|
|
| Mohamed Anwar K Abdelhalim1* and Ahmed Mohamed El-Toni2 |
| 1Department of Physics and Astronomy, College of Science, King Saud University, B.O. Box 2454, Riyadh 11451, Saudi Arabia |
| 2King Abdullah Institute for Nanotechnology, King Saud University, B.O. Box 2454, Riyadh 11451, Saudi Arabia |
| *Corresponding authors: |
Mohamed Anwar K Abdelhalim
Department of Physics and Astronomy
College of Science, King Saud University
P.O. Box 2454, Riyadh 11451, Saudi Arabia
Email: abdelhalimmak@yahoo.com |
|
| |
| Received May 29, 2012; Published August 08, 2012 |
| |
| Citation: Abdelhalim MAK, El-Toni AM (2012) Optimization of a Citrate Method for Synthesizing GNPs of Controllable Particle Size and Monodispersity: Physical Studies. 1:278. doi:10.4172/scientificreports.278 |
| |
| Copyright: © 2012 Abdelhalim MAK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Background: Gold nanoparticles (GNPs) play an important role in drug delivery, imaging and cancer therapy. This study aimed to optimize a citrate method for synthesizing GNPs of controllable particle size and monodispersity through the variation of hydrogen tetrachloroaureate (HAuCl4) and citrate concentrations. |
| |
| Methods: The GNPs were prepared by citrate reduction of HAuCl4. An aqueous solution of HAuCl4 (100 mL: 0.5, 1, 4, 7, 10 and 15 mM) was refluxed for 5-10 min, warmed at (50 - 60°C) and aqueous solution of sodium citrate (10 mL: 100 mM) was added quickly. Reflux was continued for another 30 min until a deep red solution was observed. The synthesized GNPs were characterized with Transmission Electron Microscopy (TEM), UV-Vis spectrophotometer, Fluorescence (FL), and GNPs size distributions were measured by dynamic light scattering. |
| |
| Results: At 1 mM HAuCl4 and low citrate concentration 40 mM, different particles size and non-round GNPs were formed. The particle size ranged from18-28 nm; while at 100 mM citrate solution resulted in gold formation of more round GNPs. Further increase in citrate concentration to 150 mM increased the GNPs size up to 18 nm. Variation of HAuCl4 was also significant in tuning the particle size and polydispersity of GNPs. The increase of HAuCl4 from 0.5 to 15 mM, caused growth of particle size of GNPs from 12 nm to 60 nm. Increasing the gold concentration to 1 and 4 mM HAuCl4 would result in more round and monodisperse GNPs. With exceeding 4 mM HAuCl4 threshold, the monodispersity lost. |
| |
| Conclusions: At lower citrate concentration (40 mM), the coverage of citrate is incomplete and the aggregation process leads to form in-homogenous GNPs. On the other hand, at high citrate concentrations (100 and 150 mM), the behavior of citrate as a pH mediator explains the tendency of particle size to more rounded shape. |
| |
| Keywords |
| |
| Gold nanoparticles, Colloids; Hydrogen Tetrachloroaureate; Synthesis mechanism; Sodium hydroxide |
| |
| Introduction |
| |
| Gold in its bulk form has been considered as an inert, noble metal with some therapeutic and medicinal value. The GNPs have found widespread applications in life sciences and attracted significant research interest. The GNPs have been widely exploited for use in imaging [1], biological applications [2] and photothermal effects [3]. |
| |
| There are many physical and chemical processes for synthetizing GNPs. The extensively used procedures to synthesize GNPs in aqueous solutions are the reduction of ionic gold by adding the sodium citrate, sodium borohydride, ascorbic acid and tannic acid [4]. |
| |
| The synthesis of GNPs without reducing agents or templates has induced exciting interest for material and chemical researchers. The synthesis of extremely stable size-controlled GNPs without the use of any reducing agents in the presence of polyvinylpyrrolidone (PVP) has been reported [5,6]. |
