| Research Article |
Open Access |
|
| Changbhale SS1, Chitlange NR1, Gomase VS1*, Somnath Waghmare2 and Kale KV3 |
| 1Department of Bioinformatics, Jagdishprasad Jhabarmal Tibrewala University, Jhunjhunu Rajasthan, 333001, India |
| 2Departmentof Zoology, Nowrosjee Wadia College of Arts and Science, Pune, India |
| 3Professor and Head, Department of Computer Science and Information Technology, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India |
| *Corresponding authors: |
Gomase VS
Department of Bioinformatics
Jagdishprasad Jhabarmal Tibrewala University
Jhunjhunu Rajasthan, 333001, India
Tel: 91-9987770696
Email: gomase.viren@gmail.com |
|
| |
| Received July 31, 2012; Published August 30, 2012 |
| |
| Citation: Changbhale SS, Chitlange NR, Gomase VS, Somnath Waghmare, Kale KV (2012) Prediction of Major Histocompatibility Complex Binding Peptides and Epitopes from Neurotoxin of Mesobuthustamulus. 1:288. doi:10.4172/scientificreports.288 |
| |
| Copyright: © 2012 Changbhale SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Mesobuthustamulus is one of the toxic scorpions found throughout Asia. Mesobuthustamulus toxin is active against various mammals including human. Antigenic peptides of Mesobuthustamulus toxic protein are most suitable for subunit vaccine development because withsingle epitope, the immune response can be generated in large population. Analysis shows MHC class II bindingpeptides of antigenic protein from Mesobuthustamulus are important determinant for protection against several neurotoxins including snake venoms.In this assay we predicted the binding affinity of Mesobuthustamulus genome protein having 64 amino acids, which shows 56 nonamers. In this analysis, we found the High affinity TAP Transporter peptide regions as, 19- NNYCHALCT (score- 8.861), 34- GACDWWVPY (Score- 8.219). We also found the SVM based MHCII-IAb peptide regions, 45- VCWCEDLPT, 49- EDLPTPVPI, 5- YIADGDNCT, 37- DWWVPYGVV (optimal score is 0.558); MHCII-IAd peptide regions, 1- GEDGYIADG, 56- PIRGSGKCR, 17- TFNNYCHAL, 4- GYIADGDNC (optimal score is 0.543); MHCII-IAg7 peptide regions 4- GYIADGDNC, 18- FNNYCHALC, 42- YGVVCWCED,17- TFNNYCHAL (optimal score is 1.551); and MHCII- RT1.B peptide regions 17- TFNNYCHAL, 46- CWCEDLPTP, 19- NNYCHALCT, 13- TYICTFNNY, (optimal score is 0.560) which represented predicted binders from neurotoxin. Themethod integrates prediction of peptide MHC class I binding; proteasomal C terminal cleavage and TAP transportefficiency of the antigenic protein of Mesobuthustamulus. Thus a small fragment of antigen can induce immune response against whole antigen. This theme is implemented in designing subunit and synthetic peptide vaccines. |
| |
| Keywords |
| |
| Mesobuthustamulus; Antigenic peptides; Scorpion neurotoxins; MHC-Binders; SVM; Nonamers |
| |
| Introduction |
| |
| Scorpion neurotoxins have different physiological and pharmacological activities. Mesobuthustamulus is one of the toxic scorpions found throughout Asia. Mesobuthustamulus toxin is active against various mammals including human. Scorpion toxin contains of numerous hydrophobic polypeptides, several polypeptides inhibits activity of ion channels and disturb their functional properties. Mesobuthustamulus toxin binds to sodium channels and inhibits the inactivation of the activated channels, thereby blocking neuronal transmission [1,2]. |
| |
| MHC Class Binding Peptides |
| |
