| Research Article |
Open Access |
|
| Rosemary B Bassey1*, Airat A Bakare2, Abraham AA Osinubi and Ademola A Oremosu |
| 1Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Nigeria |
| 2Department of Anatomy, College of Medicine, University of Lagos, Lagos, Nigeria |
| *Corresponding authors: |
Rosemary B Bassey
Department of Anatomy
Faculty of Basic Medical Sciences
University of Uyo, Akwa Ibom State, Nigeria
Tel: 234-8064853304, 8098555224
E-mail: Rosemary_Bassey@yahoo.com. |
|
| |
| Received December 01, 2011; Published July 27, 2012 |
| |
| Citation: Bassey RB, Bakare AA, Osinubi AAA, Oremosu AA (2012) Staining Properties of Lonchocarpus Cyanescens on the Testes. 1: 211. doi:10.4172/scientificreports.211 |
| |
| Copyright: © 2012 Bassey RB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Background: The use of non-allergic, non-toxic and eco-friendly natural dyes on textiles has become a matter of significant importance due to the increased environmental awareness in order to avoid some hazardous synthetic dyes. |
| |
| Objective: To determine the staining properties of the dye from Lonchocarpus cyanescens extract on histomorphology of the testes. |
| |
| Methodology: The freshly cut leaves of Lonchocarpus cyanescens were put into a container and boiling water was poured to cover the leaves in the jar. It was covered and left for about an hour. The liquid was strained to and potassium hydroxide was added to the strained liquid to reach a pH of 9 or higher. A whisk was used to mix air into the liquid, and then allowed to sit until the blue indigo has sunken to the bottom of the container. The dye was diluted with 70% ethanol to a concentration of 0.1 g/ml and was used to stain testes sections. Its potential for use as a counter stain was also investigated. |
| |
| Results: The testes sections were stained in shades of blue. The dye overshadowed the colours of Haematoxylin and Eosin. Preliminary phytochemical screening of Lonchocarpus cyanescens revealed that it contains alkaloids, saponins, flavonoids and tannins. |
| |
| Keywords |
| |
| Lonchocarpus cyanescens; Indigo; Dye; Stains; Testis |
| |
| Introduction |
| |
| Mankind has known about natural dyes for millennia, using it in various aspects of their lives - from tribal or funeral ceremonies to body painting, decorative art, clothing or domestic decoration [1]. |
| |
| The discovery of synthetic dyes in 1856 by William Henry Perkin gave way to a range of new synthetics that spread throughout the world [2]. With advances in chemical techniques, the manufacture of synthetic dyes became possible leading to greater production efficiency in terms of quality, quantity and the potential to produce low-cost raw materials. As a result, natural dyes were progressively replaced by synthetic dyes by the beginning of the twentieth century [3]. |
| |
| Dyes with azo bonds nitro- or amino-groups are carcinogenic, causing tumors of liver and urinary bladder in experimental animals. Reduction of azo dyes leads to formation of aromatic amines and several aromatic amines are known mutagens and carcinogens. Natural dyes offer an important alternative in these regards as these are safer in use with no health hazards, have easy disposability and are biodegradable [4]. |
| |
| Lonchocarpus cyanescens is a shrub of twining habitat, belonging to the tribe Dalbergiae of the natural order Leguminosae [5]. Lonchocarpus cyanescens is known as ‘elu’ in Yoruba, ‘anunu’ in Ibo, ‘talaki’ in Hausa, ‘suru’ in Tiv and ‘ebelu’ in Edo languages of Nigeria. The indicancontaining leaves and young sprouts are used after fermentation to obtain the blue indigo dye, which is used for colouring textile and other materials. The precipitate of the fermented material, known as ‘aro’ in Yoruba, is sold in local markets. |
| |
| Several natural dyes are used for the study of histology, histochemistry and histopathology. The most important and most used histological dye, haematoxylin is a natural dye produced from the logwood Haematoxylon campechianum and in combination with Eosin is used for the demonstration of general tissue structures [6]. |
| |
| Popular demand for more natural products and the toxicity problem in relation to synthetic dyes are the principal factors in encouraging a revival in the use of natural dyes. This study was therefore designed to study the staining properties of Lonchocarpus cyanescens, a commonly used textile dye, on the histomorphology of the testis with a view of introducing it as a histological stain for testicular tissues. |
| |
| Methodology |
| |
| Preparation of extract |
| |
