| Research Article |
Open Access |
|
| Donald I Williamson1* and Nicander G J Boerboom2 |
| 1School of Biological Sciences, University of Liverpool L69 3BX, UK |
| 2Spechtstraat 9, 6921 KP Duiven, the Netherlands |
| *Corresponding authors: |
D I Williamson
School of Biological Sciences
University of Liverpool
14 Pairk Beg, Port Erin
Isle of Man IM9 6NH, UK
Tel: 44 1624 832625
E-mail: diwilliamson@manx.net |
|
| |
| Received July 11, 2012; Published July 14, 2012 |
| |
| Citation: Williamson DI, Boerboom NGJ (2012) Experimental Hybrids between Ascidians and Sea Urchins. 1: 233. doi:10.4172/scientificreports.230 |
| |
| Copyright: © 2012 Williamson DI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| The larval transfer hypothesis claims that larvae did not evolve gradually from the same stocks as adults but were transferred by hybridization from animals in distantly related taxa. Experimental hybrids between organisms from different phyla demonstrate the feasibility of crossing distantly related animals and they provide material to study the morphologies, chromosomes and genomes of hybrids. Untreated eggs of the ascidian Ascidia mentula mixed with concentrated sperm of the sea urchin Echinus esculentus divided into 33 of 63 experiments. One experiment yielded 3,000 eight-armed pluteus larvae, a minority of which developed into sea urchins. Two pentaradial sea urchins and one tatraradial sea urchin survived more than four years and produced fertile eggs. The majority of the 3,000 plutei resorbed their arms to become spheroids. Forms resembling tadpoles developed within some of these spheroids, but no tadpoles emerged. Eggs of the sea urchin Psammechinus miliaris, pretreated with acid seawater before mixing with dilute sperm of the ascidian Ascidiella aspersa, developed into four-armed pluteus larvae, all of which resorbed their arms to become bottom-living spheroids that divided repeatedly. Some of these spheroids developed inclusions with ascidian features, but they did not develop further. Many spheroids grew into irregular shapes; others divided to produce more spheroids. The results show that, on occasion, some distantly related animals will hybridize in the laboratory and larval and adult forms can retain their identities in hybrids. The findings are consistent with the larval transfer hypothesis. Biologists are urged to conduct comparable hybridization experiments. |
| |
| Keywords |
| |
| Evolution; Larval transfer; Pluteus larvae; Tadpoles; Selfreplicating spheroids |
| |
| Introduction |
| |
| Most modern biologists follow Darwin in assuming that larvae and their corresponding adults evolved from common ancestors [1]. As a result, animals are frequently separated into protostomes and deuterostomes by their larvae; molluscs, annelids and several other phyla are thought to have evolved from a common protostome ancestor with trochophore larvae and echinoderms and hemichordates are thought to have evolved from a common deuterostome ancestor with tornaria larvae. The ‘common ancestor’ hypothesis is, however, difficult or impossible to reconcile with: |
| |
| (a) Recently acquired larvae, where animals have apparently suddenly acquired fully evolved larva, as in species of Hebella (Hydrozoa) [2,3]. |
| |
| (b) Incongruous larvae, where the larva seems to be only distantly related to the adult, as in Hebella (Hydrozoa), Dromiidae (Crustacea, Brachyura) and Rhizocephala [2,4-6]. |
| |
| (c) Multiple larvae in the same life history, as in Penaeidae (Crustacea) and some insects [2,5]. |
| |
| (d) ‘Overlapping metamorphosis’, in which the larva and juvenile live side by side, as in Luidia sarsi (Echinodermata, Asteromorpha) [2,5]. |
| |
| (e) ‘Start again metamorphosis’, in which no larval tissues or organs survive metamorphosis, as in bryozoans with trochophore or cyphonautes larvae and holometabolous insects [2-4,6]. |
| |
| These and other examples are discussed fully in previous publications and larval transfer is proffered as an alternative explanation [2-6]. Larval transfer claims that the basic forms of all larvae were later additions to animal life histories. They originated as adults in other taxa and their genomes were transferred by hybridization. Experimental hybrids test the feasibility of crossing distantly related animals and they provide material to study the fate of genes in hybrids. The experiments discussed here are not attempts to replay evolutionary history, but they demonstrate that interphyletic hybridization can occur. |