| The new paradigm in vaccine design is emerging, following essential discoveries in immunology and development of new MHC Class-I binding peptides prediction tools [3-7]. MHC molecules are cell surface glycoproteins, which take active part in host immune reactions. The involvement of MHC class-I in response to almost all antigens and the variable length of interacting peptides make the study of MHC Class I molecules very interesting. MHC molecules have been well characterized in terms of their role in immune reactions. They bind to some of the peptide fragments generated after proteolytic cleavage of antigen [8]. This binding acts like red flags for antigen specific and to generate immune response against the parent antigen. So a small fragment of antigen can induce immune response against whole antigen. Antigenic peptides are most suitable for subunit vaccine development because with single epitope, the immune response can be generated in large population. MHC peptide complexes will be translocated on the surface of antigen presenting cells (APCs). This theme is implemented in designing subunit and synthetic peptide vaccines [9-11]. One of the important problems in subunit vaccine design is to search antigenic regions in an antigen [12] that can stimulate T cells called T-cell epitopes. In literature, fortunately, a large amount of data about such peptides is available. Pastly and presently, a number of databases have been developed to provide comprehensive information related to T-cell epitopes [13-16]. |
| |
| Materials and Methods |
| |
| Protein Sequence Analysis |
| |
| The antigenic protein sequence of Mesobuthustamulus was analyzed to study the antigenicity (Saritha and Jain, 2007), solvent accessible regions and MHC class peptide binding, which allows potential drug targets to identify active sites against venom toxin. |
| |
| Prediction of Antigenicity |
| |
| Prediction of antigenicity program predicts those segments from within pathogenic protein that are likely to be antigenic by eliciting an antibody response. Antigenic epitopes is determined using the Gomase, (2007), Hopp and Woods, Welling, Parker, B-EpiPred Server and Kolaskar and Tongaonkar antigenicity methods [7,17-21]. |
| |
| Prediction of Protein Secondary Structure |
| |
| The important concepts in secondary structure prediction are identified as: residue conformational propensities, sequence edge effects, moments of hydrophobicity, positionof insertions and Deletions in aligned homologous sequence, moments of conservation, autocorrelation, residue ratios, secondary structure feedback effects, and filtering [22-24]. |
| |
| Finding the Location in Solvent Accessible Regions |
| |
| Finding the location in solvent accessible regions in protein, type of plot determines the hydrophobic and hydrophilic scales and it is utilized for prediction. This may be useful in predicting membranespanning domains, potential antigenic sites and regions that are likely exposed on the protein surface [24-32, Aboder in(1971), 33-45] |
| |
| Prediction of MHC Binding Peptide |
| |
| The MHC peptide binding is predicted using neural networkstrained on C terminals of known epitopes. In analysispredicted MHC/peptide binding is a log-transformed valuerelated to the IC50 values in nM units. MHC2Pred predicts peptide binders to MHCI and MHCII molecules from proteinsequences or sequence alignments using Position Specific Scoring Matrices (PSSMs). Support Vector Machine (SVM) based method for prediction of promiscuous MHC class II binding peptides. The average accuracy of SVMbased method for 42 alleles is ~80%. For development ofMHC binder, an elegant machine learning technique SVMhas been used. SVM has been trained on the binary input ofsingle amino acid sequence. In addition, we predicts thoseMHCI ligands whose C-terminal end is likely to be the result of proteosomal cleavage [46-49]. |
| |
| Result and Interpretation |
| |
| A antigenic sequence is 64 residues long as- |
| |
| GEDGYIADGDNCTYICTFNNYCHALCTDKKGDSGACDWW VPYGVVCWCEDLPTPVPIRGSGKCR |
| |
| Prediction of Antigenic Peptides |
| |
| |
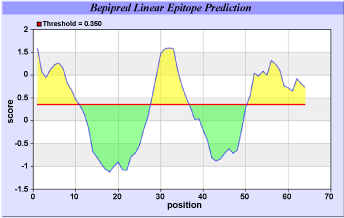