| |
| Polymers and/or ionic surfactants are used as capping agents in aqueous-solution systems to prepare the nanomaterials. Cationic surfactants such as Hexadecyl trimethyl ammonium bromide (CTAB) are usually used in aqueous solution to synthesize anisotropic GNPs [7,8]. |
| |
| Abdelhalim, 2011 and 2012, Abdelhalim and Jarrar, 2011 and 2012 [5,9-16], have reported the side effects, bioaccumulation and toxicity induced by intraperitoneal administration of GNPs in several rat tissues in vivo. |
| |
| In order to understand and categorize the mechanisms of NPs toxicity, information is needed on the response of living systems to the presence of NPs of varying size, shape, surface and bulk chemical composition, but very little information on these aspects is presently available and this implies an urgent need to find novel simple methods for synthesizing GNPs. |
| |
| It is important to synthesize and optimize novel and simple high quality GNPs. In certain applications, it is also important to prepare GNPs of high concentrations [17]. Therefore, the goal of this work is to develop a new approach to synthesize GNPs of high quality and high concentration that are appropriate for preparation of GNPs conjugates. |
| |
| In this study, the GNPs will be synthesized and optimized by adding different citrate concentrations to hydrogen tetrachloroaureate (HAuCl4) of varying concentrations. The synthesized GNPs are characterized with TEM, UV-Vis spectroscopy and fluorescence; while the GNPs particle size distributions are characterized by the dynamic light scattering. |
| |
| Experimental |
| |
| Synthesizing gold nanoparticles (GNPs) |
| |
| The GNPs were prepared by citrate reduction of HAuCl4 [18]. An aqueous solution of HAuCl4 (100 mL: 0.5, 1, 4, 7, 10 and 15 mM) was refluxed for 5-10 min and warmed at (50 - 60°C) aqueous solution of sodium citrate (10 mL: 100 mM) was added. Reflux was continued for another 30 min until a deep red solution was observed. The solution was filtered through 0.45 μm Millipore syringe filters to remove any precipitates. The pH was adjusted to 7 using dilute NaOH solution, and the filtrate was stored at room temperature [18,19]. The solutions were induced dark red color with a maximum of absorbance centered at 520 nm. |
| |
| Characterization of gold nanoparticles (GNPs) |
| |
| The synthesized GNPs were characterized with transmission electron microscopy (TEM: JEOL JSM-2100F electron microscope operated at 200 kV), UV-Vis spectrophotometer (Spectro UVD3500: SPECTRO UV-VIS double beam research spectrophotometer variable bandwidth; Labomed, INC. USA) and fluorescence (FL) (FluoroMax-4 spectrofluorometer (JOBIAN YVON-SPEX, Instruments S. A., Inc., France). GNPs size distributions were measured by dynamic light scattering (Malvern Nanosizer ZS instrument). |
| |
| Results and Discussions |
| |
| Effect of sodium citrate concentration |
| |
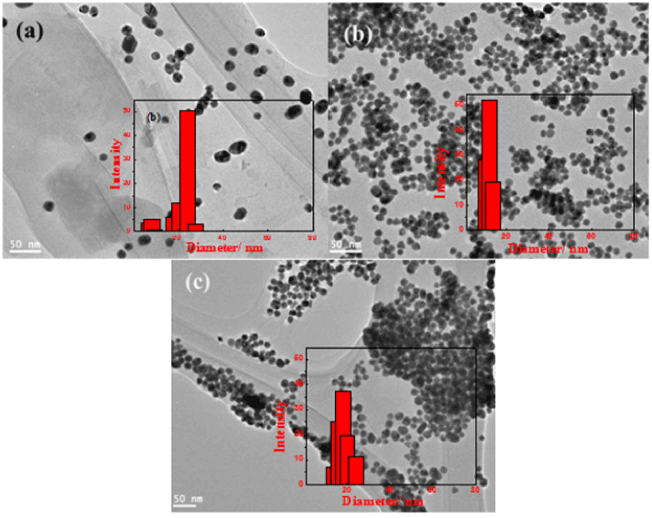
| TEM images in Figure 1a, 1b and 1c show the formation of GNPs at different sodium citrate concentrations. It is clear in this study that at 1 mM HAuCl4 and low citrate concentration 40 mM, different particles size and non-round GNPs were formed. The particle size was not homogenous and ranged from18-28 nm as shown in the particle size distribution (Figure 1a); while the addition of 100 mM citrate solution to 1 mM HAuCl4 resulted in gold formation of more round GNPs (Figure 1b). The particle size was reduced to around 12 nm with very narrow particle size distribution (Table 1). Further increase in citrate concentration to 150 mM increased the GNPs size up to 18 nm (Table 1). However, the homogeneity of the particle size distribution reduced as indicted from both TEM image (Figure 1c) and distribution profile (inset) [12]. |