| |
| Brief accounts of the crosses described here as Experiments 1 and 2 are included in two books [2,5], but they lack the experimental details. The present paper includes hitherto unpublished photographs relating to Experiment 2 and it provides evidence that some hybrids developed further than was previously realized. Experiments 3 and 4 have not been described previously. The experiments started in 1988-95 (Experiments 1 and 2) were carried out by DIW and both authors collaborated in those started in 2002 (Experiments 3 and 4). |
| |
| Materials and Methods |
| |
| We describe the following hybrid crosses: |
| |
| (a) eggs of the ascidian Ascidia mentula of Müller, 1776 (phylum Chordata, subphylum Urochordata) fertilized with sperm of the sea urchin Echinus esculentus Linnaeus, 1758 (phylum Echinodermata, Class Echinomorpha) |
| |
| (b) eggs of the ascidian Ciona intestinalis (Linnaeus, 1767) fertilized with sperm of the sea urchin Psammechinus miliaris (Gmelin, 1778) |
| |
| (c) eggs of P. miliaris fertilized with sperm of the ascidian Ascidiella aspersa (OF Müller, 1776). We discuss the relevance of these crosses to the larval transfer hypothesis. Throughout the paper we use the ‘eggs first’ convention for hybrids. The crosses described are, therefore, Ascidia mentula x Echinus esculentus, Ciona intestinalis x Psammechinus miliaris and Psammechinus miliaris x Ascidiella aspersa. |
| |
| The work took place at Port Erin Marine Laboratory (University of Liverpool), Port Erin, Isle of Man, UK, which closed in 2006. |
| |
| Filtered seawater used in all experiments had been passed through a 0.1 μm filter. Some bottom-living hybrids from the 2002 experiments were kept in running seawater passed through a 1.0 μm filter. Port Erin seawater was at pH 8.4. Acid seawater was prepared by gradually adding concentrated acetic acid to filtered seawater until the pH fell to 5.0 [7]. |
| |
| Specimens of Ascidia mentula used in the 1989-90 experiments were obtained from an old covered seawater storage tank at Port Erin Marine Laboratory. This tank was drained soon after the 1990 experiments and never refilled. After 1990, divers obtained specimens of A. mentula from a shipwreck, five km northwest of Port Erin. Specimens of Ciona intestinalis, Ascidiella aspersa and other ascidians used in 2002 were obtained from other seawater storage tanks at the Marine Laboratory and by diving on the ruined breakwater at Port Erin. Divers also collected Echinus esculentus from the breakwater. Psammechinus miliaris was obtained from a mid-tide rock pool at Gansey Point, Port St Mary and Isle of Man. |
| |
| In 1989-1995, large specimens of A. mentula were gently squeezed to express eggs or sperm. In 2002, specimens of several species of ascidians were isolated in bowls of filtered seawater. Ripe specimens shed eggs or sperm, usually overnight. Although ascidians are simultaneous hermaphrodites, they only rarely emit eggs and sperm together. Eggs were observed at intervals for at least two hours and any batch with dividing eggs was discarded. |
| |
| Ripe sea urchins will sometimes discharge gametes spontaneously when placed in seawater after collection. They will also shed eggs or sperm when removed from the water and inverted over a beaker for up to one hour, or within minutes if injected with 0.1 molar potassium chloride. Pre-treatment of eggs with acid seawater lasted 40 seconds and exposure to foreign sperm lasted 20-25 minutes, both of which were followed by several washes with filtered seawater. |
| |
| In 1989, cultures of larvae were fed with the flagellate Pseudoisochrysis and the green alga Chlorella. The diatom Nitzschia was used in 1990 and the flagellates Isochrysis and Nanochloropsis in 2002, in sufficient quantity to turn the water faintly green. |
| |
| Results |
| |
| The results are in order of their starting dates. Non-hybrid controls were started before the numbered hybridization experiments in some cases, after in others. |
| |