| In these methods we found the antigenic determinants by finding the area of greatest local hydrophilicity. The Hopp-Woods scale was designed to predict the locations of antigenic determinants in a protein, assuming that the antigenic determinants would be exposed on the surface of the protein and thus would be located in hydrophilic regions (Figure 1). Its values are derived from the transfer-free energies for amino acid side chains between ethanol and water. Welling antigenicity plot gives value as the log of the quotient between percentage in a sample of known antigenic regions and percentage in average proteins (Figure 2). We also study B-EpiPred Server, Parker, Kolaskar and Tongaonkar antigenicity methods and the predicted antigenic fragments can bind to MHC molecule is the first bottlenecks in vaccine design (Figure 3-5). |
| |
|
|
Figure 1: Hydrophobicity plot of Hopp and Woods (1981) of Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 2: Hydrophobicity plot of Welling et al. (1985) of Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 3: B-cell epitopes are the sites of molecules that are recognized by antibodies of the immune system of the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 4: Kolaskar and Tongaonkar antigenicity are the sites of molecules that are recognized by antibodies of the immune system for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 5: Hydrophobicity plot of HPLC / Parker et al. (1986) of Mesobuthustamulus neurotoxin. |
|
| |
| Secondary Alignment |
| |
| The Robson and Garnier method predicted the secondary structure of the Mesobuthustamulus neurotoxin. Each residue is assigned values for alpha helix, beta sheet, turns and coils using a window of 7 residues (Figure 6). Using these information parameters, the likelihood of a given residue assuming each of the four possible conformations alpha, beta, reverse turn, or coils calculated, and the conformation with the largest likelihood is assigned to the residue. |
| |
|
|
Figure 6: Secondary structure GOR plot of the Mesobuthustamulus neurotoxin. |
|
| |
| Solvent Accessible Regions |
| |
| Solvent accessible scales for delineating hydrophobic and hydrophilic characteristics of amino acids and scales are developed for predicting potential antigenic sites of globular proteins, which are likely to be rich in charged and polar residues. It was shown that a Mesobuthustamulus neurotoxin is hydrophobic in nature and contains segments of low complexity and high-predicted flexibility (Figure 7-27). |
| |
|
|
Figure 7: Hydrophobicity Sweet plot of OMH for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 8: Hydrophobicity plot of Kyte and Doolittle (1982) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 9: Hydrophobicity plot of Abraham and Leo (1987) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 10: Hydrophobicity plot of Bull and Breese (1974) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 11: Hydrophobicity plot of Guy (1985) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 12: Hydrophobicity plot of Miyazawa, et al (1985) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 13: Hydrophobicity plot of Roseman (1988) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 14: Hydrophobicity plot of Wolfenden et al. (1981) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 15: Hydrophobicity Wilson et al. (1981) plot of HPLC for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 16: Hydrophobicity Cowan (1990) plot of HPLC pH3.4 for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 17: Hydrophobicity plot of Rf mobility for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 18: Hydrophobicity plot of Chothia (1976) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 19: Hydrophobicity plot of Eisenberg et al. (1984) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 20: Hydrophobicity plot of Manavalan, et al (1978) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 21: Hydrophobicity plot of Black (1991) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 22: Hydrophobicity plot of Fauchere, et al (1983) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 23: Hydrophobicity plot of Janin (1979) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 24: Hydrophobicity plot of Rao and Argos (1986) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 25: Hydrophobicity plot of Tanford (1962) for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 26: Hydrophobicity Cowan (1990) plot of HPLC pH7.5 for the Mesobuthustamulus neurotoxin. |
