| Research Article |
Open Access |
|
| Chandan S, Umesha S1* and BalamuruganV2 |
| 1Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore-5700 06, Karnataka, India |
| 2Project Directorate on Animal Disease Monitoring and Surveillance (PD_ADMAS), ICAR, University of Agricultural Sciences, Hebbal, Bangalore- 560 024, Karnataka, India |
| *Corresponding authors: |
Umesha S
Department of Studies in Biotechnology
University of Mysore, Manasagangotri
Mysore-570006, Karnataka, India
Tel: +91-0821-2473983
Email: umeshgroup@yahoo.co.in, su@appbot.uni-mysore.ac.in |
|
| |
| Received June 21, 2012; Published August 10, 2012 |
| |
| Citation: Chandan S, Umesha S, Balamurugan V (2012) AntiLeptospiral, Antioxidant and DNA damaging properties of Eclipta alba and Phyllanthus amarus. 1: 231. doi:10.4172/scientificreports.231 |
| |
| Copyright: © 2012 Chandan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Leptospirosis is globally important disease affecting almost all mammals including human being. Several cases of this disease occur in India every year and studies have made to trace the epidemiology, pathology of this disease. It occurs in urban environments of industrialized and developing countries, as well as in rural regions worldwide. The disease is found mainly wherever human come in contact with the urine of infected animals and urine contaminated environment. The unsuccessful attempts to control this disease by chemo therapeutics prompted us to go for alternative control strategies. The present study comprises the use of traditional medicines to overcome the side effects caused by chemotherapeutics. In the present study an attempt has been made to use the herbal medicine to cure leptospirosis either by direct killing or by inhibiting the growth of Leptospira. Inhibition of growth has been realized as unique approach to stop the growth. A methonolic and aqueous extract of whole plants of Eclipta alba and Phyllanthus amarus assessed to determine the mechanism(s) of its antiLeptospiral and antioxidant activity. In this study the antioxidant activity and radical scavenging activity of methanolic and aqueous extracts of selected plant materials were evaluated against 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical and 2,2’azinobis- (3-ethylbenzthiazoline- 6-sulfonic acid) (ABTS) activity. DNA damaging studies on Leptospira were also investigated. Finally, extracts were tested in different concentration in their respective solvent systems by Tube Dilution Technique (TDT) and Micro Dilution Technique (MDT) tests. The antiLeptospiral activity of Eclipta alba and P. amarus were studied by both tube dilution and micro dilution techniques and the results showed better inhibitory action against various serogroups of Leptospira. DPPH free radical scavenging effect of extract was compared with standard antioxidant ascorbic acid. IC50 value was found 75μg/ml for extract and 45μg /ml for ascorbic acid and the methanol extracts of both plants had good activity and IC50 value was found 55μg/ml and 30μg/ml in ABTS assay. The plant extracts were also found to be very effective against leptospirosis. The possible use of E.alba and P.amarus extracts to control Leptospirosis are discussed in the present studies. |
| |
| Keywords |
| |
| AntiLeptospiral activity; Antioxidant activity; Eclipta alba; Phyllanthus amarus; DNA damaging studies; DPPH assay; ABTS assay. |
| |
| Introduction |
| |
| Leptospirosis is a bacterial zoonotic disease caused by Leptospira of spirochaetes that affects a wide range of animals including mammals, birds, amphibians reptiles and also human beings. Leptospirosis being recognized as the world's most common zoonoses. The infection is commonly transmitted to humans by allowing fresh water that has been contaminated by animal urine to come in contact with unhealed breaks in the skin, eyes or with the mucous membranes. Outside of tropical areas, leptospirosis cases have a relatively distinct seasonality with most of them occurring August-September/February-March months of the year [1,2]. |
| |
| The family Leptospiraceae contains two genera, Leptospira and Leptonema. Based on antigenic determinants, the genus Leptspira is classified into two species, Leptospira interrogans and Leptospira biflexa, the parasitic and saprophytic forms, respectively [3]. The classification of leptospires has been confusing and complicated. Two methods that can be used for classification of the genus Leptospira include serology and molecular biology based methods. Although over 269 serovars of Leptospira have been described, all members of the genus have similar morphology. Leptospira are spiral-shaped bacteria that are 6-20 μm long and 0.1 μm in diameter. |
| |
| Leptospiral organisms enter into the host most oftenly through cutaneous or mucosal abrasions. Oral transmission may occur when animals are feeding on contaminated pasture or feedstuffs, or drinking or standing in contaminated water [4]. Leptospirosis in cattle may appear as acute, subacute or chronic forms. The number of animals clinically affected in a cattle herd depends on the host-serovar adaptation, and the susceptibility of the herd to the infecting serovar. In all animals the incubation period is from 3 to 7 days. |
| |
| Humans are considered as "accidental hosts" and become infected by coming in contact with urine from infected animals. This is either through direct contact with urine or through contact with soil, water, or plants that have been contaminated by animal urine. Leptospira can survive for as long as six months outdoors under favorable conditions. |