| |
|
|
Figure 1: TEM images of gold nanoparticles at sodium citrate concentrations of (a) 40, (b) 100, and (c) 150 mM and 1 mM HAuCl4, (inset) their corresponding particle size distribution (Abdelhalim, 2012). |
|
| |
|
|
Table 1: Impact of different sodium citrate concentrations on the gold nanoparticle size |
|
| |
| In the preparation of GNPs by citrate reduction, citrate acts as both reducing and stabilizing agent. The adsorption of citrate on the particles as a stabilizer significantly affects the particle size band and the multirole of citrate increases the complexity of the particle preparation process. At lower citrate concentration (40 mM), the coverage of citrate is incomplete and the aggregation process leads to form in-homogenous GNPs. On the other hand, at high citrate concentrations (100 and 150 mM), the behavior of citrate as a pH mediator explains the tendency of particle size to more rounded shape [11]. |
| |
| |
| Citrate is known as a weak base which can change the solution pH to a certain extent with its concentration. The pH of the solution increases as the citrate concentration increases. Subsequently, the less reactive complexes [AuCl2(OH)2]− and [AuCl(OH)3]− are formed by the hydrolysis of AuCl4−, consequently, the nucleation rate becomes slower and the amount of Au precursor remaining in the solution will become larger. The final particle size is determined by the diffusional growth of Au precursor on the surface of Au nuclei. Because of using an excessive amount of citrate, the final particle size increases a little [11,20]. |
| |
| Effect of variation of HAuCl4 concentration |
| |
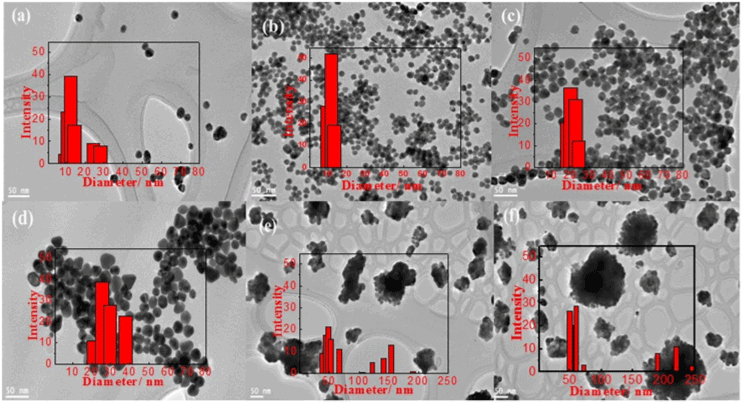
| Variation of HAuCl4 was also significant in tuning the particle size and polydispersity of GNPs. The increase of HAuCl4 from 0.5 to 15 mM (Figure 2a-2f), caused growth of particle size of GNPs from 12 nm to around 60 nm, respectively. At low concentration of HAuCl4 a non-round and non-humongous GNPs were obtained. Increasing the gold concentration to 1 and 4 mM HAuCl4 would result in more round and monodisperse GNPs. With exceeding 4 mM HAuCl4 threshold, monodispersity was lost. At 10 and 15 mM HAuCl4, a significant increase of particle size observed to reach 49 and 63 nm, respectively as shown in Table 2. Furthermore, both TEM images and particle size distributions show the formation of bigger GNPs that has particles size range from 150-300 nm. These large GNPs approaching micrometer sizes, formed from the agglomeration of smaller size of GNPs (20-50 nm). |
| |
|
|
Figure 2: TEM images of gold nanoparticles at HAuCl4 of (a) 0.5, (b)1, (c) 4, (d) 7, (e) 10, and (f) 15 and 100 mM sodium citrate, (inset) their corresponding particle size distribution. |
|
| |
|
|
Table 2: Impact of variations of HAuCl4 concentrations on the gold nanoparticle size |
|
| |
| In that regards, the particle size distribution tended to become broader with increasing the HAuCl4 concentration which means more polydisperse particles are formed. The increase of HAuCl4 concentration means that the corresponding amount of citrate, reducing agent, becomes lower which in turn causes the retardation of the nucleation step and makes the lower number of nuclei in the solution and thus increases the final particle size and polydispersity [21]. |
| |
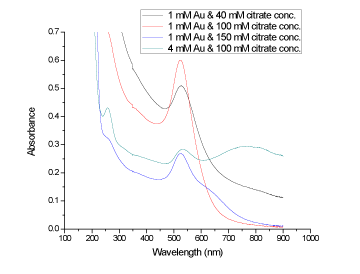
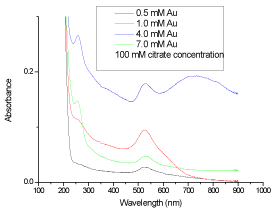
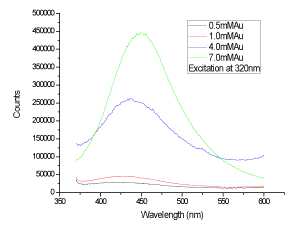