|
| |
|
|
Figure 27: Hydrophobicity plot of Rose et al. (1985) for the Mesobuthustamulus neurotoxin. |
|
| |
| Prediction of MHC Binding Peptides |
| |
| These MHC binding peptides are sufficient for elicitingthe desired immune response. The prediction is based oncascade support vector machine, using sequence and propertiesof the amino acids. The correlation coefficient of 0.88was obtained by using jack-knife validation test. In this test, we found the MHCI and MHCII binding regions (Table1,2). MHC molecules are cell surface glycoproteins, whichtake active part in host immune reactions and involvementof MHC class-I and MHC II in response to almost all antigens.In this assay we predicted the binding affinity of Mesobuthustamulus neurotoxin having 64 amino acids, which shows differentnonamers (Table1,2). For development of MHC binder prediction method, an elegant machine learning techniquesupport vector machine (SVM) has been used. SVM hasbeen trained on the binary input of single amino acid sequence.In this assay we predicted the binding affinity of Mesobuthustamulus neurotoxin sequence (IsTX) having 64 aminoacids, which shows 235nonamers. Small peptide regions found as High affinity TAP Transporter peptide regions as, 19- NNYCHALCT (score- 8.861), 34- GACDWWVPY (Score- 8.219). We also found the SVM based MHCII-IAb peptide regions, 45- VCWCEDLPT, 49- EDLPTPVPI, 5- YIADGDNCT, 37- DWWVPYGVV (optimal score is 0.558); MHCII-IAd peptide regions, 1- GEDGYIADG, 56- PIRGSGKCR, 17- TFNNYCHAL, 4- GYIADGDNC (optimal score is 0.543); MHCII-IAg7 peptide regions 4- GYIADGDNC, 18- FNNYCHALC, 42- YGVVCWCED, 17- TFNNYCHAL (optimal score is 1.551); and MHCII- RT1.B peptide regions 17- TFNNYCHAL, 46- CWCEDLPTP, 19- NNYCHALCT, 13- TYICTFNNY, (optimal score is 0.560) which represented predicted binders from Mesobuthustamulus neurotoxin (Table 2). The predicted binding affinity is normalizedby the 1% fractil. The MHC peptide binding is predicted using neural networks trained on C terminals of known epitopes. In analysis predicted MHC/peptide binding is a log-transformed value related to the IC50 values in nM units. These MHC binding peptides are sufficient for eliciting the desired immune response. Predicted MHC binding regions in an antigen sequence and there are directly associated with immune reactions, in analysis we found the MHCI and MHCII binding region. |
| |
|
|
Table 1: TAP Peptide binders of Mesobuthustamulus neurotoxin. |
|
| |
|
|
Table 2: Peptide binders to MHCII molecules of Mesobuthustamulus neurotoxin. |
|
| |
| Discussion and Conclusion |
| |
| Gomase method (2007), B-EpiPred Server, Hopp and Woods, Welling, Parker, Kolaskar and Tongaonkar antigenicity scales were designed to predict the locations of antigenic determinants in Agrobacterium tumefaciens. Nucleocapsid shows beta sheets regions, which are high antigenic response than helical region of this peptide and shows highly antigenicity (Figure 2-4,28). We also found the Sweet hydrophobicity, Kyte & Doolittle hydrophobicity, Abraham & Leo , Bull & Breese hydrophobicity, Guy, Miyazawa hydrophobicity, Roseman hydrophobicity, Cowan HPLC pH7.5 hydrophobicity, Rose hydrophobicity, Eisenberg hydrophobicity, Manavalan hydrophobicity, Black hydrophobicity, Fauchere hydrophobicity, Janin hydrophobicity, Rao & Argos hydrophobicity, Wolfenden hydrophobicity, Wilson HPLC hydrophobicity, Cowan HPLC pH3.4, Tanford hydrophobicity, Rf mobility hydrophobicity and Chothia hydrophobicity scales, Theses scales are essentially a hydrophilic index, with a polar residues assigned negative values (Figure 6-26). In this assay we predicted