| Research Article |
Open Access |
|
| Dugesh Karley* and Deepesh Gupta |
| Rajiv Gandhi Proudyogiki Vishwavidyalaya, Bhopal, India |
| *Corresponding author: |
Dugesh Karley
Rajiv Gandhi Proudyogiki Vishwavidyalaya
Bhopal, India
E-mail: dugeshkarley@gmail.com |
|
| |
| Received April 28, 2011; Published October 25, 2012 |
| |
| Citation: Karley D, Gupta D (2012) Analysis of Toll-like Receptor-4 Activation in Akt Phosphorylation and Cancer Related Characteristics in Renal Cells 1:405. doi:10.4172/scientificreports.405 |
| |
| Copyright: © 2012 Karley D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| The Toll-like receptors the name comes from fruit-fly receptor Toll, which was first discovered because of its important role in early development of fly and it was afterward identify as an important factor in innate immunity in adult flies. It recognizes a variety of pathogen-associated molecular patterns (PAMPs) through evolutionaryconserved motifs. Binding of PAMPs on TLRs classically leads to activation of inflammation-associated genes. TLR4-mediated signalling also leads to rapid activation of PI3K, one of the families of kinases involved in regulation of cell growth, apoptosis, and motility. LPS stimulates phosphorylation of Akt, a downstream target of PI3K. Akt phosphorylation has been shown in many type of cancer like lung, ovary, stomach and colon. In this study the relation between Akt phosphorylation and LPS treatment has been shown including the role of LPS in the cancer related activity. TLR4 activation in immune cells is an important factor but it did not show any major effect in increase of cancer related activity. |
| |
| Keywords |
| |
| Inflammation; Toll-like receptors; Akt; Renal cancer |
| |
| Introduction |
| |
| Cancer is a well known mortal disease that is caused due to the inability of some feverishly growing cells to die. More than 100 types of cancer have been acknowledged till date, classified on the basis of the type of the initially pretentious cell. Cancer usually forms tumors of cells that get in the way with the nervous, digestive and circulatory systems of the body and sometimes alter the functions by releasing unnecessary hormones. They are usually benign and are limited to particular area. The more treacherous is the other kind of tumors, i.e. the malignant ones. It occurs when a cancerous cell manages to move throughout the body through the circulatory system and destroy vigorous tissues in its path. Cancer usually develops due to mutation in the genes of the cell making it to forget to die. The cell goes on grow rapidly and does not die as it had to. Finally, it starts forming a mass. These mutations are caused by scores of different stimuli like x-rays, radiations, different chemicals etc [1]. |
| |
| In analogous from these factors a new finding suggests the involvement of TLRs (Toll like Receptors) in the progression of colorectal as well as in other kind of cancers [2]. TLR family includes family member TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10 and TLR11. Each member of this family is specific for individual microbial pattern; unfortunate activation or regulation of TLR signalling may lead to chronic inflammatory conditions and diseases [3]. While others have verified a role for TLR2 and TLR5, the natural ligands of which are peptidoglycan and bacterial flagellin respectively, we focused on TLR4 since the natural ligand is lipopolysaccharide (LPS), a bacterial component. Toll like receptor 4 is a protein that in humans is encoded by the TLR4 gene. TLR-4 is a tolllike receptor detects Lipopolysaccharide from Gram-negative bacteria and is thus vital in the initiation of the innate immune system. TLR4 has also been designated as CD284 (cluster of differentiation 284). Toll receptor was originally identified in Drosophila as an essential receptor for the establishment of the dorso-ventral pattern in developing embryos [4]. In signalling pathways via TLRs, a common adaptor, MyD88, was first characterized as a crucial component for the activation of innate immunity by all the TLRs. However, accumulating evidence indicates that individual TLRs show signs of specific responses. Furthermore, they have their own signalling molecules to manifest these specific responses. A mammalian homologue of Drosophila Toll receptor (now termed TLR4) was shown to induce the expression of genes involved in inflammatory responses [5]. |