| |
| An effective course of treating leptospirosis still remains unsolved problem. Leptospirosis usually response to treatment with the antibiotics, provided they are administered in enough doses early in the infection. Benzyl penicillin should be administered intravenously for upto 7 days in a daily dose of 6-8 mega units (3.6-4.8 g) but penicillin may cause a temporary exacerbation of the symptoms. Tetracyclin should be administered if there is evidence of renal failure. Continuous renal replacement therapy is supposed to be superior to conventional haemodialysis in leptospirosis. Vaccines are currently available in a very limited availability outside certain geographical areas and few licensed in developed countries. In recent years, there has been a global trend towards the use of natural phytochemicals present in natural resources, such as herbs, fruits and vegetables, as antioxidant agents. India is a vast country having wide diversity in eco climatic conditions, botanical and mineral wealth, flora and fauna. Inspite of modern developments medical facilities, about 80% of Indian population are depend on traditional system of medicines because of severe adverse reactions by western medicines [5]. To overcome the adverse reaction by the above drugs, herbal-based therapeutics had been used in treating leptospirosis [6]. |
| |
| Since the available therapies for curing Leptospira in modern medicine are very limited, potential alternatives from traditional medicine and their respective mechanisms of the action is worth investing. Accumulated evidence suggests that Reactive Oxygen Species (ROS) can be scavenged through chemoprevention utilizing natural antioxidant compounds present in foods and medicinal plants. India is blessed with enormous biodiversity resources, but plagued with several diseases, including those with ROS as the etiological factor. The antioxidant activity and radical scavenging activity of overproduction of ROS can damage cellular biomolecules like nucleic acid, protein, lipids, carbohydrates, proteins and enzymes, resulting in several diseases. Living system can overcome by these adverse effects of damage. However, sometimes these repair mechanisms fail to keep pace with such deleterious effects. Free radicals are responsible for aging and causing various human diseases. Antioxidant-based drug formulations are used for the prevention and treatment of complex diseases like atherosclerosis, stroke, diabetes, zoonatic disease like leptospirosis, Alzheimer’s disease and cancer [7]. |
| |
| DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method is an antioxidant assay based on electron-transfer that produces a violet solution [8]. This free radical, stable at room temperature, is reduced in the presence of an antioxidant molecule, giving rise to colorless solution. The use of the DPPH assay provides an easy and rapid way to evaluate antioxidants by spectrophotometry , so it can be useful to assess various products at a time. Due to the lack of evidence about which solution can be more effective as an antioxidant or even if there are other solutions with equal or more capacity to eliminate free radicals. |
| |
| The most commonly used antioxidant methods are 2,2’azinobis- (3-ethylbenzthiazoline- 6-sulfonic acid) (ABTS) activity and (2,2-diphenyl-1-picryl-hydrazyl-hydrate) (DPPH). Both of them are characterized by excellent reproducibility under certain assay conditions, but they also show significant differences in their response to antioxidants. The DPPH free radical does not require any special preparation, while the ABTS radical cation (ABTS_+) must be generated by enzymes or chemical reactions [9]. Another important difference is that ABTS_+ can be dissolved in aqueous and organic media, in which the antioxidant activity can be measured, due to the hydrophilic and lipophilic nature of the compounds in samples. In contrast, DPPH can only be dissolved in organic media, especially in ethanol, this being an important limitation when interpreting the role of hydrophilic antioxidants. Both radicals show similar bi-phase kinetic reactions with many antioxidants. Traditional medicine has remained as the most affordable and easily accessible source of treatment in the primary healthcare system of resource poor communities and the local therapy is the only means of medical treatment for such communities. However, since cultural systems are dynamic [10], the skills are fragile and easily forgettable as most of the indigenous knowledge transfer in the country is based on oral transmission [11]. The information about the role of medicinal plants against leptospirosis cases are scanty. To our knowledge, there are no published data regarding the traditional medicinal plant use in Mysore District, Karnataka, India to cure this disease. In view of the above, the current study was carried out to evaluate antiLeptospiral and antioxidant activity of selected medicinal plants. |
| |
| Eclipta alba (L.) belong to the family Asteraceae commonly called as Bhringaraj in India, that is of herbaceous type and spreads on ground or partly ascending with its stem and small leaves are succulent and are found mostly in tropical and subtropical regions where water logging condition is very high. Phyllanthus amarus (L.) belongs to the family Euphorbiaceae commonly called as Bahupatra in India. Traditionally, these plants are ayurvedic herb used in southern India for the treatment of liver related diseases [12]. |