| Results of 63 attempts to fertilize untreated eggs of Ascidia mentula with concentrated sperm of Echinus esculentus in 1989-1995 are summarized in Table 1. No eggs divided in 30 experiments and one or more eggs divided but did not hatch in 16 cases. On 9 March 1994 many eggs divided once and re-fused and on 26 April 1994 one egg divided twice and then re-fused. In an attempt to fertilize eggs of Ciona intestinalis with sperm of E. esculentus on 6 April 1992, two eggs were rapidly changing shape and repeatedly dividing within the egg membrane into two and four cells and re-fusing, 24 hours after exposure to Echinus sperm. Nothing is known of the behaviour of the chromosomes in these cases. In the other 17 A. mentula x E. esculentus experiments, eggs hatched as blastulas (4 cases), tadpoles (10 cases) or a mixture of blastulas and tadpoles (3 cases). In ten attempts to fertilize untreated E. esculentus eggs with concentrated A. mentula sperm, no eggs divided. Experiments 1 and 2 (described below) are cases in which more than 100 A. mentula eggs hatched as ciliated blastulas after exposure to E. esculentus sperm. |
| |
|
|
Table 1: Attempts to fertilize Ascidia mentula eggs with Echinus esculentus sperm in 1989-1995. |
|
| |
| Control for experiment 1, started 27 March 1989: Echinus x Echinus |
| |
| |
| 27 March 1989. Several newly collected specimens of Echinus esculentus started to spawn spontaneously when placed in seawater. Gametes were collected from three males and one female. One drop of sperm was mixed with about 300 eggs in a litre of seawater in a homospermic fertilization. The remainder of the sperm was stored at 3-5°C until the next day. |
| |
| 28 March: at 10-12°C, 99% of eggs hatched as ciliated blastulas, 27 hours from fertilization. 29 March: larvae gastrulated by invagination, then became prism-shaped. 30 March: Pseudoisochrysis added as food. 31 March: mouth formed. 2 April: four-armed plutei. 5 April: Pseudoisochrysis and Chlorella added as food. 9 April: six-armed plutei. 12 April: eight-armed plutei. 19 April: anterior epaulettes in some larvae. 6 May: one larva with juvenile rudiment. Mortality was high and no free-living juveniles were obtained. |
| |
| Experiment 1, started 28 March 1989: Ascidia x Echinus. |
| |
| 28 March 1989: laboratory temperature 10-12°C. 14.00: specimen of Ascidia mentula, gently squeezed, yielded 250 eggs. Eggs washed in filtered seawater. None divided over three hours. Seawater drained from eggs. 17.00-17.20: eggs immersed in concentrated sperm from three E. esculentus, obtained on 27 March. Eggs then repeatedly washed in filtered seawater to remove excess sperm. Re-count confirmed 250 eggs. Several started to divide 50 minutes from first contact with Echinus sperm. Great majority divided in next 15 minutes. |
| |
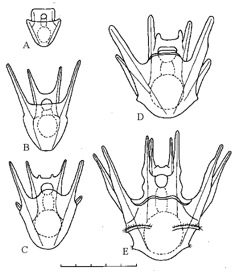
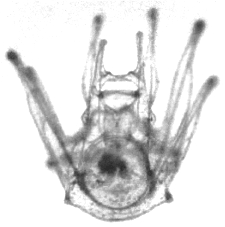
| 29 March-30 May 1989. Of the 250 eggs, 230 hatched, all as ciliated blastulas, about 18 hours after fertilization. Most blastulas 0.18–0.20 mm; a few 0.10 mm. Blastulas gastrulated, then became prism-shaped, developed internal rods and grew as pluteus larvae. Larvae kept at 15°C, fed on Pseudoisochrysis from 30 March and Pseudoisochrysis + Chlorella from 5 April. About 75% of hybrid pluteus larvae stunted or deformed, but 25% indistinguishable from those from plutei from homospermic fertilization on 27 March (Figures 1,2). |
| |
|
|
Figure 1: Experiment 1: Ascidia x Echinus, 1989. Drawings of hybrid larvae. A, 2 days from hatching; B, 7 days; C, 14 days; D, 21 days; E, 28 days. Scale=0.5 mm. |
|
| |
|
|
Figure 2: Experiment 1: Ascidia x Echinus, 1989. Photograph of hybrid larva, 28 days from hatching. |
|
| |
| Mortality high in hybrid larvae. No hybrid developed Echinus rudiments. Pluteal arms started to shorten in fifth week from hatching. Several died with short stumps. One became spheroidal and settled. Last hybrid larva died 30 May. |
| |
| Control for experiment 2, started 2 February 1990: Echinus x Echinus. |
| |
| 1 February 1990. Several freshly collected specimens of E. esculentus started to spawn spontaneously when placed in seawater, as on 27 March 1989. Sperm from three males and eggs from one female kept at 3-5°C until following day. |
| |
| 2 February 1990: about 500 Echinus eggs exposed to diluted Echinus sperm. 3 February 1990: >95% of eggs hatched, 25-27 hours after exposure to sperm. Larvae kept at 18°C and fed on Nitzschia. Development faster and mortality less than Echinus x Echinus started 27 March 1989 (at 10°C, fed on Pseudoisochrysis and Chlorella). 16 February: many eight-armed, with anterior epaulettes. 29 February: several with small juvenile rudiments. No free-living juvenile Echinus obtained. |
| |
| Experiment 2, started 2 February 1990: Ascidia x Echinus. |
| |