| The leaves of Lonchocarpus cyanescens were collected locally from Ikono local government area of Akwa Ibom State and authenticated at the Botany department of the University of Uyo, Nigeria. |
| |
| The freshly cut leaves of Lonchocarpus cyanescens were put into a container and boiling water was poured to cover the leaves in the container. It was covered and left for about an hour. The liquid was strained and potassium hydroxide was added to the strained liquid to reach a pH of 9 or higher. A whisk was used to mix air into the liquid, and then allowed to sit until the blue indigo has sunken to the bottom of the container. It was left to dry and 50 g of dyestuff was obtained. |
| |
| Preparation of sections |
| |
| The testes were carefully dissected out, trimmed of all fat and blotted dry to remove any blood. They were fixed in 10% formal saline. The fixed tissues were transferred to a graded series of ethanol. On day 1, they were placed in 70% alcohol for 7 hours, then transferred to 90% alcohol and left in the latter overnight. On day 2, the tissues were passed through three changes of absolute alcohol for an hour each then cleared in xylene. Once cleared, the tissues were infiltrated in molten paraffin wax in the oven at 58°C. Three changes of molten paraffin wax at one-hour intervals were made, after which the tissues were embedded in wax and blocked out. Prior to embedding, it was ensured that the mounted sections to be cut by the rotary microtome were orientated perpendicular to the long axis of the testes. The sections were designated «vertical sections». Serial sections of 5 μm thick were obtained from a solid block of tissue using rotary microtome (SAKURA FINE TECH, Netherlands) and attached to sections and dried at 65°C for 45 min. |
| |
| Preparation of Lonchocarpus cyanescens staining solution and staining methods |
| |
| 1 g of the dried ethanolic extract of Lonchocarpus cyanescens was dissolved in 10 ml of 1% acetic acid in 70% ethanol, 1% sodium hydroxide in 70% alcohol and water respectively. |
| |
| Sections were dewaxed in xylene for 4 min and hydrated through descending grades of alcohol. Sections were then stained with each of the prepared solutions of Lonchocarpus cyanescens for 5, 10 and 15 minutes. The sections were finally rinsed in water, dehydrated, cleared and mounted in a synthetic mountant. |
| |
| Some sections of the testis were stained with Haematoxylin for 15 minutes and counterstained with Lonchocarpus cyanescens 1 minute; Lonchocarpus cyanescens was used to stain the testes for 5 minutes and counterstained with Eosin for 1 minute. |
| |
| Preliminary phytochemical screening |
| |
| The extract of Lonchocarpus cyanescens was screened to determine the presence of the following metabolites through preliminary phytochemical screening. Alkaloids were detected using the Dragendoff’s reagent, Mayer’s reagent, Wagner’s reagent and tannic acid. Flavonoids were determined by the ferric chloride test, lead acetate test, sodium hydroxide test and ethyl acetate test. Tannin detection was by ferric chloride test and bromine water test, while phlobotannins was with hydrochloric acid. Saponin was determined with the froth tests and haemolytic test. |
| |
| Results |
| |
| The dye from Lonchocarpus cyanescens was observed to be insoluble in water. The dye dissolved in 1% sodium hydroxide in 70% alcohol stained the testes blue but was washed off the tissue completely when rinsed in water and dehydrated in descending grades of alcohol. |
| |
| The best results however were observed in the sections stained with the dye dissolved in 1% acetic acid in 70% alcohol as it was colour fast. It stained the nuclear structures in the testes sections blue within 5 to 15 minutes. |
| |
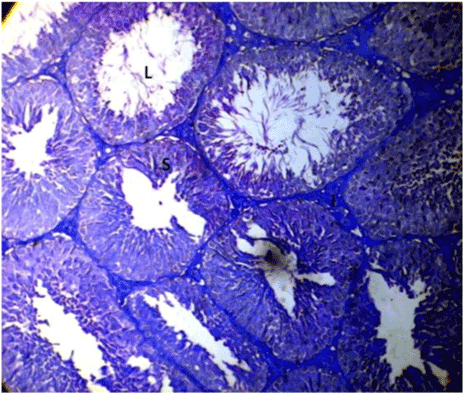
| Optimal staining intensity was achieved at staining time of 5 minutes (Figure 1). At 10, the staining was too intense and differentiating the different cells was difficult (Figure 2). At 15 minutes, the stain completely overshadowed the cellular structures in the testis (Figure 3). |
| |
| |
|
|
Figure 1: Photomicrograph of testes stained with Lonchocarpus cyanescens arrows head show the deeply stained cells of the spermatogenic series and interstitium. |
|
| |
|
|
Figure 2: Photomicrograph of testes stained with Lonchocarpus cyanescens. |
|
| |
|
|
Figure 3: Photomicrograph of testes stained with Lonchocarpus cyanescens. |
|
| |
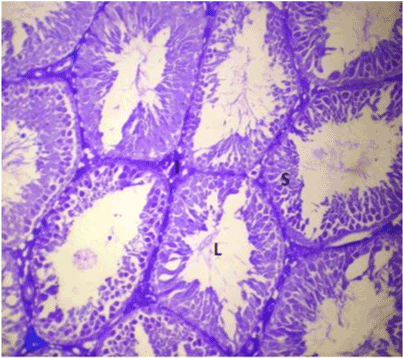