| |
| Antioxidant that neutralize free radicals are key elements in the treatment of several diseases. The evaluation of anti-Leptospiral properties of herbal preparations requires understanding of its potential to scavenge reactive oxygen species and enhance antioxidant defense in the body. The primary objective of the present study is to evaluate the antioxidant property of Eclipta alba and Phyllanthus amarus based on its ability to scavenge free radicals. The other objective is to study the efficacy of the of Eclipta alba and Phyllanthus amarus against Leptispira species by evaluating the minimum inhibitory concentration (MIC) of the extracts by Tube Dilution Technique (TDT) and Micro Dilution Technique (MDT) tests. |
| |
| Materials and Methods |
| |
| Preparation of plant extracts |
| |
| The plants Eclipta alba and Phyllanthus amarus were collected from Sri Rangapattana, Karnataka, India during the months of May- September’ 2011. The voucher specimen was submitted to the Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore- 570 006, Karnataka, INDIA. Vocher No. UOMABBC 201 and 202 respectively. |
| |
| The fresh whole plant of E. alba and P. amarus were washed with distilled water, minced into small pieces, air dried at room temperature for about 10 days, ground into powder and extracted with various solvents viz., Hexane, Methanol, Chloroform and water (Both hot and cold) by a Soxhlet apparatus at 450C. The extracts were evaporated under reduced pressure below 500C through rotatory vacuum evaporator (BioLinx India, Mumbai, INDIA). The concentrated extracts were stored at 40C until further use. |
| |
| In vitro assay for antioxidant activity of Eclipta alba and Phyllanthus amarus |
| |
| Free radical-scavenging ability by the use of a stable DPPH radical |
| |
| The antioxidant activity of E. alba and P. amarus extracts were assessed in comparison to standard antioxidant ascorbic acid (Sigma, St. Louis, USA) on the basis of scavenging effect of the stable 2,2- diphenyl-1-picrylhydrazyl (DPPH) free radical according to established procedure [13]. Standard ascorbic acid solution (1ml) and different concentrations (25, 50, 75, 100, 125 and 150 μg/ml in methanol) of 1ml of E. alba solution were mixed with 3 ml of 0.002% DPPH solution. The mixtures were kept in dark for 30 min to measure the absorbance at 517 nm using UV-Visible Spectrophotometer (UV 3600, Shimadzu Corporation, Tokyo, Japan). Lower absorbance of the reaction mixture indicated higher free radical-scavenging activity[13]. The inhibitory effect of DPPH was calculated according to the following formula: |
| |
| Inhibition (%) of DPPH activity =A-B/A * 100 |
| |
| *Where A is Absorbance of control and B is Absorbance of Test. |
| |
| 50% of the radicals scavenging by test samples are regarded as IC50 value. Experiments were conducted in duplicates and were repeated for three times. |
| |
| Free radical-scavenging ability by the use of a stable ABTS radical cation |
| |
| The free radical-scavenging activity was determined by 2,2’azinobis- (3-ethylbenzthiazoline- 6-sulfonic acid (ABTS) radical cation decolorization assay [14]. lM ABTS was mixed with 2.45 mL of 1M potassium per sulphate and kept in dark at room temperature for 12-16 h before use. The radical was stable in this form for more than two days when stored in the dark at room temperature. Different concentrations of test samples (5μl) were added to 2.95 ml of ABTS·+ solution to give a final volume of 3 ml. The absorbance was recorded after incubation at room temperature for 30 min at 734 nm with Gallic acid as standard [15]. The percent inhibition was calculated from the following formula: |
| |
| Inhibition (%) of DPPH activity =A-B/A*100 |
| |
| Where A is Absorbance of control and B is Absorbance of Test. |
| |
| 50% of the radicals scavenging by test samples are regarded as IC50 value. Experiments were conducted in duplicates and were repeated for three times. |
| |
| Collection of Leptospira |
| |
| A total of eight Leptospira species namely, L. icterohaemorrhagiae, L. canicola, L. pamona, L. autumnalis, L. javanica, L. pyrogenes, L. australis and L. hardjo were procured from the repository of Project Directorate on Animal Disease Monitoring and Surveillance (PD_ADMAS), ICAR, University of Agricultural Sciences, Hebbal, Bangalore- 560 024, Karnataka, INDIA |
| |
| DNA preparation and Polymerase Chain Reaction for confirmation of Leptospira |
| |
| Template genomic DNA was prepared from pure culture of L. icterohaemorrhagiae, using QIAamp DNA minikit (QIAGEN, Hilden, Germany) and PCR was standardized to achieve the best sensitivity and tested a primer pair that enabled us to amplify a 600 bp segment of Leptospira rpoB. Primer sequence used in the present study; Forward-5’-CCTCATGGGTTCCAACATGCA-3’ and Reverse-5’- CGCATCCTCRAAGTTGTAWCCTT-3’ [16]. In order to achieve the best sensitivity for the PCR using primer rpoB, several combinations of annealing temperatures from 580C to 680C were tested. The following combinations of reaction mixture and conditions were chosen: PCR was performed in 25 μl reaction volume containing 10x Taq buffer with KCl (100mM Tris HCl, 50mM KCl, 0.8% Nonidet P40), 1.5mM MgCl2, 2.5mM of each dNTPs, 2U of Taq polymerase, 25pmoles of each primer and 0.25μl of DNA (40ng/μl) extract from L. icterohaemorrhagiae. All the reagents and chemicals were procured from Fermantas, USA. The reactions were carried out in a thermocycler (Eppendorf, Hamburg, Germany). Initially, PCR conditions were narrowed down by gradient PCR. The reaction started with an initial polymerase activating temperature of 940C for 3 min followed by 40 cycles of denaturing at 940C for 30 sec, annealing at 630C for 1 min at G ± 50C, elongation step at 720C for 1 min and a final elongation at 720C for 20 min. Finally annealing temperature was standardized to 550C for 1 min. The same procedure was adapted for isolation of DNA and also for the PCR with all the remaining seven species of Leptospira Species. Experiments were conducted in duplicates and repeated twice. |