| 2 February 1990. Laboratory temperature about 12°C. 12.15: large specimen of Ascidia mentula yielded about 3,700 eggs and a little sperm when gently squeezed. Eggs washed in filtered seawater; none divided over next three hours. 15.10-15.35: eggs immersed in concentrated Echinus sperm collected previous day, washed, split between three bowls. First cleavage in different eggs from 15.40-16.40. |
| |
| 3 February 1990. Total of 3,438 eggs (about 93%) hatched as ciliated blastulas, 21-28 hours after contact with Echinus sperm. One egg hatched as tadpole larva. Blastulas mostly 0.2 mm, but some 0.1 mm. Transferred to controlled temperature (CT) room at 18°C. Nitzschia supplied as food. |
| |
| 4 February - 20 February 1990. About 30% of larvae deformed or stunted. Other 70% developed as pluteus larvae indistinguishable from Echinus x Echinus controls, started on 2 February. Most plutei eightarmed on 20 February. From this date hybrid larvae developed either as (a) sea urchins or (b) spheroids. |
| |
| (a) Experiment 2 (continued): hybrids that developed as sea urchins: 21 February 1990 - 20 April 1994. |
| |
| About 8% of larvae each developed an Echinus rudiment in left mesocoel sac. Two developed Echinus rudiments in both left and right mesocoel sacs, as occasionally happens in non-hybrid Echinus [8]. Juvenile urchins settled and crawled away from remains of plutei 37-50 days from hatching. |
| |
| 13 March 1990. 74 juveniles, 1.2 mm between tips of spines, 0.5 mm across disc placed in small tank at 15°C with seawater drip. |
| |
| 1 October 1990. Four surviving urchins, approximately 10, 10, 9 and 3 mm across disc. Two largest pentaradial, two smallest tetraradial urchins measured at monthly intervals. |
| |
| 15 November 1991. Tank moved to CT room at ambient temperature of sea at Port Erin. Diameters of urchins: 27 mm with spines (18 mm across disc), 25 mm (17 mm), 18 mm (13 mm), 12 mm (8 mm). Urchins inverted over beakers for discharge of gametes at monthly intervals. |
| |
| 1 April 1993: urchins measured 45 mm with spines (34 mm across disc), 45 mm (34 mm), 35 mm (24 mm) and 12 mm (9 mm). Three largest produced eggs when inverted over beakers. |
| |
| 30 April 1993: smallest urchin disintegrated. It had shown no growth for 18 months. |
| |
| 2 September 1993: a pentaradial urchin produced 12 eggs. 6 December 1993: the tetraradial urchin produced eggs. 7 February 1994: all three urchins produced eggs. Each batch of eggs fertilized with sperm from wild E. esculentus. All hatched as ciliated blastulas that developed into plutei indistinguishable from Echinus x Echinus. 14 February 1994: plutei preserved. |
| |
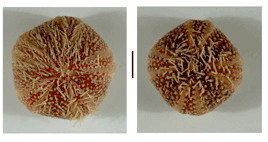
| 20 April 1994: urchins died as result of water stoppage. Diameters (across discs) 64, 52 and 43 mm. Preserved (Figure 3). |
| |
|
|
Figure 3: Experiment 2: Ascidia x Echinus, 1990. Sea urchins preserved 4 years 3 months from hatching. A, pentaradial form. B, tetraradial form. Scale=1 cm. |
|
| |
| (b) Experiment 2 (continued): hybrids that developed as spheroids: 21 February 1990 - 13 May 1990. |
| |
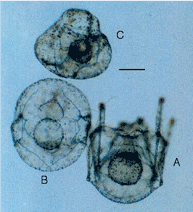
| 21 February - 2 April 1990: about 92% of plutei resorbed arms to become spheroids, taking 2 - 4 weeks to complete resorption (Figure 4). |
| |
|
|
Figure 4: Experiment 2: Ascidia x Echinus, 1990. Transition from pluteus to spheroid in three hybrids, photographed together 54 days from hatching. A, pluteus resorbing arms. B, spheroid with vestiges of arms. C, spheroid without arms. Scale=0.1 mm. |
|
| |
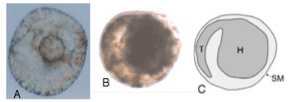
| 2 April - 13 May 1990. Spheroids 0.3-0.15 mm, most 0.25 mm, each with small protuberance (Figure 5A). Spheroids could swim or attach by protuberance. Inclusions in some spheroids resemble coiled tadpoles (Figure 5B, 5C), but no tadpoles emerged. Last spheroids died 13 May 1990, without developing further. |
| |
|
|
Figure 5: Experiment 2: Ascidia x Echinus, 1990. Spheroids 56 days from hatching. A, photograph of surface, from below. B, photograph showing inclusion. C, drawing of B showing tadpole-like features of inclusion (H=head of tadpole, T=tail of tadpole, SM=spheroid membrane). Scale=0.1 mm. |
|
| |
| Experiment 3, started 16 September 2002: Ciona x PsammEchinus |
| |
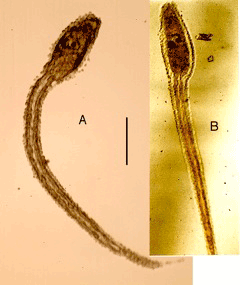
| Untreated eggs and concentrated sperm used. Eggs hatched as tadpoles in 22 hours (Figure 6A). Tadpoles died in 72 h without attaching to glass and without metamorphosing. They resembled non-hybrid tadpole larvae of C. intestinalis (Figure 6B), but with rougher surface. |