the binding affinity of Mesobuthustamulus neurotoxin having 243 amino acids, which shows 235 nonamers. Small peptide regions found as, 19- NNYCHALCT (score- 8.861), 34- GACDWWVPY (Score- 8.219). Adducts of MHC and peptide complexes are the ligands for T cell receptors (TCR) (Table1). MHC molecules are cell surface glycoproteins, which take active part in host immune reactions and involvement of MHC class-I and MHC II in response to almost all antigens (Table2). Kolaskar and Tongaonkar antigenicity are the sites of molecules that are recognized by antibodies of the immune system for the Mesobuthustamulus neurotoxin, analysis shows epitopes present in the Mesobuthustamulus neurotoxin the desired immune respons. The region of maximal hydrophilicity is likely to be an antigenic site, having hydrophobic characteristics, because C- terminal regions of Mesobuthustamulus neurotoxin is solvent accessible and unstructured, antibodies against those regions are also likely to recognize the native protein. For the prediction of antigenic determinant site of Mesobuthustamulus neurotoxin, we got eighteen antigenic determinant sites in the sequence. The SVM based MHCII-IAb peptide regions, 45- VCWCEDLPT, 49- EDLPTPVPI, 5- YIADGDNCT, 37- DWWVPYGVV (optimal score is 0.558); MHCII-IAd peptide regions, 1- GEDGYIADG, 56- PIRGSGKCR, 17- TFNNYCHAL, 4- GYIADGDNC (optimal score is 0.543); MHCII-IAg7 peptide regions 4- GYIADGDNC, 18- FNNYCHALC, 42- YGVVCWCED, 17- TFNNYCHAL (optimal score is 1.551); and MHCII- RT1.B peptide regions 17- TFNNYCHAL, 46- CWCEDLPTP, 19- NNYCHALCT, 13- TYICTFNNY, (optimal score is 0.560) which represented predicted binders from Mesobuthustamulus neurotoxin (Table2).Which is a larger percentage of their atoms are directly involved in binding as compared to larger molecules. |
| |
|
|
Figure 28: Secondary Structure of a Neurotoxin from Mesobuthustamulus at 2.2a Resolution Showing hydrophobicity. |
|
| |
| Future Perspectives |
| |
| This method will be useful in cellular immunology, Vaccine design, immunodiagnostics, immunetherapeutics and molecular understanding of autoimmune susceptibility. Mesobuthustamulus neurotoxin sequence involved multiple antigenic components to direct and empower the immune system to protect the host from the neurotoxin. MHC molecules are cell surface proteins, which take active part in host immune reactions and involvement of MHC class in response to almost all antigens and it give effects on specific sites. Predicted MHC binding regions acts like red flags for antigen specific and generate immune response against the parent antigen. So a small fragment of antigen can induce immune response against whole antigen. The method integrates prediction of peptide MHC class binding; proteosomal C terminal cleavage and TAP transport efficiency. This theme is implemented in designing subunit and synthetic peptide vaccines. |
| |
| |
| References |
| |
- Badhe RV, Thomas AB, Deshpande AD, Salvi N, Waghmare A (2007) The Action of Red Scorpion (MesobuthusTamuluscoconsis, Pocock) Venom and Its Isolated Protein Fractions on Blood Sodium Levels. J Venom Anim Toxins Incl Trop Dis 13: 82-93.
- Lala K, Narayanan P (1994) Purification, N-terminal sequence and structural characterization of a toxic protein from the Indian scorpion venom Buthustamulus. Toxicon 32: 325-338.
- Bhasin M, Singh H, Raghava GPS (2003) MHCBN: acomprehensive database of MHC binding and non-bindingpeptides. Bioinformatics 19: 666-667.
- Singh H, Raghava GPS (2001) Pro Pred: prediction of HLA-DR binding sites. Bioinformatics 17: 1236-1237.
- Cui J, Han LY, Lin HH, Tang ZQ, Jiang L, et al. (2006) MHC-BPS: MHC-binder prediction server for identifyingpeptides of flexible lengths from sequence-derived physicochemical properties. Immunogenetics 58: 607-13.
- Ponomarenko JV, Bourne PE (2007) Antibody-protein interactions: benchmark datasets and prediction tools evaluation. BMC Struct Biol 7: 64.
