| Research Article |
Open Access |
|
| Guo-Qiang HOU, Xuan PAN, Cong-Shu LIAO and Dong-Feng LI* |
| School of Life Science, South China Normal University, China |
| *Corresponding author: |
Dong-Feng LI
School of Life Science
South China Normal University, China
Tel: +86 20 8521372
E-mail: dfliswx@126.com |
|
| |
| Received November 24, 2011; Published October 30, 2012 |
| |
| Citation: Guo-Qiang HOU, Xuan PAN, Cong-Shu LIAO and Dong-Feng LI (2012) SK Channels Modulate the Excitability and Firing Precision of Projection Neurons in the Robust Nucleus of the Arcopallium (RA) in Adult Male Zebra Finches. 1:403. doi:10.4172/scientificreports.403 |
| |
| Copyright: © 2012 Guo-Qiang HOU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| The firing activities of neurons encode the information of motor control. SK channels maintain the regularity and precision of firing by contributing to the after hyperpolarization (AHP) of action potential in mammals. But it is not clear that how SK channels regulate the output of vocal motor in songbirds. The premotor robust nucleus of the arcopallium (RA) in zebra finch is responsible for the output of song information. Temporal pattern of spike bursts in RA projection neurons is associated with the timing of acoustic features of birdsong. The present study showed that SK channel blockade decreased the amplitude of AHP and increased the firing rate in RA projection neurons, and caused a reduction in the regularity and precision of firing. RA projection neurons display regular action potentials spontaneously. SK channel blocker apamin made spontaneous firing more irregular, but had no effect on the spontaneous firing rate. In the absence of synaptic inputs, RA projection neurons still have spontaneous firing, and apamin had a similar effect on the firing activities, compared with apamin application alone. Therefore, SK channels contribute to maintaining the regularity of firing in RA projection neurons, suggesting that SK channels ensure the precision of song encoding. |
| |
| Keywords |
| |
| SK channels; Excitability; Firing precision; RA; Zebra finches |
| |
| Introduction |
| |
| Small-conductance Ca2+-activated K+ channels (SK channels) are insensitive to the change of membrane potential and widely expressed throughout the nervous system in mammals [1-4]. There are three subtypes (SK1, SK2 and SK3) of SK channels and all of them have been found to express in the mammalian brain [1]. These channels are activated by rises in intracellular calcium concentration and can be specifically blocked by the bee toxin apamin. SK channels contribute to the after hyperpolarization (AHP) following action potential [5]. In central nervous system, SK channels are important in controlling firing frequency of neurons, regulating dendritic excitability, synaptic transmission and synaptic plasticity [6-9]. In rat globus pallidus neurons, blockade of SK channels by apamin reduces the amplitude of AHP and increases the firing rate. Apamin also varies spike threshold and disrupts the precision of firing. These changes make it difficult for neurons to encode accurate information through spike time and firing rate [10]. 10-fold overexpression of SK2 subunits causes an approximately 4-fold increase in the apamin-sensitive current in CA1 neurons of transgenic mice, compared with wild-type littermates. EPSPs synaptically evoked from SK2 overexpressed CA1 neurons are increased in amplitude after SK channel blockade by 2-fold over that in wild-type CA1 neurons. In addition, SK2 overexpression decreases long-term potentiation (LTP) and weakens hippocampus- and amygdala-dependent learning, as compared to wild-type littermates [11]. Therefore, SK channels play a critical role in regulating synaptic transmission, synaptic plasticity and learning and memory. |
| |
| Songbirds learn their songs by imitation and auditory feedback [12]. They provide an excellent model for studying the neural modulation of a complex learned behavior [13]. The song system in songbird’s brain is composed of a discrete set of interconnected nuclei, which underlie song learning and production [14,15]. These nuclei with their connection can be divided into two pathways: the vocal motor pathway (VMP), which contributes to song production, and the anterior forebrain pathway (AFP), which is necessary for song learning and plasticity [16,17]. The robust nucleus of the arcopallium (RA) in the VMP receives input from the AFP as well, via the lateral magnocellular nucleus of the anterior nidopallium (LMAN) in the AFP [16,18,19]. Therefore, RA, as a premotor nucleus, occupies an important position in the song system, integrating information from both pathways. |
| |