| The synthesized GNPs are further characterized by UV-Vis spectrophotometer at the room temperature. Abdelhalim, 2012 [11], has reported that when Au increases from 1 mM (at citrate concentration: 40, 100 and 150 mM) to 4 mM (at citrate concentration 100 mM)), the peak shifted from 527, 523 and 523 to 530 nm, respectively. A typical surface absorption band (λmax) at about 523, 527 and 530 nm for GNPs dependent on Au and citrate concentrations observed [11]. Because of the special shape and structure of GNPs, it often exhibited distinctive SPR peaks in the UV-Visible region, which was coincident with the plasmon band of the spherical GNPs [22], (Figure 3 and Figure4). Indeed, it is well established that at 100 mM citrate concentration and with increasing Au from 1 to 4 mM concentration, the band width increased from 110 to 190 accompanied with a decrease in the absorbance from 0.60 to 0.28 and an increase in particle size of GNPs from 12 to 18 nm [11]. At 100 mM citrate concentration and 4 mM Au concentration, 256, 530, 772 peaks were observed with 0.43, 0.28 and 0.29 absorbances and 50, 65 and 190 bandwidths, respectively [12]. |
| |
|
|
Figure 3: The absorbance of synthesized gold nanoparticles at different citrate and gold concentrations against the wavelength (nm). |
|
| |
|
|
Figure 4: The absorbance of synthesized gold nanoparticles at different gold concentrations against the wavelength (nm). |
|
| |
|
|
Figure 5: The fluorescence of different gold concentrations at excitation wavelength 320 nm. |
|
| |
| The bandwidth is inversely proportional to the particle size smaller than about 20 nm and the plasmon absorption maximum is red-shifts with increasing particle size [22]. |
| |
| This study suggests that additional synthesizing methods of GNPs are needed without using reducing agents; such as the addition of different sodium hydroxide (NaOH) concentrations to HAuCl4 and Hexadecyl trimethyl ammonium bromide (CTAB). In addition to the comparison that must be done between both of the 2 methods. Moreover, additional experiments are now performed taken in consideration the use of GNPs as diagnostic and therapeutic tool for treatment of the cancer disease. |
| |
| Conclusions |
| |
| This study aimed to optimize a citrate method for synthesizing GNPs of controllable particle size and monodispersity through the variation of hydrogen tetrachloroaureate (HAuCl4) and citrate concentrations. Optimization of precursor concentration indicates that the sodium citrate concentration is more effective in obtaining monodispersed and round GNPs. Furthermore, at high citrate concentrations (100 and 150 mM), the behavior of citrate as a pH mediator explains the tendency of particle size to more rounded shape. On the other hand, the HauCl4 concentration seems to provide monodsipersed GNPs at concentration range from 1-4 mM while the higher HAuCl4 concentrations 10 and 15 mM resulted in formation of bigger GNPs with bi-particle size distribution around 45-65 and 150-250 nm, respectively. |
| |
| Author's Contributions |
| |
| AMAK and EAM have analyzed data, interpreted and written the final draft of this manuscript. All materials used in synthesizing GNPs have been purchased from Sigma Chemicals; Sigma Companies Group Pty Ltd; Perth, Western Australia. AMAK has conceived the study and its design and obtained research grants for this study. The authors have read and approved the final manuscript. |
| |
| Acknowledgements |
| |
| The author is very grateful to the National Plan of Science and Technology (NPST). This research was financially supported by the National Science and Technology Innovation Plan (NSTIP), Research No. 09-NAN670-02, College of Science, King Saud University, Saudi Arabia. |
| |
| |
| References |
| |
- Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS, Rusling JF (2009) Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano 3: 585-594.