| |
|
|
Figure 6: Experiment 3: Ciona x Psammechinus, 2002. Tadpole larvae. A, recently hatched larva. B, Control: Ciona x Ciona, larva one day from hatching. Scale=1 mm. |
|
| |
| Control for Experiment 3, started 16 September 2002: PsammEchinus x PsammEchinus |
| |
| Homospermic (non-hybrid) fertilization, with untreated eggs. Eggs hatched as ciliated blastulas, which went through normal series: gastrula, prism-shaped larva, pluteus. Pluteus larvae all eight-armed on 15 October, when they were found dead, possibly as result of overnight drop in temperature from 16°C to 14°C. |
| |
| Control for Experiment 3, started 24 September 2002: Ciona x Ciona |
| |
| Homospermic (non-hybrid) fertilization, with untreated eggs. Eggs hatched as tadpole larvae within 24 hours (Figure 6B). Tadpoles attached 3-5 days after hatching, cast their tails and gradually developed into small ascidians. |
| |
| Experiment 4, started 19 September 2002: PsammEchinus x Ascidiella |
| |
| Seawater cloudy in bowl containing specimen of Ascidiella aspersa. Microscopic examination confirmed presence of active sperm. Several specimens of P. miliaris injected with potassium chloride; one produced eggs. About 200 P. miliaris eggs decanted into sieve and rinsed with filtered seawater. Following method of Raff et al. [7], eggs in sieve immersed in acid seawater (pH 5.0) for 40 seconds, followed by several rinses of filtered seawater. Seawater with slightly cloudy suspension of A. aspersa sperm sieved to remove any possible eggs, then PsammEchinus eggs in sieve immersed in sperm suspension for 20 minutes. Eggs rinsed repeatedly with filtered seawater, then washed into bowl. First cell division 69 minutes after first contact with Ascidiella sperm. Virtually all eggs divided in next 15 minutes. |
| |
| PsammEchinus eggs treated with acid seawater (as above) but not exposed to sperm did not divide. |
| |
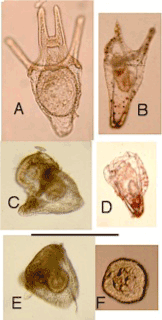
| In PsammEchinus x Ascidiella culture, first ciliated blastula hatched 23.5 hours after first contact with Ascidiella sperm; practically all eggs hatched within next 1.5 hours. Hybrids developed into pluteus larvae, which, for next fortnight, showed no consistent differences from the P. miliaris x P. miliaris larvae (control, started 16 September). At 14 days, hybrid and non-hybrid larvae were all four-armed plutei (Figure 7A). Non-hybrid controls gradually developed eight pluteal arms over next fortnight, but hybrids resorbed their four pluteal arms (Figures 7B-E) to become spheroids (Figure 7F). In some but not all cases, rods protruded from shrinking arms. Some resorbed all four arms together; others showed various forms of asymmetrical loss of arms. All cases resulted in swimming spheroids of diameter 0.1-0.2 mm. No obvious organ of attachment, but spheroids soon attached firmly to glass and did not swim again. These bottom-living spheroids could crawl at about 1 mm per hour. |
| |
|
|
Figure 7: Experiment 4: Psammechinus x Ascidiella, 2002. Formation of spheroid. A, 4-armed pluteus, age 14 days. B-E, resorption of arms, age 16- 25 days. F, spheroid, age 40 days. Scale=0.5 mm. |
|
| |
| 15 October 2002: all eight-armed non-hybrid plutei (controls) had died overnight, possibly due to a drop in temperature to 14°C. No deaths in hybrid spheroids. |
| |
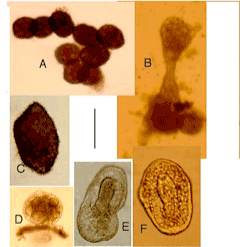
| In following months, many spheroids divided to produce more spheroids (Figure 8A,B); others grew larger. Most remained smooth, but some developed dense covering of small spines (Figure 8C). Some developed various shapes within outer body wall (Figure 8D-8F). Some inner shapes appeared to show features of developing ascidians, including siphons (Figure 8E,8F), but none developed into free-living ascidians. After 22 months, hybrid cultures contained spheroids, some still dividing and a range of larger irregular shapes. Some of these are shown in Figure 9, which shows no details, but illustrates the variety of shapes and sizes attained. Hybrid in 9C almost flat; those in Figure 9C- 9E rigid, apparently calcified; largest (Figure 9E) approximately 6 mm long. Cultures preserved in ethanol at 28 months. |
| |
|
|
Figure 8: Experiment 4: Psammechinus x Ascidiella, 2002. Spheroids and other hybrids, 7-9 weeks from hatching. A, B, dividing spheroids; C, hybrid with spines; D-F, hybrids with inclusions. Scale=0.2 mm. |