| Two cell types have been identified in RA, projection neurons and interneurons. Both types receive excitatory glutamatergic input from HVC (used as a proper name) and LMAN [20-23]. They have different electrophysiological properties. The glutamatergic RA projection neurons display regular firings spontaneously, and can be induced to generate a time-dependent inward rectification by hyperpolarizing current injection. They have bigger input resistance and longer duration of AHP than interneurons. The identical depolarizing current injection induces a higher firing rate in GABAergic interneurons and less regular frequency than that in projection neurons [22,24]. |
| |
| RA projection neurons are responsible for transmitting song information to midbrain and brainstem vocal and respiratory structures [25]. Temporal pattern of spike bursts in RA projection neurons is associated with the timing of acoustic features of birdsong. Precise timing of individual spikes has a close relationship with stereotypic behavior, which suggests that the song is represented in RA by a temporal code [26,27]. Calcium-activated SK channels have been reported to regulate the excitability and firing precision of central neurons by contributing to the AHP of action potential in mammals. In zebra finch, apamin-sensitive neurons have been identified in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) [28], which suggests that SK channels may participate in the activities of neurons in the song system. Although it is known that RA, as an important premotor nucleus, controls the output of song information encoding, the involvement of SK channels is not clear. Therefore, in this study we tested whether and how SK channels modulate the excitability and firing precision of RA projection neurons in adult male zebra finches. |
| |
| Materials and Methods |
| |
| Slice preparation |
| |
| Adult male zebra finches (Taeniopygia guttata) were obtained from a commercial supplier and housed in group cages. All experiments were carried out in accordance with the university and national animal guidelines. Brain slices were prepared as previously described [29]. Briefly, birds were anesthetized with 10% chloral hydrate and then rapidly decapitated. Their brains were dissected into ice-cold, oxygenated (95%O2 and 5% CO2) slice solution, consisting of (in mM) 62.5 NaCl, 5 KCl, 28 NaHCO3, 10 glucose, 1.3 MgSO4•7H2O, 1.26 NaH2PO4•H2O, 248 sucrose [30,31]. Standard artificial cerebrospinal fluid (ACSF) consisted of (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.27 NaH2PO4•H2O, 1.2 MgSO4•7H2O, 2 CaCl2, 25 glucose, and was adjusted with sucrose to a final osmolarity of 350 mOsm [30]. Coronal brain slices (250-300 μm thick) containing RA were cut with a vibrating microtome (NVSLM1, WPI, USA) and collected in standard ACSF that had been warmed to 37°C. Slices were allowed to recover in the holding chamber for at least 1h and cool to room temperature before recordings were performed. |
| |
| Electrophysiological recordings |
| |
| Electrophysiological recordings were performed at room temperature (24-28°C) in oxygenated ACSF. RA and the surrounding tissues were distinguished under BX51WI microscope connected with a DIC-IR video camera (Olympus, Japan). Recording pipettes were fabricated from borosilicate glass (Sutter Instruments, USA) using a Flaming-Brown puller (model P-97, Sutter Instruments, USA) and were filled with the pipette solution containing (in mM): 120 K MeSO4, 5 NaCl, 10 HEPES, 2 EGTA, 2 ATP, 0.3 GTP (pH 7.2-7.4, and 340 mOsm). The recording pipettes, which had resistances ranging from 4-7 MΩ, were positioned using an integrated motorized control system (Sutter Instruments, USA). Whole-cell and cell attached recordings were obtained using standard techniques. Two cell types in RA were distinguished according to their distinct differences in electrophysiological properties. |
| |
| Drugs |
| |
| All agents were applied by changing the bath perfusate from standard ACSF to modified ACSF, in which the drugs were simply added. Unless indicated otherwise, all solutions were continuously bubbled with 95% O2 and 5% CO2. Apamin, APV, DNQX and picrotoxin were obtained from Sigma-Aldrich. Apamin is a noncompetitive selective antagonist of SK channels, and resisted washout of our slice preparation. |
| |
| Data analysis and statistics |
| |
| The Clampfit 9.2 (Axon Instruments, USA) and OriginPro 8 (OriginLab, Nothampton, MA, USA) were used for data analysis. Measurements of firing rate, coefficient of variation of interspike intervals (CV of ISIs), spike threshold and CV of spike threshold were conducted with Clampfit 9.2. The amplitude of AHP was quantified as the difference between spike threshold and the lowest point of AHP. All statistics were performed using the software Excel and OriginPro 8. Data were graphed using OriginPro 8. Two-tailed paired-sample t-test was used to evaluate the differences between the groups. Differences were considered to be significant when p<0.05, and very significant when p<0.01. All numerical data are presented as the mean±SEM. |