| |
| Anti-Leptospiral activity of Eclipta alba and Phyllanthus amarus |
| |
| Effect of Eclipta alba and Phyllanthus amarus on Leptospira DNA cleavage |
| |
| DNA of L. icterohaemorrhagiae (1μg) which is isolated and confirmed by PCR assay was incubated with the presence or absence of Eclipta alba and Phyllanthus amarus extracts with different concentration viz., 25, 50,75,100, 125 and 150 μg/ml in methanol for 30 min at room temperature. After incubation, each sample was mixed with loading buffer and was analyzed by electrophoresis on agarose gel (1%), along untreated DNA as a normal control. The DNA bands were visualized and analyzed using a gel documentation system (BIO-RAD, Hercules, California, USA). Experiments were conducted in duplicates and were repeated for thrice. |
| |
| Tube Dilution Technique and Micro Dilution Technique |
| |
| The Tube Dilution Technique was carried out by adding various concentrations of plant extracts in the Ellinghausen, McCullough, Jensen and Harris (EMJH) liquid medium [17]. The EMJH (Difco) modified, semi-solid medium was prepared with the addition of 15% rabbit serum (Sigma, USA) and enriched with L-asparagin (3%), calcium chloride (1%), magnesium chloride (1%), pyruvate sodium (1%) and 0.2% agar with the addition of 5-fluoruracil (300 mg/L) named as the selective medium[18]. After sterility checking of the medium by placing in the room temperature for 48h, all the Leptospira species were inoculated with syringe filter. The tubes were incubated at room temperature for 7 days. The inhibition patterns for each species in different concentrations were observed under dark field microscope by placing a loopful of suspension on the clear glass slide and observed under different magnifications of the dark field microscope (Zeiss,USA). The total viable counts per microscopic fields were compared with the positive control samples and images were captured. Experiments were carried out in duplicates and repeated for three times. |
| |
| In Micro Dilution Technique, the viable Leptospiral species were taken in all the wells of micro titer plate and the different concentrations of plant extracts (5μL in each well) were mixed in all the wells. This culture extract mixture was well mixed and the microtitre plate was covered with clean aluminum foil and kept under dark condition for incubation at room temperature. After 30 min, the samples were spread on a slide by micro diluter and observed under dark field microscope (Zeiss, USA) to study the inhibitory activity on Leptospira species. Experiments were carried out in duplicates. |
| |
| In both these techniques percentage of Leptospiral inhibition was calculated and tabulated. |
| |
| Results and Discussion |
| |
| In vitro assay for antioxidant activity of Eclipta alba and Phyllanthus amarus |
| |
| DPPH and ABTS radical scavenging assays |
| |
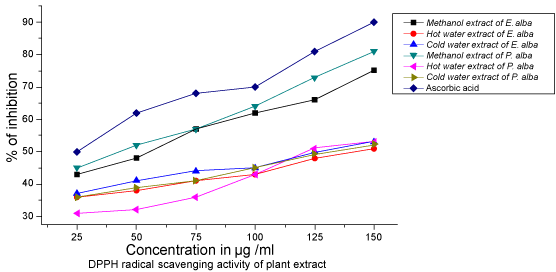
| The antioxidants react with the stable free radical DPPH (deep violet color) and convert it to 1, 1-diphenyl- 2-picryl hydrazine with decolouration. The scavenging effects of extract increased with their concentrations to similar extents. |
| |
| |
| The hexane and chloroform extracts of E.alba and P. amarus selected plants exhibited very negligible or no antioxidant activity. Methanol and aqueous extracts of these plants showed interesting and consisting results. Hence, methanol and aqueous extracts were selected to evaluate their antiLeptospiral and DNA damaging studies. Among the two extracts tested for the in vitro antioxidant activity using the DPPH method, the crude methanolic and aqueous extracts of E. alba and P. amarus showed antioxidant activity with IC50 values. The DPPH activity of E.alba methanol, hot water and cold water extracts were found to be 75μg/ml, 130μg/ml and 150μg/ml respectively. Similarly methanol, hot water and cold water extracts of P.amarus were found to be 40 μg/ml followed by 120 μg/ml and 145μg/ml respectively shown in Table1.The IC50 value for ascorbic acid was 25μg/ml. The results indicate that the antioxidant activity of the crude extract of P.amaurus is almost similar to that of ascorbic acid. The antioxidant activity of E. alba was nearly the same when compared to ascorbic acid. However, the other solvent extracts from E. alba and P. amarus were found to be less active than ascorbic acid since their IC50 values were found to be higher shown in Table1. Hence, the free radical scavenging activity of methanol extract exhibited promising antioxidant activity, which was further selected which for further antiLeptospiral activity and DNA damage studies. |
| |
|
|