|
| |
|
|
Figure 9: Experiment 4: Psammechinus x Ascidiella, 2002. Hybrids 22 months from hatching. Scale=2.5 mm. |
|
| |
| Discussion |
| |
| The spheroids in Experiment 2 (1990: Ascidia x Echinus) usually swam too actively to see any shape within the spheroid. They could attach firmly by the small protuberance and, when attached, they were always viewed in the same orientation, with no indication of a shape within the spheroid. Figure 5B shows a slowly moving spheroid with the protuberance to the upper right. We interpret the form within this spheroid as a larva with a tail (Figure 5C), but this is not immediately obvious. These tadpole-like features were not noticed under the microscope and, between taking this batch of photographs and development of the film, while collecting Echinus at low water spring tide, I (DIW) slipped, fell, hit my head on a rock and had a stroke. I got the photographs six months later, on my next visit to the laboratory and I examined them only briefly. I did not notice the supposed coiled tadpole, shown here as Figure 5B, until all the relevant photographs were re-examined in 2008. Our interpretation of Figure 5B may be questioned and we urge others to carry out comparable experiments to confirm or refute our view. All the spheroids in Experiment 2 had developed from pluteus lavae, which, in turn, were the products of Ascidia mentula eggs fertilized with Echinus esculentus sperm. We suggest that most spheroids in Experiment 2 probably contained tadpoles, but they were not viewed from the critical angle to show the enclosed shape. |
| |
| Hart [9], investigated nucleotide sequences for the COI mitochondrial gene and the 28S ribosomal gene extracted from tubefeet of the three hybrid urchins from Experiment 2 that survived into their fourth year. In each case, he found near identity with wild Echinus esculentus, with no ascidian components. He concluded that the putative hybrid urchins could not have hatched from ascidian eggs and he suggested that “a hermaphrodite [Echinus] used in the crossfertilization experiments to provide sperm may have provided eggs as well.” The only known hermaphrodite E. esculentus was reported by Moore [10], who investigated 3,000 specimens, but Hart [9], implies that hermaphrodite E. esculentus were used in 1989 and again in 1990 and were undetected in microscopic examination of the gametes. He also implies that eggs from the alleged hermaphrodite E. esculentus did not divide when exposed to concentrated E. esculentus sperm for 24 hours, but did so when mixed with the same number of Ascidia mentula eggs, which they supposedly supplanted. Neither the hermaphrodite Echinus hypothesis nor Hart’s alternative suggestion of contaminated cultures would explain the fact that some of the plutei from these eggs metamorphosed into tetraradial urchins (Figure 3) and others metamorphosed into spheroids that contained tadpole-like shapes (Figures 5B, 5C). We can find no record of wild tetraradial urchins, but Hinegardner obtained ‘square’ specimens of LytEchinus pictus (Verrill, 1867) by crossing adults reared from larvae with developmental abnormalities [11]. Hart’s results show that the urchins in question had Echinus mitochondrial DNA and he argues that this precludes them from being hybrids. The male mitochondrion disintegrates in early cleavage in most mammals (e.g. [12]), but it survives in some bivalve molluscs [13]. Hart has provided evidence that it can also survive in some Ascidia x Echinus hybrids. The material that he examined was from urchins from the minority (about 8%) of hybrid plutei that developed Echinus rudiments. The great majority of the plutei in this experiment had no Echinus rudiments and they developed into spheroids, but their mitochondrial DNA was not investigated. |
| |
| The tadpole larvae produced by several Ascidia x Echinus crosses (Table 1) and by crossing Ciona x PsammEchinus (Experiment 3) died without metamorphosing. They may have been true hybrids, with genes from either parents, or the ascidian eggs may have developed by pseudogamy: a form of parthenogenesis in which the egg is stimulated to divide by sperm which makes no genic contribution to the embryo. The larvae produced by crossing PsammEchinus x Ascidiella in Experiment 4 could not have been pseudogamous, although the initial larvae were maternal. These plutei developed only four arms, which they resorbed to become self-replicating spheroids and some of these spheroids developed into forms with inclusions resembling parts of ascidians. Parthenogenetic development in the related species Echinus esculentus shows little difference from normal homosperm development [14]. These parthenogenetic larvae all grew into eight-armed plutei, none of which became spheroids and some developed Echinus rudiments. |