| |
| Several finding suggest that TLR4 is responsible for detection of LPS. In one study the C3H/HeJ mouse strain is characterized by hyporesponsiveness to LPS [6]. Macrophages from C3H/HeJ mice fail to induce inflammatory cytokines, including TNF-a, IL-1, and IL-6. Their splenic B cells do not proliferate after exposure to LPS. The molecular basis of this hypo-responsiveness is unknown, but it may result from defective membrane signal transduction after LPS binding. The hyporesponsive phenotype of the C3H/HeJ mouse maps to the LPS locus (endotoxin unresponsive gene locus) on mouse chromosome4 [7] the corresponding chromosomal location in the human genome is chromosome 9q32–33; that is the same region to which human TLR4 has been mapped [8]. Recently genetic and physical mapping of the LPS locus has been identifies TLR4 as a contender gene in the important region [7]. Recognition of several microbial products is mediated by members of the Toll like receptor (TLR) family [9,10] Cytokine. |
| |
| Materials and Methods |
| |
| Renal cell lines |
| |
| Three cell lines human embryonic kidney (HEK 293), Rabbit Kidney Epithelial Cells (RK13) and normal rat kidney (NRK52E) cell lines were maintained for the experiments outlined (all from NCCS, Pune, India). HEK cells will be grown in a DMEM supplemented with 100 units/ml penicillin G sodium, 100 μg/ml streptomycin, 4mM L-glutamine, and 10% fetal bovine serum. For RK13 cell lines the base medium is Eagle's Minimum Essential Medium. To make the complete growth medium various components have been added to the base medium those are fetal bovine serum to a final concentration of 10%, and NRK-52E cells were grown with Dulbecco's modified Eagle's medium with 4 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate and 4.5 g/L glucose, 95%; fetal bovine serum. All three Cell lines were incubated in 5% of carbon dioxide (CO2) with of temperature 37°C [11-13]. |
| |
| LPS preparation and treatment |
| |
| LPS 055:B5 variant (Invitrogen) was prepared in phosphate-buffered saline (PBS) to 1 mg/ml stock, aliquoted and frozen at -20°C until ready for use. For experiments, cells were cultured into T-25 flask and allowed to adhere and grow until approximately 80% confluent. Cells were then washed three times in PBS to remove residual complete media and incubated overnight in serum-free media. The next day, cells were treated at different timepoints with LPS (final concentration=1 μg/ml). After treatment cells were washed with PBS to remove LPS and cell lysates were immediately prepared using a Triton-X-based Cell Lysis Buffer supplemented with protease inhibitor and 1 mM phenylmethyl sulfonyl fluoride (PMSF; Sigma-Aldrich) [14-16]. |
| |
| Western blot analysis (cDNA synthesis and reverse transcription PCR) |
| |
| First a cell lysis buffer was prepared as described previously [17]. This solution was aliquoted and stored at 20°C. Before use the following protease inhibitors were added: leupeptin (2 μg/ml), pepstatin-A (1 μg/ml), aprotinin (2 μg/ml) and phenylmethylsulphonyl fluoride (100 μg/ml). All cell lines they were grown to 70-90% confluence in a 75 cm2 flask, washed with ice cold PBS on ice and 1 ml of the lysis buffer was added to cells. They were incubated on ice with rocking for 5 min before transfer to a -80°C freezer. The flasks were removed from -80°C and defrosted on ice, cells were scraped from the flask and the contents transferred to 1.5 ml eppendorfs. These were vortexes for 30s then centrifuged at 13000 RCF at 4°C for 10 min. The remaining solution was aliquoted and stored at -80°C until use in western blot experiments. Blots were probed with phosphorylated and total Akt (Invitrogen), exposed to HRP-conjugated secondary antibodies (sigma), and imaged. |