Table 1: DPPH activity of Eclipta alba and Phyllanthus amarus. |
|
| |
| In Both aqueous and methanolic extracts of these two medicinal herbs were used for comparison. IC50 values for ABTS activity of Eclipta alba methanolic, hot water and cold water extracts were found to be 60 μg/ml, 150 μg/ml and 125 μg/ml. whereas in P. amarus methanolic, hot water and cold water extracts were found to be 35 μg/ml, 110 μg/ ml and 125 μg/ml respectively. In ABTS radical scavenging assay, the methanol extracts of both plants had good activity, reference standard gallic acid showed 50% inhibition at 25μg/ml in ABTS model. Lower IC50 value implies higher antioxidant power shown in Table 1 and 2; (Figure 1 & 2). |
| |
|
|
Figure 1: DPPH radical scavenging activity of different extracts of E.alba and P.amarus. Each value represents the percentage of inhibition for DPPH scavenging activity. Different concentration of 25-150 μg/ml was used for the assay. Methanol and aqueous extracts and they are compared with standard ascorbic acid. |
|
| |
|
|
Figure 2: ABTS radical scavenging activity of different extracts of E.alba and P.amarus. Each value represents the percentage of inhibition for ABTS scavenging activity. Different concentration of 25-150 μg/ml was used for the assay. Methanol and aqueous extracts and they are compared with standard gallic acid. |
|
| |
|
|
Table 2: ABTS activity of Eclipta alba and Phyllanthus amarus. |
|
| |
| In both the assays methanolic and aqueous extracts of both the plants showed a better result of antioxidant, therefore the methanolic and aqueous extract of both the plants were used for further studies. |
| |
| Confirmation of Leptospira species by PCR with specific primers |
| |
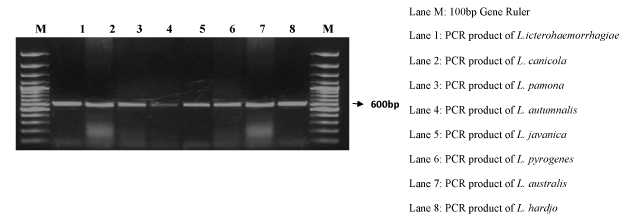
| Initially, PCR using rpoB primer, rpoB is the gene that encodes the β subunit of bacterial RNA polymerase was standardized by carrying out reactions at various annealing temperatures ranging from 58° to 68°C on L. icterohaemorrhagiae species which showed amplification at 55°, 58.7° and 59.2°C (Figure 3). A single band of approximately 600bp was observed at 55°C annealing temperature. A set of PCR reactions were done by varying the template concentration from 5ng to 60ng and could amplify the specific band at all the template concentrations (Figure 3). |
| |
|
|
Figure 3: Specificity and detection of annealing temperature of rpoB gene. Annealing temperature was standardized to 550C for 1 min. Lane1-9, PCR products using DNA template of L. icterohaemorrhagiae of each lane corresponding to 580C-680C, respectively. Lane M- 100bp DNA ladder. |
|
| |
| The PCR reaction for all the Leptospira species was done and the amplification was obtained at 600 bp and the Leptospira DNA was confirmed for the DNA damaging studies (Figure 4). |
| |
|
|
Figure 4: The specificity of rpoB primer tested on eight different species under optimized conditions for rpoB gene. Lane 1-8 different species, LaneM- 100bp Gene Ruler and lane 8 Negative control. |
|
| |
| Anti-Leptospiral activity of Eclipta alba and Phyllanthus amarus |
| |
| Effect of E.alba and P. amarus on Leptospira DNA cleavage |
| |
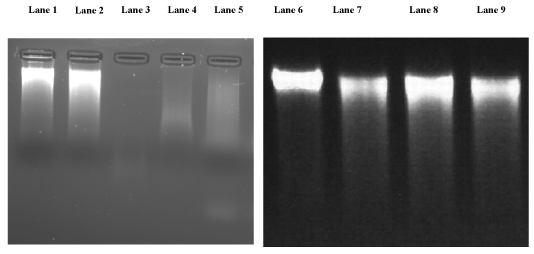
| In this study, confirmed DNA of L. icterohaemorrhagiae was selected to study DNA cleavage. Exposure of the DNA of L. icterohaemorrhagiae to plant extracts resulted in DNA cleavage as evident from the Figure (Figure 5). Methanol extracts of E. alba and P. amarus dose induced DNA cleavage. The methanol extract of E. alba at the concentration of 1 μg/mL induced DNA cleavage. The increased concentration of methanolic extracts of E. alba did not show any DNA cleavage the methanolic extract of 1 μg/mL cleaved Leptospira DNA completely. Similarly P. amarus the methanolic extracts at the concentration of 1 μg/ mL cleaved Leptospira DNA completely and the increased concentration of P. amarus extracts did not show any changes (Figure 5). |
| |
|
|
Figure 5: Effect of E. alba and P. amarus on Leptospira DNA cleavage; Lane 1 and 2 : Normal DNA of L. icterohaemorrhagiae, Lane 3,4 and 5: Normal DNA of L. icterohaemorrhagiae + E. alba extract with different concentration ranging from 1 μg/mL, 5 μg/mL and 10 μg/mL. Lane 6: Normal DNA of L. icterohaemorrhagiae, Lane 7, 8 and 9: Normal DNA L. icterohaemorrhagiae + P. amarus extract with different concentration ranging from 1 μg/mL, 5 μg/ mL and 10 μg/mL. |
|
| |
| Tube Dilution Technique and Micro Dilution Technique |
| |
| In the case of tube dilution technique the methanol and aqueous extracts of both the plants showed the better inhibitory property. The aqueous and methanol extract at the concentration of 50 and 100 μg/ mL showed the significant antiLeptospiral activity especially on the species of L. icterohaemorrhagiae, L. canicola, L. pamona, L. javnanica and L. hardjo, species remaining recorded not significant inhibition but did not inhibit the growth of L. australis in E. alba plant extract. The methanol and aqueous extract of P. amarus showed the better inhibition rate at 50 μg/mL against L. icterohaemorrhagiae, L. canicola, L. pamona. Not significant reduction was observed in the remaining species. In aqueous extract under micro dilution technique, the minimum inhibitory concentration like 50 μg/mL showed 100% inhibition against species like L. icterohaemorrhagiae, L. canicola, L. pamona. Along with these species, L. hardjo is also inhibited by the aqueous extract shown in Table 3 and 4. |
| |
|
|