| |
| The 1989/1990 experiments (Experiments 1 and 2), in which ascidian eggs were fertilized with sea urchin sperm, show similarities to and differences from the near reciprocal cross in 2002 (Experiment 4) in both methods and results. In Experiments 1 and 2 untreated ascidian eggs were mixed with undiluted sea urchin sperm, but in Experiment 4 sea urchin eggs were treated with acid seawater before exposure to dilute ascidian sperm. Pluteus larvae were produced in all cases, but these were paternal in Experiments 1 and 2 and maternal in Experiment 4. In Experiment 2, a small minority of the larvae eventually produced fertile adults, similar to the paternal parent (Echinus esculentus), but most larvae became spheroids, at least some of which contained tadpole-like inclusions. In Experiment 4, all the plutei developed into spheroids, a few of which temporarily contained forms resembling parts of juvenile ascidians. The spheroids in Experiments 2 and 4 were generally similar in size, but those in Experiment 2 could swim or attach and they did not divide. Those in Experiment 4 were permanently bottom-living and they divided repeatedly. |
| |
| There were many attempts to hybridize animals in the late 19th and the 20th centuries and Giudice [15], listed examples involving sea urchins. Prior to 1910, there were few phytoplankton cultures to feed any resulting larvae [16], but little attempt was made to rear hybrid larvae when cultured phytoplankton became available. For example, crosses between the regular sea urchin Strongylocentrotus purpuratus (Stimpson, 1857) and the sand-dollar Dendraster excentricus (Eschscholtz, 1831), both from the Pacific coast of North America, have been reported by three authors [17-19]. They noted paternal features in some of the larvae and maternal features in others, but in no case these were kept alive for more than 12 days. It would be of great interest to see if such hybrids could metamorphose and into what Hinegardner [11], reared experimental hybrids between the sand dollars Encope micropora L Agasiz, 1841 (as E. californicus) and Dendraster excentricus and the reciprocal cross and the resulting adults from both crosses showed a preponderance of paternal characteristics. Hybrids between a regular sea urchin and a heart urchin (Spatangoidea) would be of particular interest, because the plutei of regular urchins have eight arms (or fewer) while heart urchin plutei have 13 arms. |
| |
| The larval transfer hypothesis requires only about ten interphyletic hybrids in the last 400 million years that have given rise to lineages of animals with larvae, although more hybrids between less distantly related animals are postulated [2,20]. We suggest that, over the ages, many hybrids comparable to the self-perpetuating spheroids described in this paper could have been produced. Such forms could have existed indefinitely in the absence of competition, but most would soon have become extinct in the competitive world of natural selection. The rare survivors gave some animals new larvae and new life histories. |
| |
| We should be wary of making generalizations from so few laboratory hybridizations, but our results show that some remotely related animals may, on occasion, hybridize, that hybrid eggs may hatch in a form resembling the larvae of either parent and that gene complexes coded for larval and adult features can retain their independence in hybrids. This independence is consistent with the larval transfer hypothesis, which claims that larvae had their origins in animals distantly related to the species that acquired larvae. They are difficult to reconcile with the widely accepted assumption that adult and their corresponding larvae evolved from the same genome. |
| |
