enhances TRAIL-induced cell death in macrophage by down-regulating decoy receptor expression and generation of reactive oxygen species. PLoS One 6: e18291.
- Tkaczyk J, Vizek M (2007) Oxidative stress in the lung tissue--sources of reactive oxygen species and antioxidant defence. Prague Med Rep 108: 105-114.
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, et al. (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939-1945.
- Hekimi S, Guarente L (2003) Genetics and the specificity of the aging process. Science 299: 1351-1354.
- Olofsson P, Holmberg J, Tordsson J, Lu S, Akerström B, et al. (2003) Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet 33: 25-32.
- Semchyshyn HM, Lozinska LM, Miedzobrodzki J, Lushchak VI (2011) Fructose and glucose differentially affect aging and carbonyl/oxidative stress parameters in Saccharomyces cerevisiae cells. Carbohydr Res 346: 933-938.
- Garrison WM (1987) Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem Rev 87: 381-398.
- Stadtman ER, Oliver CN (1991) Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem 266: 2005-2008.
- Kaur S, Rana S, Singh HP, Batish DR, Kohli RK (2011) Citronellol disrupts membrane integrity by inducing free radical generation. Z Naturforsch C 66: 260-266.
- Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91: 179-194.
- Mattill HA (1947) Antioxidants. Annu Rev Biochem 16: 177-192.
- Moreau, Dufraisse (1922) Comptes Rendus of the Sessions and Memoirs of the Society of Biology 86: 321.
- German JB (1999) Food processing and lipid oxidation. Adv Exp Med Biol 459: 23-50.
- Gutteridge JMC, Halliwell B (1994) Antioxidants in Nutrition, Health and Disease. Oxford University Press, Oxford, UK.
- Bose KS, Vyas P, Singh M (2012) Plasma non-enzymatic antioxidants-vitamin C, E, beta-carotenes, reduced glutathione levels and total antioxidant activity in oral sub mucous fibrosis. Eur Rev Med Pharmacol Sci 16: 530-532.
- Singh RP, Khanna R, Kaw JL, Khanna SK, Das M (2003) Comparative effect of benzanthrone and 3-bromobenzanthrone on hepatic xenobiotic metabolism and anti-oxidative defense system in guinea pigs. Arch Toxicol 77: 94-99.
- Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300: 535-543.
- Vertuani S, Angusti A, Manfredini S (2004) The antioxidants and pro-antioxidants network: an overview. Curr Pharm Des 10: 1677-1694.
- Kibanova D, Nieto-Camacho A, Cervini-Silva J (2009) Lipid Peroxidation Induced by Expandable Clay Minerals. Environ. Sci. Technol 43: 7550-7555.
- Halliwell B (2000) The antioxidant paradox. Lancet 355: 1179-1180.
- Herbert V (1996) Prooxidant effects of antioxidant vitamins. Introduction. J Nutr 126: 1197S-1200S.
- Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18: 685-716.
- Vendemiale G, Grattagliano I, Altomare E (1999) An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res 29: 49-55.
- Shu YZ (1998) Recent natural products based drug development: a pharmaceutical industry perspective. J Nat Prod 61: 1053-1071.
- Gale CR (2001) Dietary antioxidants and dementia. Int Psychogeriatr 13: 259-262.
- Dempster WS, Sive AA, Rosseau S, Malan H, Heese HV (1995) Misplaced iron in kwashiorkor. Eur J Clin Nutr 49: 208-210.
- Wu G, Flynn NE, Flynn SP, Jolly CA, Davis PK (1999) Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J Nutr 129: 1347-1354.
- Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiss C, et al. (2002) Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest 109: 1241-1248.
- Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, et al. (2002) Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr 75: 767-772.
- Garcion E, Nataf S, Berod A, Darcy F, Brachet P (1997) 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res 45: 255-267.
- Gurujeyalakshmi G, Wang Y, Giri SN (2000) Suppression of bleomycin-induced nitric oxide production in mice by taurine and niacin. Nitric Oxide 4: 399-411.
- Sano M, Fujita H, Morita I, Uematsu H, Murota S (1999) Vitamin K2 (menatetrenone) induces iNOS in bovine vascular smooth muscle cells: no relationship between nitric oxide production and gamma-carboxylation. J Nutr Sci Vitaminol (Tokyo) 45: 711-723.
- Chow CK, Reddy K, Tappel Al (1969) Effect of dietary vitamin E on the activity of glutathione peroxidase in vitro and in vivo studies. J Clin Invest 48:1957.
- Yunzhong F, Yefu L, Weiqun C (1983) The effect of vitamin B_(12) and folic acid on radiation damage 3. Nitrogen metabolism. Acta Nutrimenta Sinica.
- Yunzhong F, Ycfu L, Bin H, Shafei H, Weiqun C, et al. (1984) Studies on the effect of vitamin B_(12) and folic acid on radiation damage4. Body weight, leucocyte counts and mortality of rats. Acta Nutrmenta Sinica.
- Hammermueller JD, Bray TM, Bettger WJ (1987) Effect of zinc and copper deficiency on microsomal NADPH-dependent active oxygen generation in rat lung and liver. J Nutr 117: 894-901.
- Youdim KA, Shukitt-Hale B, MacKinnon S, Kalt W, Joseph JA (2000) Polyphenolics enhance red blood cell resistance to oxidative stress: in vitro and in vivo. Biochim Biophys Acta 1523: 117-122.
- Aqil F, Ahmad I, Mehmood Z (2006) Antioxidant and Free Radical Scavenging Properties of Twelve Traditionally Used Indian Medicinal Plants. Turk J Biol 30: 177-183.
- Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, et al. (1999) Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med 27: 1208-1218.
|
| |
| |