| |
| Results |
| |
| SK channel blockade reduces the amplitude of AHP and increases the firing rate of RA projection neurons |
| |
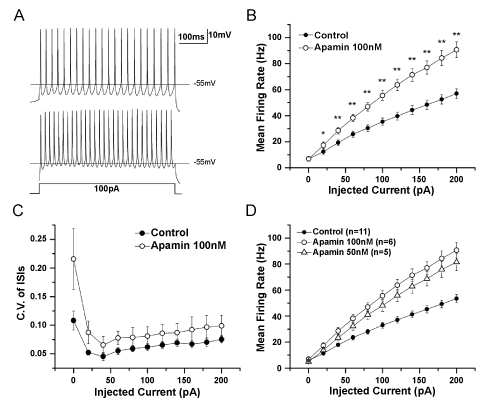
| To test whether or not SK channels contribute to the AHP and play a role in regulating excitatory activities of RA projection neurons in adult male zebra finches, we made plots of injected currents from 0 pA to 200 pA, 500ms and step 50 pA with 10s intervals, against mean action potential firing rate. An example response to a 100 pA current injection is shown in figure 1A. Application of 100nM apamin, a specific antagonist of SK channels, caused an increase in firing rate (Figure 1B). Apamin increased the mean firing rates induced by 150 pA and 200 pA current injection significantly (p<0.05; n=10). Apamin led to an increase in firing rate by reducing the amplitude of the AHP of action potential. The interspike interval was shortened because the AHP duration was decreased (Figure 1C). The average amplitude of AHP was reduced after 100 nM apamin application, in particular, significantly at the plots of 100 pA, 150 pA and 200 pA current injection (p<0.05; n=8) (Figure 1D). These results implied that SK channels contributed to the AHP of action potential and controlled the firing rate of RA projection neurons. |
| |
|
|
Figure 1: Blockade of SK channels reduces the amplitude of AHPs and increases the firing rate in RA projection neurons. |
|
| |
| On the other hand, to test whether or not SK channels also contribute to activities below the membrane potential in RA projection neurons, we compared the current-voltage relationships between control and 100nM apamin group by injecting hyperpolarizing current pulses. An example response to hyperpolarizing current injection is shown in figure 1E. Apamin had no influence on the slope of I-V curve by linear fit (Figure 1F), suggesting that SK channels was activated only by Ca2+ influx when the membrane potential was depolarized in RA projection neurons. |
| |
| |
| SK channel blockade disrupts firing regularity of RA projection neurons |
| |
| The AHP, as an essential part of action potential, provides time to allow ion channels to recover from inactivation on each cycle. The results of this study showed that the firing regularity was disrupted by blockade of SK channels (Figure 2). The spontaneous firings became even more irregular after 100nM apamin application (Figure 2A). Apamin significantly increased the coefficient of variation of interspike intervals (CV of ISIs) induced by ≥100 pA current injection (p<0.05; n=10) (Figure 2B). The CV of ISIs of spontaneous firings was bigger than that of evoked firings in control solutions. Apamin caused a small, but obvious increase in CV of ISIs of spontaneous firings, from 0.1385 ± 0.037 in control to 0.1643 ± 0.0282 in apamin, though not significant (p>0.05; n=10) (Figure 2B). |
| |
|
|
Figure 2: SK channels maintain the regularity of firings in RA projection neurons. |
|
| |
| The production of spontaneous action potential is more complex than firings evoked by injecting current. To test the effects of apamin on spontaneous firings for each neuron, we made plots of mean firing rate against the CV of ISIs. Spontaneous firings were recorded for at least 80s. The results indicated that the average CV of ISIs of spontaneous firings was increased by apamin (100nM), whereas the mean spontaneous firing rate was decreased, though both were not significant (p>0.05; n=7) (Figure 2D). In our experiments, we found that spontaneous firing rates were gradually reduced with the time in control conditions. It could not be avoided for whole-cell recording to break a little piece of cell membrane under the tip of electrode, which may weaken the cell and reduce the spontaneous firing rate. To confirm the effect of apamin on the spontaneous activities of RA projection neurons, we recorded spontaneous currents in cell attached voltage-clamp configuration. An example of spontaneous currents in cell attached recording is shown in Figure 2C. On average, apamin had no significant effect on the mean spontaneous firing rate, comparing 7.18 ± 0.87Hz in control with 7.3 ± 0.96 Hz in apamin (p>0.05; n=8). But the CV of ISIs was distinctly increased from 0.2946 ± 0.0495 in control to 0.4532 ± 0.0777 in apamin (p<0.05; n=8) (Figure 2E). These results suggest that SK channels enhanced the regularity of spontaneous firings in RA projection neurons. |
| |