| The pre-treatment of eggs with acetic acid seawater before exposure to dilute sperm of another species was initially used by Raff et al. [7], to hybridize congeneric sea urchins, but our successful use of the same technique in an interphyletic cross (Experiment 4) suggests that it could be used in other crosses between remotely related animals. We would welcome more attempts to cross animals in different orders, different classes and different phyla and urge experimenters to try to rear any hybrids through metamorphosis. I (DIW) call for attempted crosses between onychophorans and insects, relevant to the hypothesis that caterpillars are transferred onychophorans [6]. Experimental hybrids are pertinent not only to the larval transfer hypothesis but also to the suggestion that component transfer may have played a significant part in evolution [3]. Lophophores occur in brachiopods, bryozoans, phoronans and pterobranch hemichordates and a similar organ is present in entoprocts. Even if consideration is limited to brachiopods, bryozoans and phoronans, descent of these taxa from a common ancestor is precluded in the studies on their morphology, palaeontology and genetics [3,21]. Laboratory hybrids between animals with and without lophophores might shed light on the inheritance of this organ. |
| |
| Acknowledgements |
| |
| We are grateful to Sebastian Holmes for the loan of several pieces of vital equipment in 2002, including photomicrography apparatus and a computer and for rescuing specimens and photographs relating to Experiment 2 when the Port Erin Marine Laboratory was closed in 2006. We also thank Lynn Margulis and Wolfgang Krumbein for financial support in 2002, Trevor Norton for facilities at Port Erin Marine Laboratory, David Woodworth for providing ascidians from seawater tanks, Ian Allen for acid seawater, Clemente Graziono and Nicholas Fullerton for cultured phytoplankton, Enid Williamson for maintaining hybrid cultures from Experiment 4 for two years and Farley Fleming for constructive contributions to the manuscript. |
| |
| |
| References |
| |
- Darwin C (1859) On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. John Murray, London.
- Williamson DI (2003) The origins of larvae. kluwer academic publishers, Dordrecht, Netherlands.
- Williamson DI (2006) Hybridization in the evolution of animal form and life-cycle. Zool J Lin Soc 148: 585-602.
- Williamson DI (1987) Incongruous larvae and the origin of some invertebrate life-histories. Prog Oceanogr19: 87-116.
- Williamson DI(1992) Larvae and evolution: toward a new zoology. Chapman and Hall, New York and London.
- Williamson DI (2009) Caterpillars evolved from onychophorans by hybridogenesis. Proc Natl Acad Sci USA 106: 19901-19905.
- Raff EC, Popodi EM, Sly BJ, Turner FR, Villinski TJ, et al. (1999) A novel ontogenetic pathway in hybrid embryos between species with different modes of development. Development 126: 1937-1945.
- MacBride EW (1911) Two abnormal plutei of Echinus, and the light which they throw on the factors in the normal development of Echinus. Q J Microsc Sci 57: 235-250.
- Hart MW (1996) Testing cold fusion of phyla: maternity in a tunicate x sea urchin hybrid determined from DNA comparisons. Evolution 50: 1713-1718.
- Moore HB (1932) A hermaphrodite sea urchin. Nature 130: 59.
- Hinegarner RT(1975) Morphology and genetics of sea urchin development. Amer Zool 15: 679-689.
- Szollosi D (2005) The fate of sperm middle-piece mitochondria in the rat egg. J Exp Zool 159: 367-377.
- Curole JP, Kocher TD (2002) Ancient sex-specific extension of the cytochrome c oxidase II gene in bivalves and the fidelity of doubly-uniparental inheritance. Mol Biol Evol 9: 1323-1328.
- Shearer C, Lloyd DJ (1913) Memoirs: On methods of producing artificial parthenogenesis in Echinus esculentus and the rearing of the parthenogenetic plutei through metamorphosis. Q J Microsc Sci s2-58: 523-552.
- Giudice G (1973) Developmental biology of the sea urchin embryo. Academic Press, New York and London.
- Allen EJ, Nelson EW (1910) On the artificial culture of marine plankton organisms. J Mar Biol Ass UK8: 421-474.
- Flickinger RA (1957) Evidence from sea urchin - sand dollar hybrid embryosfor a nuclear control of alkaline phosphatase activity. Biol Bull 112: 21-27.
- Moore AR (1957) Biparental inheritance in the interordinal cross of sea urchin and sand dollar. J Exp Zool 135: 75-83.
- Brookbank JW (1970) DNA synthesis and development in reciprocal interordinal hybrids of a sea urchin and a sand dollar. Dev Biol 21: 29-47.
- Williamson DI (2012) The origins of chordate larvae. Cell Development Biol 1: 1.
- Passamaneck Y, Halanych KM (2006) Lophotrochozoan phylogeny assessed with LSU and SSU data: evidence of lophophorate polyphyly. Mol Phylogenet Evol 40: 20-28.
|
| |
| |