| |
| Wound healing assay |
| |
| It is simple laboratory technique use to detect growth rate of cells. In this process cell has been cultured with 60% confluency (approx). A scratch has been made in centre of plate to make gap in plate. The time taken by the cells to fill that gap was calculated with and without treatment of LPS in the terms of confluency rate. |
| |
| Results |
| |
| Renal cell lines express TLR4 |
| |
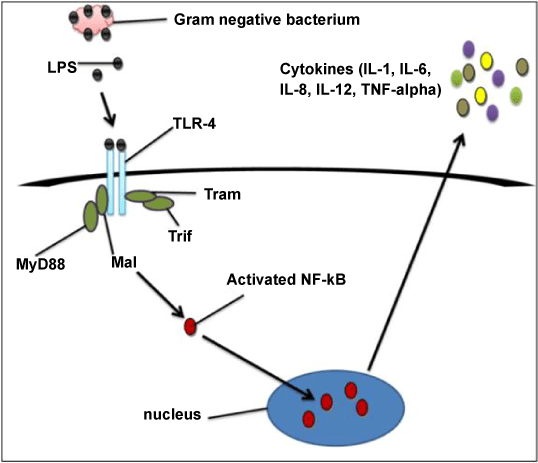
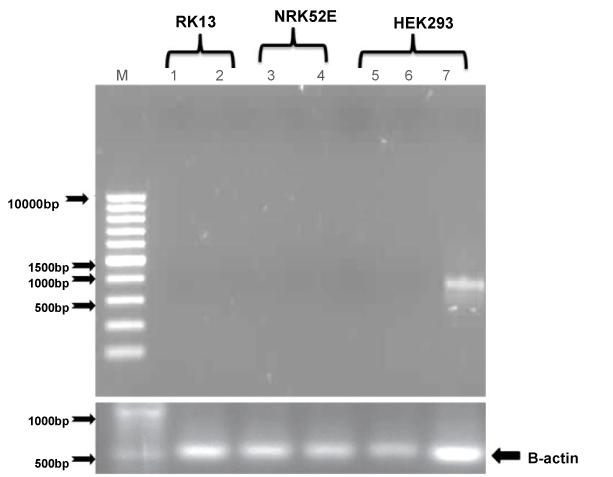
| After treatment of all three cell lines with LPS, TLR4 expression studies were conducted in HEK-293, RK-13 and NRK-52E renal cell lines by using PCR. HEK293 showed slightly less expression whereas RK-13 and NRK52E cells did not show any expression. These results confirmed that TLR4 is not expressed in renal cells suggesting that TLR4 do not play any role in renal cell tumorigenesis. Colonal cells shows abundant expression of TLR4 in the finding of Doan et al. [2] and similarly High TLR4 expression was found in the majority of lung cancer specimens in study of Zhang et al. [18]. The controversial result of this experiment did not justify why these three cell lines shows no expression (except HEK293 which shows a negligible expression). LPS is a bacterial component which was responsible for activation of inflammatory response in Colon cancer and the colon is exposed to a high bacterial load but in the case of kidney the exposure with bacterial component was less and that was probable reason of less expression of TLR4 in all these three cells (Figure 1). |
| |
|
|
Figure 1: Signalling pathway of TLR4. |
|
| |
| Stimulation of TLR4 with LPS increased phosphorylated Akt |
| |
| |
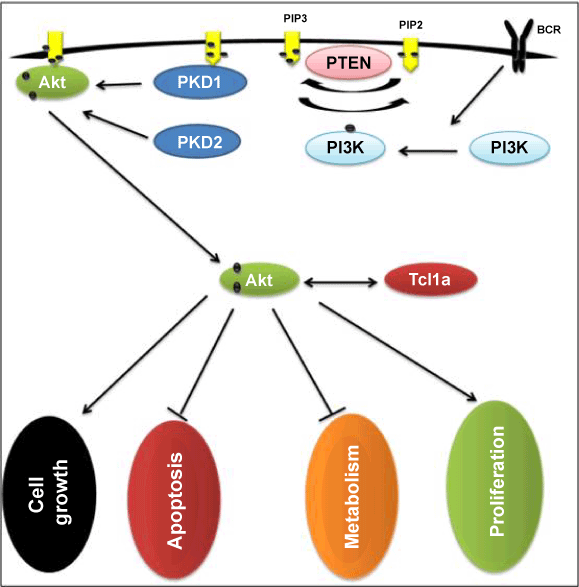
| A significant role for PI3K/Akt pathway in different kinds of cancerous cells progression and metastases was determined by different kind of studies [19-21]. The present study determined whether PI3K activation occurs after treating cells with LPS which acts through TLR4. HEK-293, RK-13 and NRK-52E were treated over a time course with LPS (1 μg/ml). Protein was extracted and western blots were assessed for expression of phosphorylated Akt. The maximum activation of phosphorylated Akt was observed at 60 min, 10 min and 30 min of LPS treatment in HEK293, RK-13 and NRK52E cell lines respectively. To control protein loading, the blots were stripped and reprobed for total Akt, These results demonstrated LPS-mediated phosphorylation of Akt. Result of PCR justify the no expression of TLR4 in renal cells in these three cell lines but result of western blot shows phosphorylation of Akt after treatment of LPS and these two are contentious. Studies conducted by Chen and Camps et al. [22,23] have implicated the PI3K/Akt pathway in inflammatory mediated disease. The result of western blot suggest LPS related activity of Akt phosphorylation of all three cell lines but as our previous result with PCR do not show any related activity with these cell lines. These two results did not match with each other which define that TLR4 did not participate in Akt phosphorylation in renal cells and the factor responsible for Akt phosphorylation is beyond the level of this study (Figures 2-4). |
| |
|
|
Figure 2: Role of PI3K/Akt phosphorylation pathway in various cell functions. |
|
| |
|
|
Figure 3: In order to evaluate whether renal cell lines express TLR4, reverse transcription PCR was performed as described in Materials and Methods; β-Actin primers were used as a control. |
|
| |
|
|
Figure 4: Activation of TLR4 by LPS activates Akt phosphorylation. Western blot analysis of renal cells treated at the indicated time points with LPS (1 μg/ml). Lysates were prepared as described in Materials and Methods to determine Akt phosphorylation. |
|
| |
| Stimulation TLR4 with LPS increased cancer related activity |
| |
| A scratch has been made on the surface of cultured cell lines HEK 293, RK13 and NRK52E. Time to fill the gap was calculated in terms of confluence rate of cell lines. Cells become confluence with respect to time as shown in figure 5. |