| Apamin also caused a frequency-dependent depolarization in spike threshold and increased the variability of spike threshold in the whole-cell configuration (Figure 3). An example of the change in spike threshold variability is shown in figure 3A. The average spike threshold was significantly increased by 200 pA current injection (p<0.05; n=8) (Figure 3B). But spontaneous spike threshold had a small hyperpolarization, probably resulting from the decrease of spontaneous firing rate after 100nM apamin. The average CV of spontaneous spike threshold slightly increased, though not significantly (p>0.05; n=8). It was consistent with the change in the CV of ISIs of spontaneous firings after apamin application. Apamin also led to a frequency-dependent increase in the average CV of evoked spike threshold (Figure 3C). The variability of spike threshold determined the regularity of spike firings. These results indicate that SK channels probably ensure the precision of spike threshold and firing regularity by contributing to the AHP of action potential. |
| |
|
|
Figure 3: SK channels enhance the precision of spike threshold to ensure the regularity of firings. |
|
| |
| Effect of apamin on neuron without spontaneous synaptic inputs |
| |
| RA projection neurons receive glutamatergic inputs from HVC, LMAN and axon collaterals of other RA neurons, as well as GABAergic inputs from local interneurons. These synaptic inputs may influence the neuron’s response to apamin. To confirm the effect of SK channel blockade on RA projection neurons without spontaneous synaptic transmission, we made plots of depolarizing current from 0 pA to 200 pA, 500 ms and step 20 pA with 10s intervals, against mean firing rate or CV of ISIs in the presence of 50μM APV (a NMDA receptor blocker), 20μM DNQX (a non-NMDA receptor blocker) and 150μM picrotoxin (a GABA receptor blocker) (Figure 4). An example response to a 100pA current injection is shown in figure 4A. Apamin (100nM) caused an extremely significant increase in average firing rates when the sample was injected with ≥40 pA currents (p<0.01; n=6) (Figure 4B). Apamin also increased the CV of ISIs obviously, though they were not statistically significant (p>0.05; n=6) (Figure 4C). In addition, apamin is a noncompetitive selective antagonist of SK channels, and resisted washout of the slice preparation in this study. To make sure apamin on SK channels was efficiently working, we examined the effect of apamin in 50% concentration on the firing rate in the presence of 50μM APV, 20μM DNQX and 150μM picrotoxin. The firing rate in 50 nM apamin was smaller than that in 100 nM apamin (Figure 4D). These results demonstrate that postsynaptic SK channels may play a critical role in modulating the firing pattern and enhancing the precision of information encoding. |
| |
|
|
Figure 4: Blockade of SK channels increases the firing rate significantly and decreases the regularity of firings in the absence of synaptic inputs. |
|
| |
| Discussion |
| |
| In this study, the contribution of SK channels to modulating the excitability and firing precision of RA projection neurons in zebra finches was investigated. The results indicate that SK channels play a critical role in maintaining the regularity and precision of firing pattern in RA. These findings are similar to those in rat globus pallidus (GP) neurons [10]. Both RA projection neurons and rat GP neurons have rhythmic spontaneous action potentials, even in the absence of synaptic inputs. Though there is no evidence of their homology, they both play an important role in motor control. In RA, SK channels contribute to the AHP of action potential, and provide time to allow Na+ channels to recover from inactivation [8,32]. They also ensure the regularity and precision of firing. And RA projection neurons may encode information of birdsong through changes in spike time and firing rate. |
| |
| The present study shows that SK channel blockade caused a small reduction of the AHP amplitude of spontaneous action potential, while spontaneous firing rate was also reduced in whole-cell configuration. This may be due to lesion by rupturing a little piece of cell membrane. RA projection neurons fired action potentials autonomously, even without synaptic transmission. This may be induced by the activities of subthreshold-activated Na+ and K+ channels, as suggested in rat GP neurons. These ion channels bring the membrane potential to threshold on each cycle of oscillation [10,33]. Changes in intracellular constituents may disrupt the mechanism of membrane potential oscillation. Cell attached recording provides a way to record spontaneous firing activity without breaking the membrane [34]. The results from cell attached recording suggest that the SK channel blocker apamin significantly decreases the regularity of spontaneous currents, and slightly increases the firing rate. In addition, the results in the presence of APV、DNQX and picrotoxin show that the spontaneous firing rate had no significant change after apamin application in the whole-cell configuration. Thus, the rise in spontaneous firing rate by apamin may just offset the fall induced by the whole-cell model. Therefore, in RA, SK channels maintain the regularity of spontaneous firing, which is associated with the stability in spontaneous spike threshold. |