Table 3: Effect of various extracts of Eclipta alba against selective species of Leptospira. |
|
| |
|
|
Table 4: Effect of various extracts of Phyllanthus amarus against selective species of Leptospira. |
|
| |
| The present study established that E.alba and P. amarus, the potentially useful anti-oxidant and anti-Leptospiral tribal herbs, possessed significant antioxidant, anti-Leptospiral and DNA damaging properties. The antioxidant, anti-Leptospiral and DNA damaging properties of the plant were evident from the fact that dose-dependently scavenged free radicals and damaged the DNA from reactive oxygen species induced cleavage. The antioxidant properties of E.alba and P. amarus might be an essential underlying mechanism for its antioxidant and anti-Leptospiral effects suggesting that the plant has the potential to be developed as an antioxidant, anti-Leptospiral medication to enhance the defense system in the infected animals. |
| |
| Recently, much attention has been directed towards plant extracts and biologically active compounds isolated from popular plant species. The use of medicinal plants plays a vital role in covering the basic health needs in developing countries and these plants may offer a new source of antibacterial, antifungal and antiviral agents with significant activity against infective microorganisms [19,20]. |
| |
| The reactive oxygen species or oxidants, which are formed in the human body due to exogenous and endogenous factors, are found to be responsible for many diseases. Day- by- day, a number of researches have shown the potential of phytochemical antioxidants as health benefactors because of their ability to neutralize free radicals, reactive oxygen species, or oxidants responsible for the onset of cell damage. Theantioxidant parameters DPPH radical scavenging activity and ABTS assay, plant extracts can be the potent antimicrobial and antioxidant activity suitable for the prevention of zoonatic disease like leptospirosis as it is evident from the present studies. |
| |
| In the present paper, we have evaluated the free radical scavenger activity of methanolic extract of E.alba and P. amarus. Among the 4 different extracts from two plants and standard tested for the in vitro antioxidant activity using the DPPH method, the crude methanolic extracts of both E.alba and P. amarus showed significant activity. IC50 values for DPPH activity of E. alba methanol, hot water and cold water extracts were found to be 75 μg/ml, 130 μg/ml and 150 μg/ml respectively. Similarly P. amarus extract for methanol, hot water and cold water extracts were found to be 40 μg/ml followed by 120 μg/ml and 145 μg/ml respectively. The IC50 value for ascorbic acid was 25μg/ ml. IC50values for ABTS activity of Eclipta alba methanolic, hot water and cold water extracts were found to be 60 μg/ml, 150 μg/ml and 125 μg/ml. where as in Phyllanthus amarus methanolic, hot water and cold water extracts were found to be 35 μg/ml, 110 μg/ml and 125 μg/ml respectively. The free radical scavenging activity of methanolic extract was confirmed in the present investigation. However, the chemical constituents present in the extract, which are responsible for this activity, need to be investigated, and it is obvious that the constituents like tannins, reducing sugars and proteins present in the extract may be responsible for such activity. The phytochemical tests indicated the presence of alkaloids, glycosides, tannins, and flavonoids in the crude methanolic extract. Several of such compounds are known to possess potent antioxidant activity [21]. Some of these constituents have already been isolated from this plant [22]. Hence, the observed antioxidant activity may be due to the presence of any of these constituents. The plant exhibited strong antibacterial, anticancer, hepato-protective, antiviral and several other activities. These properties may be due to its antioxidant activity. The crude methanolic extract merits further experiments in vivo. |
| |
| Our study clearly demonstrated that the plant extracts have good antioxidant properties when assessed by DPPH and ABTS models. Natural antioxidants that are present in herbs are responsible for inhibiting or preventing the deleterious consequences of oxidative stress. Herbs contain free radical scavengers like polyphenols, flavonoids and phenolic compounds. In the present paper, we have evaluated the free radical scavenger activity of various extract of E. alba and P. amarus [20,23]. |
| |
| ABTS method was not only a rapid and reliable test of total antioxidant capacity but also an advantageous assay applicable to both hydrophilic and lipophilic antioxidants/systems. The whole plants of E. alba and P. amarus can be used to treat/prevent various bacterial disease that includes leptospirosis. |
| |
| On the basis of the result obtained in this present investigation, we conclude that the methanol and aqueous extract of E. alba and P. amarus had significant in vitro antiLeptospiral activity. This implied that even gram-positive bacteria were more susceptible to the extract than the gram-negative bacteria, because of the presence of outer membrane that serves as an effective barrier in gram negative species [23,24], but the extract of E. alba and P. amarus showed significant activity against Leptospira a gram negative bacteria. |
| |