| |
|
|
Figure 5: Filling of gap with respect to time in increasing order from 1 to 4. |
|
| |
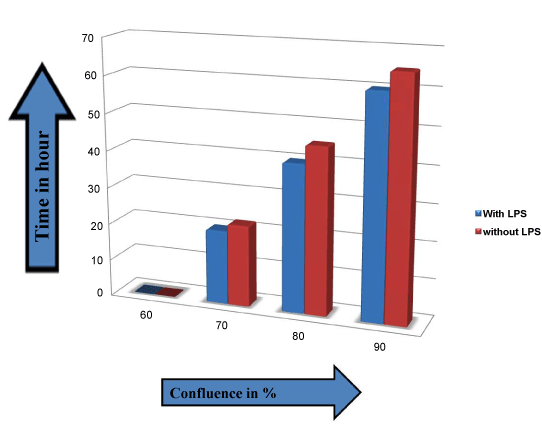
| On the basis of calculation of time for cell lines to become confluence, a graph has been drawn which defines the difference of confluence rate of cell lines with and without treatment of LPS (1 μg/ml). The graph has been made on the basis of time taken by HEK293 cell lines to become confluence, data with NKR52E and RK13 are similar (data not shown). As the graph shows that there is a slight variation in time taken to become confluence in both conditions which were with and without treatment of LPS. No strong relationship in between growth rate and LPS treatment of renal cells was observed as reported difference was negligible. Relationship of rate of confluence with time after treatment of LPS was not evaluated previously in this manner in any kind of studies. In figure 6 growth of cells has been shown in graphical manner in which X-axis and Y-axis shows confluence rate and time (in hour) respectively. The difference in confluence rate with respect to time in both of the condition (with and without treatment of LPS) was negligible. As TLR4 did not illustrate activation (Figure 6), thus LPS did not affect cancer related activity. Therefore, from the present investigation this clearly indicates that LPS is unable to activate TLR4 in renal cells. |
| |
|
|
Figure 6: Comparison of HEK293 confluence rate with and without treatment of LPS with respect to time. Here Y-axis is for time interval and X-axis is for confluence rate. |
|
| |
| Discussion and Conclusion |
| |
| There is a clear link between inflammation and the development of cancer in a multitude of organs. For example, chronic inflammatory bowel disease is associated with increased incidence of CRC compared with the normal population [24,25]. This link between inflammation and cancer suggests that the mechanisms contributing to inflammation may also be critical for tumor formation. In our current study, we try to find out possible expression of TLR4 in Renal cell lines of human, rabbit and rat. According to our finding TLR4 expression was noted in renal cell lines. The same kind of study was done in various kind of cancer those suggest possible role of inflammation in cancer development but here our result show no expression. As LPS is natural ligand for TLR4 activation there result is little bit controversial as compare to other studies similar to this but we know that LPS is natural component of bacteria those are responsible for various contamination but as we know kidney do not face any direct contact with bacterial infection so our finding is positively counterpart with the logic. These findings suggest that TLR4 is possibly did not participate in renal cell carcinoma like other cancers like colorectal tumorigenesis and other cancers. |
| |
| On the other hand when we talk about phosphorylation of Akt, the result of western blot suggest LPS related activity of Akt phosphorylation of all three cell lines but as our previous result with PCR do not show any related activity of TLR4 with these cell lines. These two results are contentious which suggest there are other factors responsible for Akt Phosphorylation and those factors are beyond the limit of this study. Third experiment suggest that the rate of confluency did not affected with treatment of LPS as shown in graph the deviation in time period is negligible. |
| |
| Relationship in between inflammation and cancer are not new as I have already discussed factors responsible for inflammation may take part in cancer development [26] but when we deliberate specific factor we did not get the piece of evidence, on other hand this results be evidence for development of renal cell carcinoma TLR4 did not play any direct role, alternatively Akt phosphorylation suggest there are certain other probable factor which may possibly alter the expression level. |
| |
| |
| References |
| |
- Cancer Treatment. Cited on 10-12-2011.