| |
| In the absence of synaptic inputs, SK channels still contribute to the AHP of action potential, suggesting that postsynaptic SK channels have a critical role in modulating the excitability of RA projection neuron. Recent studies in rat indicate that SK channels are located postsynaptically [7,34-37] . Blockade of SK channels has no influence on neurotransmitter release in hippocampal CA1 pyramidal neurons [6]. In songbirds, HVC has neurons that project to RA. Neighbouring RA-projecting neurons in HVC do not burst simultaneously, but burst one after another with regular intervals. Each burst may represent a different syllable in a motif. Every projection neuron in RA receives the time code of song from a population of neurons in HVC. The activities of individual RA projection neurons probably encode a whole song precisely [38]. Precision in behavior should be reflected in precision in neuronal control elements [27]. Thus, postsynaptic SK channels in RA may play a critical role so that the bird can produce a stereotyped song every time. |
| |
| Furthermore, it has been reported that Ca2+ influx through postsynaptic NMDA receptors can activate SK channels, especially in dendritic spines. SK channels repolarize the membrane potential and drive Mg2+ back to block the NMDA receptor channels. The Mg2+ lock limits NMDA receptor-dependent Ca2+ influx. Thus, SK channels and NMDA receptors form a Ca2+-mediated negative feedback loop in dendritic spines [6,9,35,39-41]. In excitatory synaptic transmission, SK channels, activated by Ca2+ influx through NMDA receptors, contribute to the excitatory postsynaptic potential (EPSP). Calcium influx through NMDA receptors is essential for multiple forms of synaptic plasticity, including long-term potentiation (LTP) and longterm depression (LTD) [42-45]. The SK channel blocker apamin causes an increase in EPSP, which requires NMDA receptor activity [7,35-37]. |
| |
| In songbirds, the nucleus RA receives two different synaptic inputs from HVC and LMAN. Both are excitatory glutamatergic, but have distinct postsynaptic properties. HVC-RA is largely mediated by AMPA receptor, whereas LMAN-RA is almost completely mediated by NMDA receptor [21,20,46]. LMAN is the last output nucleus of the anterior forebrain pathway which is necessary for song learning and plasticity [15-17]. It can be inferred that the NMDA receptor-SK channel negative feedback loop may have an effect on the complex procedure of song learning and plasticity in songbirds. Therefore, in addition to contributing to the precision of song encoding, SK channels may also coordinate with NMDA receptors to modulate synaptic transmission and plasticity in the song system of songbirds. |
| |
| Acknowledgements |
| |
| This work was supported by the National Natural Science Foundation of China (30970363, 31172092). The authors thank Dr.Zhang Ren and Dr.Feng Qili for critical reading and helpful comments on this paper. |
| |
| |
| References |
| |
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, et al. (1996) Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709-1714.
- Stocker M, Pedarzani P (2000) Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 15: 476-493.
- Sah P, Faber ES (2002) Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345-353.
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, et al. (2002) Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci 22: 9698-9707.
- Castle NA (1999) Recent advances in the biology of small conductance calcium-activated potassium channels [J]. Perspectives in Drug Discovery and Design 15/16: 131-154.
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, et al. (2002) Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 22: 10163-10171.
- Faber ES, Delaney AJ, Sah P (2005) SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala [J]. Nat Neurosci 8: 635-641.
- Faber ES, Sah P (2007) Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol 34: 1077-1083.
- Gu N, Hu H, Vervaeke K, Storm JF (2008) SK (KCa2) channels do not control somatic excitability in CA1 pyramidal neurons but can be activated by dendritic excitatory synapses and regulate their impact. J Neurophysiol 100: 2589-2604.
- Deister CA, Chan CS, Surmeier DJ, Wilson CJ (2009) Calcium-activated SK channels influence voltage-gated ion channels to determine the precision of firing in globus pallidus neurons. J Neurosci 29: 8452-8461.