| Lonchocarpus cyanescens counter-stained with Eosin produced a blend in colours to form a purple stain, with Lonchocarpus cyanescens taking up the basic staining character and Eosin taking up the acidic staining character (Figure 4). In the counter-staining of Haematoxylin with Lonchocarpus cyanescens, Haematoxylin took up the basic staining character, Lonchocarpus cyanescens blended in as an acid dye but its blue colour overpowered that of Haematoxylin thereby preventing proper differentiation (Figure 5). |
| |
|
|
Figure 4: Photomicrograph of testes stained with Lonchocarpus cyanescens counter-stained with Eosin. |
|
| |
|
|
Figure 5: Photomicrograph of testes stained with Haematoxylin; counter-stained with Lonchocarpus cyanescens. |
|
| |
| Preliminary phytochemical screening of Lonchocarpus cyanescens revealed that it contains alkaloids, saponins, flavonoids and tannins. |
| |
| Discussion |
| |
| In recent years, there has been a revival of the use of dyes and color of natural origin for coloring food, pharmaceutical, cosmetic and textile products. This increasing demand for material of natural origin is because of the toxic nature of many of the synthetic dyes; and natural dyes are becoming widely recognized throughout the world [7]. The present scenario is focused more towards the utilization of the vast diversity of natural resources of color pigments in place of their synthetic counterparts [8]. |
| |
| The findings from this study strongly suggest that Lonchocarpus cyanescens has great potential for use as stains for testicular histology. Bettinger & Zimmerman [9] have suggested that several cationic dye complexes existing at low pH contribute to the colour of dyes. The pH of the staining solution also influences this reaction and glacial acetic acid can be added to enhance nuclear staining as seen in this study. This occurs because the pH of such acidified solutions is higher than the iso-electric point of nucleic acids which will thus express a negative charge` and more readily attract and bind the cationic dye lake [10]. Another factor affecting staining reactions is the staining time; which can stain quickly up to a point at which all dye uptake sites have been utilized. |
| |
| Phytochemical screening of the dye confirmed the presence of saponins, tannins, flavonoids and alkaloids. Among these compounds, tannins and flavonoids are the substances which can give the colour. Tannins are the most important ingredients which are necessary for dyeing with natural dyes [11]. |
| |
| Conclusion |
| |
| Lonchocarpus cyanescens dye is a promising histological stain that can serve as a useful stain for histological examination of tissues and histopathological diagnoses. Many natural dyes however, contain impurities and several dye fractions [12]. These will be further investigated through thin layer chromatography and spectrophotometric studies. |
| |
| Conflict |
| |
| None. |
| |
| |
| References |
| |
- Cardon D (2003) Le Monde des Teintures Naturelles, E´ ditions Belin, Paris.
- Mati E, de Boer H (2010) Contemporary Knowledge of Dye Plant Species and Natural Dye Use in Kurdish Autonomous Region, Iraq. Economic Botany 64: 137-148.
- Kyritsis (2001) 1st World Congress on Biomass for Energy and Industry. 2: 1148.
- Jiang N (2008) Effect of Technical Barriers to Trade on Chinese Textile Product Trade.International Business Research 3: 91-97.
- Ogungbaro ST (2010) Extract of Lonchocarpus cyanescens and Crassocephalum crepidioides leaf extracts on monoamine oxidase activity from rat brain. Thesis submitted to the Department of biochemistry, University of Agriculture, Abeokuta, Ogun state in partial fulfillment of the requirement for the award of Bachelor of Science (B.Sc) degree in Biochemistry. Pp: 24.
- Avwioro OG (2002) Histochemistry and tissue pathology. Claverianun press, Nigeria; 134-213.
- Nilani P, Duraisamy B, Dhamodaran P, Kasthuribai N, Alok S, et al. (2008) A Study on the effect of marigold flower dye with natural mordant on selected fibers. Journal of Pharmacy Research 1: 175-181.
- Jothi D (2008) Extraction of Natural Dyes from African Marigold Flower (Tagetes ereecta L) for Textile Coloration. AUTEX Research Journal 8: 49-53.
- Bettinger CL, Zimmermann HW (1991) New investigations on hematoxylin, hematein, and hematein-aluminium complexes II. Hematein-aluminium complexes and hemalum staining. Histochem 96: 215-228.
- Prentø P (2001) A contribution to the theory of biological staining based on the principles for structural organization of biological macromolecules. Biotechnic and histochemistry 76: 137-161.
- Win ZM, Swe MM (2008) Purification of the Natural Dyestuff Extracted from Mango Bark for the Application on Protein Fibres. World Academy of Science, Engineering and Technology 46: 536-540.
- Banerjee A and Mukherjee AK (1981) Chemical aspects of santalin as a histological stain. Stain Technol 56: 83-85.
|
| |
| |