- Gomase VS (2006) Prediction of Antigenic Epitopes of Neurotoxin Bmbktx1 from Mesobuthusmartensii. Curr Drug Discov Technol 3: 225-229.
- Kumar M, Gromiha MM, Raghava GP (2007) Identification of DNA-binding proteins using support vector machines and evolutionary profiles. BMC Bioinformatics 8: 463.
- Gomase VS, Kale KV, Chikhale NJ, Changbhale SS (2007) Prediction of MHC Binding Peptides andEpitopes from Alfalfa mosaic virus. Curr Drug Discov Technol 4: 117-125.
- Gomase VS, Kale KV (2008) Insilico prediction of epitopes: a new approach for fragment based viral peptide vaccines. Int J of Applied Computing 1: 39-46.
- Gomase VS, Kale KV, Shyamkumar K (2008) Prediction of MHC Binding Peptides and Epitopes from Groundnut Bud Necrosis Virus (GBNV). J of Proteomics & Bioinformatics 1: 188- 205.
- Schirle M, Weinschenk T, Stevanovic S (2001) Combining computer algorithms with experimental approaches permits the rapid and accurate identification of T cell epitopes from defined antigens. J Immunol Methods 257: 1-16.
- Rammensee H, Bachmann J, Emmerich NP, BachorOA, Stevanovic S (1999) SYFPEITHI: database forMHC ligands and peptide motifs. Immunogenetics 50: 213-9.
- Blythe MJ, Doytchinova IA, Flower DR (2002) JenPep:a database of quantitative functional peptide data forimmunology. Bioinformatics 18: 434-439.
- Schonbach C, Koh JL, Flower DR, Wong L, Brusic V (2002) FIMM, a database of functional molecular immunology: update. Nucleic Acids Res 30: 226-229.
- Korber TMB, Brander C, Haynes BF, Koup R, KuikenC, et al. (2001) Los Alamos National Laboratory, Theoretical Biology and Biophysics, Los Alamos, NewMexico. LA-UR 02-4663.
- Hopp TP, Woods KR (1981) Prediction of Protein Antigenic Determinants from Amino Acid Sequences Proc Natl Acad Sci U S A 78: 3824-3828.
- Welling GW, Weijer WJ, van der Zee R, Welling-Wester S (1985) Prediction of sequential antigenic regions inproteins. FEBS Lett 188: 215-218.
- Jens EPL, Ole L, Morten N (2006) Improved methodfor predicting linear B-cell epitopes. Immunome Res 2: 2.
- Parker JMR, Guo D, Hodges RS (1986) New Hydrophilicity Scale Derived from High-Performance Liquid Chromatography Peptide Retention Data: Correlation of Predicted Surface Residues with Antigenicity and Xray-Derived Accessible Sites. Biochemistry 25: 5425-5431.
- Kolaskar AS, Tongaonkar PC (1990) A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett 276: 172-174.
- Garnier J, Osguthorpe DJ, Robson B (1978) Analysis ofthe accuracy and implications of simple methods for predictingthe secondary structure of globular proteins. JMolBiol 120: 97-120.
- Robson B, Garnier J (1993) Protein structure prediction. Nature 361: 506.
- Gomase VS, Tandale JP, Patil SA, Kale KV (2006) “Automatic modeling of protein 3D structure Nucleoplasmin-like viral coat protein from Cucumber mosaic virus”. 14th International Conference on Advance Computing & Communication, Published by IEEE Computer Society in IEEE Xplore USA 614-615.
- Sweet RM, Eisenberg D (1983) Correlation of sequence hydrophobicities measures similarity in three-dimensional protein structure. J Mol Biol 171: 479-488.
- Kyte J, Doolittle RF (1982) A Simple Method for Displaying the Hydropathic Character of a Protein. J MolBiol 157: 105-132.