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, et al. (2006) Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci 26: 1844-1853.
- Mooney R (2009) Neural mechanisms for learned birdsong. Learn Mem 16: 655-669.
- Mooney R (1992) Synaptic basis for developmental plasticity in a birdsong nucleus. J Neurosci 12: 2464-2477.
- [No authors listed] (2004) Behavioral Neurobiology of Birdsong. Proceedings of a conference. New York, USA, 12-14 December 2002. Ann N Y Acad Sci 1016: xiii-xvii, 1-788.
- Nottebohm F, Stokes TM, Leonard CM (1976) Central control of song in the canary, Serinus canarius. J Comp Neurol 165: 457-486.
- Nottebohm F (2005) The neural basis of birdsong. PLoS Biol 3: e164.
- Bottjer SW, Miesner EA, Arnold AP (1984) Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224: 901-903.
- Scharff C, Nottebohm F (1991) A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11: 2896-2913.
- Kao MH, Doupe AJ, Brainard MS (2005) Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433: 638-643.
- Sizemore M, Perkel DJ (2008) Noradrenergic and GABA B receptor activation differentially modulate inputs to the premotor nucleus RA in zebra finches. J Neurophysiol 100: 8-18.
- Mooney R (1992) Synaptic basis for developmental plasticity in a birdsong nucleus. J Neurosci 12: 2464-2477.
- Mooney R, Konishi M (1991) Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci USA 88: 4075-4079.
- Spiro JE, Dalva MB, Mooney R (1999) Long-range inhibition within the zebra finch song nucleus RA can coordinate the firing of multiple projection neurons. J Neurophysiol 81: 3007-3020.
- Stark LL, Perkel DJ (1999) Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch. J Neurosci 19: 9107-9116.
- Liao SQ, Hou GQ, Liu XL, Long C, Li DF (2011) Electrophysiological properties of neurons in robust nucleus of the arcopallium of adult male zebra finches [J]. Neurosci Lett 487: 234-239.
- Wild JM (1993) Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol 338: 225-241.
- Yu AC, Margoliash D (1996) Temporal hierarchical control of singing in birds. Science 273: 1871-1875.
- Chi Z, Margoliash D (2001) Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron 32: 899-910.
- Gale SD, Perkel DJ (2006) Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol 96: 2295-2306.
- Liao SQ, Liu XL, Li DF (2009) Whole-cell recording of the robust nucleus of the arcopallium neurons in the adult zebra finch [J]. Neural Regen Res 4: 623-628.
- Bottjer SW (2005) Silent synapses in a thalamo-cortical circuit necessary for song learning in zebra finches. J Neurophysiol 94: 3698-3707.
- Wang J, Hessler NA (2006) Coordination of presynaptic and postsynaptic maturation in a zebra finch forebrain motor control nucleus during song learning. Eur J Neurosci 24: 2859-2869.
- Patlak J (1991) Molecular kinetics of voltage-dependent Na+ channels. Physiol Rev 71: 1047-1080.
- Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ (2007) Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci 27: 13552-13566.
- Perkins KL (2006) Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods 154: 1-18.
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, et al. (2005) SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8: 642-649.
- Brosh I, Rosenblum K, Barkai E (2007) Learning-induced modulation of SK channels-mediated effect on synaptic transmission. Eur J Neurosci 26: 3253-3260.
- Faber ES (2010) Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. J Physiol 588: 1281-1292.
- Hahnloser RH, Kozhevnikov AA, Fee MS (2002) An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: 65-70.
- Bloodgood BL, Sabatini BL (2007) Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol 17: 345-351.
- Bloodgood BL, Sabatini BL (2007) Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron 53: 249-260.
- Higley MJ, Sabatini BL (2008) Calcium signaling in dendrites and spines: practical and functional considerations. Neuron 59: 902-913.
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F (1983) Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305: 719-721.
- Bröcher S, Artola A, Singer W (1992) Intracellular injection of Ca2+ chelators blocks induction of long-term depression in rat visual cortex. Proc Natl Acad Sci USA 89: 123-127.
- Malenka RC, Lancaster B, Zucker RS (1992) Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron 9: 121-128.
- Mulkey RM, Malenka RC (1992) Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9: 967-975.
- Kubota M, Saito N (1991) NMDA receptors participate differentially in two different synaptic inputs in neurons of the zebra finch robust nucleus of the archistriatum in vitro. Neurosci Lett 125: 107-109.
|
| |
| |