- Doan HQ, Bowen KA, Jackson LA, Evers BM (2009) Toll-like receptor 4 activation increases Akt phosphorylation in colon cancer cells. Anticancer Res 29: 2473-2478.
- Hazeki K, Nigorikawa K, Hazeki O (2007) Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull 30: 1617-1623.
- Hashimoto C, Hudson KL, Anderson KV (1988) The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52: 269-279.
- Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394-397.
- Morrison DC, Ryan JL (1979) Bacterial endotoxins and host immune responses. Adv Immunol 28: 293-450.
- Poltorak A, Smirnova I, He X, Liu MY, Van Huffel C, et al. (1998). Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis 24: 340-355.
- Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF, et al. (1998) A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A 95: 588-593.
- Kopp EB, Medzhitov R (1999) The Toll-receptor family and control of innate immunity. Curr Opin Immunol 11: 13-18.
- Means TK, Golenbock DT, Fenton MJ (2000) The biology of Toll-like receptors. Cytokine Growth Factor Rev 11: 219-232.
- Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A (1998) The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6). J Exp Med 187: 2097-2101.
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, et al. (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2: 253-258.
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, et al. (2001) Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 17: 615-675.
- Burgering BM, Coffer PJ (1995) Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376: 599-602.
- Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655-1657.
- Kean T, Miller JH, Skellern GG, Snodin D (2006) Acceptance criteria for levels of hydrazine in substances for pharmaceutical use and analytical methods for its determination. Pharmeur Sci Notes 2006: 23-33.
- Earl TM, Nicoud IB, Pierce JM, Wright JP, Majoras NE, et al. (2009) Silencing of TLR4 decreases liver tumor burden in a murine model of colorectal metastasis and hepatic steatosis. Ann Surg Oncol 16: 1043-1050.
- Zhang H, Chen X, Bollag WB, Bollag RJ, Sheehan DJ, et al. (2009) Lasp1 gene disruption is linked to enhanced cell migration and tumor formation. Physiol Genomics 38: 372-385.
- Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM (2006) Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg 243: 833-842.
- Sheng H, Shao J, Townsend CM Jr, Evers BM (2003) Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut 52: 1472-1478.
- Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, et al. (2008) Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci U S A 105: 20315-20320.
- Chen K, Iribarren P, Gong W, Wang JM (2005) The essential role of phosphoinositide 3-kinases (PI3Ks) in regulating pro-inflammatory responses and the progression of cancer. Cell Mol Immunol 2: 241-252.
- Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, et al. (2005) Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11: 936-943.
- Fracasso P, Assisi D, Stigliano V, Casale V (1999) Colorectal cancer complicating ulcerative colitis: an institutional series. J Exp Clin Cancer Res 18: 29-32.
- Itzkowitz SH (1997) Inflammatory bowel disease and cancer. Gastroenterol Clin North Am 26: 129-139.
- Karley D, Gupta D, Tiwari A (2011) Tlr4 and pi3k/akt pathway activation: new approach for cancer detection. IJBR 2: 334-345.
|
| |
| |