- Park SJ, Taton TA, Mirkin CA (2002) Array-based electrical detection of DNA with nanoparticle probes. Science 295: 1503-1506.
- Llorca J, Casanovas A, Dominguez M, Casanova I, Angurell I, et al. (2008) Plasma-activated core-shell gold nanoparticle films with enhanced catalytic properties. J Nanopart Res 10: 537-542.
- Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104: 293-346.
- Abdelhalim MAK (2012) Optimizing a novel method for synthesizing gold nanoparticles: Biophysical studies. J Cancer Sci Ther 4: 140-143.
- Zhou M, Wang B, Rozynek Z, Xie Z, Fossum JO, et al. (2009) Minute synthesis of extremely stable gold nanoparticles. Nanotechnology 20: 505606 .
- Min-Chen H, Liu RS, Tsai DP (2009) A versatile route to the controlled synthesis of gold nanostructures. Cryst Growth Des 9: 2079-2087.
- Sau TK, Murphy CJ (2004) Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 20: 6414-6420.
- Abdelhalim MAK (2011) Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure. Lipids Health Dis 10: 205.
- Abdelhalim MA (2011) Gold nanoparticles administration induces disarray of heart muscle, hemorrhagic, chronic inflammatory cells infiltrated by small lymphocytes, cytoplasmic vacuolization and congested and dilated blood vessels. Lipids Health Dis 10: 233.
- Abdelhalim MAK (2012) Exposure to gold nanoparticles produces pneumonia, fibrosis, chronic inflammatory cell infiltrates, congested and dilated blood vessels, and hemosiderin granule and emphysema foci. J Cancer SciTher 26 March, 4: 46-50.
- Abdelhalim MAK, Jarrar BM (2012) Histological alterations in the liver of rats induced by different gold nanoparticles size, dose and exposure duration. J Nanobiotechnology 10: 5.
- Abdelhalim MAK, Jarrar BM (2011) Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia. Lipids Health Dis 10: 133.
- Abdelhalim MAK, Jarrar BM (2011) The appearance of renal cells cytoplasmic degeneration and nuclear destruction might be an indication of GNPs toxicity. Lipids Health Dis 10: 147.
- Abdelhalim MAK, Jarrar BM (2011) Renal tissue alterations were size-dependent with smaller ones induced more effects and related with time exposure of gold nanoparticles. Lipids Health Dis 10: 163.
- Abdelhalim MA, Jarrar BM (2011) Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis10: 166.
- Kim JY, Nelson AL, Algon SA, Graves O, Sturla LM, et al. (2003) Medulloblastoma tumorigenesis diverges from cerebellar granule cell differentiation in patched heterozygous mice. Dev Biol 263: 50-66.
- Lévy R, Thanh NT, Doty RC, Hussain I, Nichols RJ, et al. (2004) Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Am Chem Soc 126: 10076-10084.
- Kogan MJ, Bastus NG, Amigo R, Grillo-Bosch D, Araya E, et al. (2006) Nanoparticle-mediated local and remote manipulation of protein aggregation. Nano Lett 6: 110-115.
- Ji X, Song X, Li J, Bai Y, Yang W, et al. (2007) Size control of gold nanocrystals in citrate reduction: the third role of citrate. J Am Chem Soc 129: 13939-13948.
- Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, et al. (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B 110: 15700-15707.
- Link S, El-Sayed MA (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 103: 8410-8426.
|
| |
| |