| The primary and secondary metabolites those are present in methanolic extracts viz., anthraquinones and alkaloids were found to be absent in the natural plant. In an earlier study, the extracts of the leaves of E. alba tested positive for anthraquinones steroid, reducing sugars, alkaloids, phenolics, saponins and tannins, but flavonoids were detected [22]. |
| |
| In Gujrat and Punjab, India E. alba is used externally for ulcers and as an antiseptic for wounds in cattle and is reported to treat many microbial infections in rural areas [25]. The results from the current studies revealed that the wedelolactone may be the main constituent responsible for antimicrobial activity. There are various reports that crude extract from E. alba and E. prostrata showed antibacterial, antifungal and anti viral activity [26, 27, 28, 29 and 30]. The presence of wedelolactone in the plant of E.alba might be responsible for the DNA damage of Leptospira. |
| |
| Wedelolactone exhibited effective antibacterial activity against different Solmonella species. It proved highly effective against S. epidermidis and S. typhimurium demonstrating the specificity of wedelolactone activity [30] reported in vitro antimicrobial activities of ethanolic extract of E. prostrate. It indicated good activity against S aureus and for P. aeruginos, at 50 μg concentration. While the present studies instead of agar well diffusion method DNA damaging study was carried out by taking Leptospiral DNA of a specific strain and different concentration of two plants E.alba and P.amarus were taken for the studies. The results from the current studies based on the reported studies on the wedelolactone compound could be the main constituent responsible for these treatments for antibacterial as it exhibited good activity against them; P.amarus was also taken for studies because this plant extract was also used for kidney and liver related disease management. |
| |
| The bioactive principle from E. alba and P. amarus was extracted by various solvents and inoculated with already standardized Leptospiral cultures. By following the periodic observation of the tubes under dark field microscopy, the inhibitory activity of the plant compound was easily detected by the reduction in the numbers and its motility of the leptospires compared with control (without plant extract) (Data not shown). The lowest concentration of 50 μg/mL itself showed the complete reduction in water extract against certain species of leptospires and this much inhibition level is not observed in any solvents used under this study. On comparing with the efficacy of P. amarus, the E. alba showed the better result by aqueous extract. The methanolic extract showed the accurate inhibitory results same like aqueous extract. So, the MIC by both the techniques of methanol extract was 100 μg. |
| |
| The standard methods to be followed for the study of efficacy of drugs against Leptospiral members are tube dilution technique (TDT) and micro dilution technique (MDT). On comparing with tube dilution technique, micro dilution was found to be better that it cleared the leptospires even during the study period of 30 min, which might make this method to be better suited for performing antiLeptospiral studies. By observing all the above results, the in vitro antiLeptospiral activity of E. alba and P. amarus was well studied and proved as a best antiLeptospiral drug [31]. |
| |
| The results of present research highlights, the fact that the organic solvent extracts exhibited greater antiLeptospiral activity because the antimicrobial principles were either polar or non-polar and they were extracted only through the organic solvent medium [32,33]. The present observation suggests that the organic solvent extraction was suitable to verify the antimicrobial properties of medicinal plants and they supported by many investigators. The present study justifies the claimed uses of E. alba and P. amarus in the traditional system of medicine to treat various infectious diseases caused by the microbes. This study also encourages cultivation of the highly valuable plant in large-scale to increase the economic status of cultivars in the country. |
| |
| The obtained results may provide a support to use of the plant in traditional medicine. Based on this, further chemical and pharmacological investigations to isolate and identify minor chemical constituents in E.alba and P. amarus and to screen other potential bioactivities may be recommended. |
| |
| According to our knowledge this is the first report on P. amarus and E. alba, This is first kind in antimicrobial history of introducing and analyzing E. alba against Leptospirosis. It provides an idea in the improvement of medicinal herbs against Leptospiral members to overcome the adverse reaction and also identify the presence of bioactive compounds in the E. alba and P. amarus as therapeutics. |
| |
| Acknowledgements |
| |
| The authors would like to thank Indian Council of Medical Research (ICMR) for the financial assistance in the form of Senior Research Fellowship. The authors wish to thank the Project Director, Project Directorate on Animal Disease Monitoring and Surveillance (PD_ADMAS), ICAR, Hebbal, Bangalore-24, Karnataka, INDIA. |
| |
| |
| References |
| |
- Abdollahpour G (1990) A review on Leptospirosis. Proceedings of Leptospirosis Research Conference, Japan, Matsoyama.
- Zhang Y, Dai B (1992) Detection of Leptospiral DNA in the serum of 175 patients with early leptospirosis by polymerase chain reaction. Hua Xi Yi Ke Da Xue Xue Bao 23: 256-260.