- Abraham DJ, Leo AJ (1987) Extension of the fragment method to calculate amino acid zwitterions and side chainpartition coefficients. Proteins 2: 130-152.
- Bull HB, Breese K (1974) Surface tension of aminoacid solutions: A hydrophobicity scale of the amino acidresidues. Arch Biochem Biophys 161: 665-670.
- Guy HR (1985) Amino acid side chain partition energiesand distributions of residues in soluble proteins. Biophys J 47: 61-70.
- Miyazawa S, Jernigen RL (1985) Estimation of Effective Interresidue Contact Energies from Protein Crystal Structures: Quasi-Chemical Approximation. Macromolecules 18: 534-552.
- Roseman MA (1988) Hydrophilicity of polar amino acid side-chains is markedly reduced by flanking peptide bonds. J Mol Biol 200: 513-522.
- Wolfenden RV, Andersson L, Cullis PM, Southgate CCF (1981) Affinities of amino-acid side-chains for solvent water. Biochemistry 20: 849-855.
- Wilson KJ, Honegger A, Stotzel RP, Hughes GJ (1981) The behaviour of peptides on reverse-phase supports during high-pressure liquid chromatography. Biochem J 199: 31-41.
- Chothia C (1976) The nature of accessible and buriedsurfaces in proteins. J Mol Biol 105: 1-14.
- Eisenberg D, Schwarz E, Komaromy M, Wall R (1984) Analysis of membrane and surface protein sequenceswith the hydrophobic moment plot. J Mol Biol 179: 125-142.
- Manavalan P, Ponnuswamy PK (1978) Hydrophobic character of amino acid residues in globular proteins. Nature 275: 673-674.
- Black SD, Mould DR (1991) Development of Hydrophobicity Parameters to Analyze Proteins Which BearPost- or Cotranslational Modifications. Anal Biochem 193: 72-82.
- Fauchere JL, Pliska VE (1983) Hydrophobic parameters of amino-acid side-chains from the partitioning of N-acetyl-amino-acid amide. Eur J Med Chem 18: 369-375.
- Janin J (1979) Surface and inside volumes in globular proteins. Nature 277: 491-492.
- Rao MJK, Argos P (1986) A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta 869: 197-214.
- Tanford C (1962) Hydrophobicity scale (Contribution of hydrophobic interactions to the stability of the globular conformation of proteins. J Am Chem Soc 84: 4240-4274.
- Cowan R, Whittaker RG (1990) Hydrophobicity indicesfor amino acid residues as determined by HPLC. PeptRes 3: 75-80.
- Rose GD, Geselowitz AR, Lesser GJ, Lee RH, Zehfus MH (1985) Hydrophobicity of amino acid residues in globular proteins. Science 229: 834-838.
- Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, et al. (1999) Protein identification and analysis tools in the ExPASy server. Methods Mol Biol 112: 531-552.
- Eisenberg D, Weiss RM, Terwilliger TC (1984) The hydrophobicmoment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A 81: 140-144.
- Brusic V, Rudy G, Honeyman G, Hammer J, Harrison L (1998) Prediction of MHC class II-binding peptides usingan evolutionary algorithm and artificial neural network.Bioinformatics 14: 121-130.
- Bhasin M, Raghava GP (2005) Pcleavage: an SVMbased method for prediction of constitutive proteasomeand immunoproteasome cleavage sites in antigenic sequences. Nucleic Acids Res 33: W202-207
- Gomase VS, Kale KV, Shyamkumar K, Shankar S (2008) Computer Aided Multi Parameter Antigen Design: Impact of Synthetic Peptide Vaccines from Soybean Mosaic Virus. ICETET, IEEE Computer Society in IEEE Xplore, Los Alamitos, California 629-634.
- Gomase VS, Kapoor RA, Ladak SS (2010) Eimeriaacervulina analysis for binding peptides using protein profiling for target validation. International Journal of Machine Intelligence 2: 01-08.
|
| |
| |