- Gangadhar NL, Prabhudas K, Bhushan S, Sulthan M, Barbuddhe SB (2008) Leptospirainfection in animals and humans: a potential public health risk in India. Rev Sci Tech OIE 27: 885-892.
- Muñoz-Mingarro D, Acero N, Llinares F, Pozuelo JM, Galán de Mera A,et al. (2003) Biological activity of extracts from Catalpa bignonioides Walt. (Bignoniaceae). J Ethonopharmacol 87: 163-167.
- Shah NC (1981) Need of cultivation of medicinal herbs used in indigenous and traditional medicine. I Drugs 18: 210-217.
- Emmanouilides CE, Kohn OF, Garibaldi R (1994) Leptospirosis complicated by a Jarisch-Herxheimer reaction of adult respiratory distress syndrome: Case report. Clin Infect Dis 18: 1004-1006.
- Huang DJ, Ou BX, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53: 1841-1856.
- Arnao MB (2000) Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci Tech 11: 419-421.
- Ellis WA, Little TWA (1984) The present state of leptospirosis diagnosis and control. The diagnosis of leptospirosis in farm animals. Veterinary research laboratory, Department of Agriculture Stoney Road 25-31.
- Koleva II, Van Beek TA, Linssen JPH, Groot AD, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochemistry 13: 8-17.
- Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF (2001) Antioxidant activity of extracts from Acacia confusabark and heartwood. J Agric Food Chem 49: 3420-3424.
- Portillo A, Vila R, Freixa B, Adzet T, Cañigueral S (2001) Antifungal activity of Paraguyan plants used in traditional medicine. J Ethnopharmocol 76: 93-98.
- Song FL, Gan RY, Zhang Y, Xiao Q, Kuang L, et al. (2010) Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int J Mol Sci 11: 2362-2372.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231-1237.
- Russo A, Izzo AA, Borrelli F, Renis M, Vanella A (2003) Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res 17: 870-875.
- Slack AT, Dohnt MF, Symonds ML, Smythe LD (2005) Development of multiple-locus variable number of tendem repeat analysis (MLVA) for Leptospira interrogans and its application to Leptospira interrogansserovar Australis isolates from far north Queensland, Australia. Ann clin Microbiol Antimicrob 4: 10.
- Mathew LM (2001) Comparative analysis of Acetone, Water and Saponified extracts of Phyllanthusniruri against Leptospira interrogansserogroups invitro (Dissertation) Salem, Tamilnadu, India: Periyar University.
- Scola BL, Bui LTM, Baranton G, Khamis A, Raoult D (2006) Partial rpoB gene sequencing for identification of Leptospira species. FEMS Microbiology Letters 263: 142-147.
- Coelho de Souza G, Haas APS, Von Poser GL, Schapoval EES, Elisabetsky E (2004) Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol 90: 135-143.
- Nikaido H (1999) Microdermatology: Cell surface in the interaction of microbes with the external world. J Bacteriol 181: 4-8.
- Lee J, Koo N, Min DB (2004) Reactive oxygen species, aging and antioxidative neutraceuticals. Comp Rev Food Sci F 3: 21-33.
- Gopalakrishnan S, Solomon MJ (1992) Comparative Pharmacognostic Studies on the Leaves of Eclipta alba and Wedelia calendulacea. Pharm Biol 30: 33-38.
- Adesokan AA, MA Akanji, MT Yakubu (2007) Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. Afr J Biotechnol; 6: 2502-2505.
- Mohanasundari C, Natarajan D, Srinivasan K, Umamaheswari S, Ramachandran A (2007) Antibacterial properties of Passiflora foetidaL. –a common exotic medicinal plant. African Journal of Biotechnology 6: 2650-2653.
- Warrier PK (1994) “Indian medicinal plants”. Orient Longman Ltd, Chennai.
- Kosuge T, Yokota M, Sugiyama K, Yamamoto T, Ni MY, et al. (1985) Studies on antitumor activities and antitumor principles of Chinese herbs. I. Antitumor activities of Chinese herbs. Yakuga Zasshi 105: 791-795.
- Wiart C, Mogana S, Khalifah S, Mahan M, Ismail S, et al. (2004) Antimicrobial screening of plants used for traditional medicine in the state of Perak, Penisular Malaysia. Fitoteropia 75: 68-73.
- Venkatesan S, Ravi R (2004) Antifungal activity of Eclipta alba. IndiaJ Pharam Sci 66: 97-98.
- Ma-Ma K, Nayunt N, KM Tin (1978) The protective effect of Eclipta alba in CCL acute liver damage. Toxicol Appl Pharmacol 45: 723-728.
- Karthikumar S, Vigneswari K, Jegatheesan K (2007) Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrata (L). Scientific Research Essay2: 101-104.
- Prabhu N, Innocent JP, Chinnaswamy P, Natarajaseenivasa K, Sarayu L (2008) In vitro evaluation of Eclipta alba against serogroup of Leptospira interrogans. Indian J Pharm Sci 70: 788-791.
- Britto JS, Swamy J (2001) Comparative antibacterial activity study of Solanum incanum L. Botany Club 18: 81-82.
- Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, et al. (2004) Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India 52: 794-804.
|
| |
| |