| Research Article |
Open Access |
|
| Ahmed Moussa1*, Djebli Noureddine2, Aissat Saad1, Meslem Abdelmelek1 |
| 1Institute of Veterinary Sciences University Ibn-Khaldoun Tiaret, Algeria |
| 2Departments of Biology, Faculty of Sciences, Mostaganem University, Algeria |
| *Corresponding authors: |
Dr. Ahmed Moussa
Institute of Veterinary Sciences University Ibn-Khaldoun Tiaret (14000)
Algeria
Tel: +213 65234059
Fax: +213 46 425001
E-mail: moussa7014@yahoo.fr |
|
| |
| Received April 19, 2012; Published July 30, 2012 |
| |
| Citation: Moussa A. Noureddine D. Saad A, Abdelmelek M (2012) Anti-Aspergillus niger of eucalyptus honey influenced by thermal treatment. 1: 175. doi:10.4172/scientificreports.175 |
| |
| Copyright: © 2012 Moussa A et al., This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| The objective of this study was to evaluate the effects of heat processing on the antifungal activity of honey against Aspergillus niger. A sample of eucalyptus honey was divided into four portions of 250 g each. One of the four honey portions was not heated (Room temperature: 25°C), The other portions were placed in water bath during 24 hours at 40°C, 60°C and 80°C temperatures. The HMF rates, Acidity, pH and the refraction index were determined by harmonized methods. The antifungal tests (Minimum Inhibitory Concentration) were carried out on Sabouraud agar medium embedded with honey according to dilution test. The moisture shows values of 15.65% and 15.83%, pH between 4.10 and 4.24, the free acidity ranges between 33.8 and 38.36 meq kg-1, Hydroxymethylfurfural (HMF) content shows values between 28.8 and 103.44 mg kg-1. |
| |
| The antifungal action of the non-heated fraction (Fc) of honey in vitro was marked 46 % (vol/vol) than heated fractions of honey (47%, 48%, and 50%) vol/vol. respectively the antifungal activity of each fraction decreased in the following order: 46% (F25°C), 47% (F40°C), 48% (F60°C) and 50% (F80°C). Our findings indicate that different levels of parameters physical-chemical properties of honey to different temperatures showed inhibitory activity against A.niger with variable degrees but heating honey diminish its antifungal capacity. |
| |
| Keywords |
| |
| Honey; Aspergillus niger; Antifungal activity; Thermal treatment |
| |
| Introduction |
| |
| Therapeutic limitations, development of fungal drug resistance, drug-related toxicity, drug interactions and insufficient bioavailability of the currently available antifungal drugs have made the development of drugs necessary that would be able to treat the emerging fungal infections [1]. Honey forms part of traditional medicine in many cultures [2], although it is most widely used as sweetener. It is composed of at least 181 components and is basically a solution supersaturated in sugars, fructose (38%) and glucose (31%) are the most important [3], the moisture content is about 17.7%, total acidity 0.08%, and ashes constitute 0.18% [4]. In addition, there is a great variety of minor components, including phenolic acids and flavonoids, the enzymes glucose oxidase and catalase, ascorbic acid, carotenoids, organic acids, amino acids, proteins, and α-tocopherol [5]. |
| |
| Acidity and pH of honey are mainly due to the content of the gluconolactone ⁄ gluconic acid present as result of the enzymatic action of glucose-oxidase [6]. Honey moisture content is a quality criterion that determines its shelf-life during storage because of resistance to spoilage by yeast fermentation. Differences on moisture content also depend on the harvest season and the degree of maturity reached in the hive [7]. |
| |
| Hydroxymethylfurfural (HMF) content is one of the most important quality parameters of the quality and health safety of honey. This cyclic aldehyde develops in honey either by hexose dehydration (glucose and fructose) in acidic environment or as a result of Maillard’s reaction. HMF content in fresh honey is very low or nonexistent, its concentration increases in the course of storing (in relation to pH, the length of storing) and also in the course of the honey heating [8]. |
| |
| Several types of honey are produced in Algeria, where honey production is a traditional practice, well implanted in several regions. The Tiaret region is located in the west of Algeria, where, due to its edaphoclimatic conditions and flora diversity, Hedysarum coronarium, Eucalyptus camaldulensis and E. globuluss , Pimpinella anisum, and Trifolium alexandrinum are the principal honey types produced, being Eucalyptus honey the most important unifloral one. |
| |
| The aim of the present work was to detect which level of physicochemical parameter values Anti-Aspergillus niger of eucalyptus are honey influenced by thermal treatment |
| |
| Materials and Methods |
| |
| Heating treatment |
| |
| A sample of eucalyptus honey was divided into four portions of 250 g each. One of the four portions was no heated (room temperature: 25°C), the other portions were placed in a water bath for 24 hours at 40°C, 60°C, 80°C. The four fractions of honey were examined immediately after heating for their moisture content, pH, free acidity, HMF and antifungal Activity. All experiments were conducted in duplicate. |
| |
| Determination of moisture, pH, free acidity and HMF contents in honey |
| |
| Physico-chemical parameters were analysed using The Official Methods of Analysis of Association of Official Analytical Chemists [10] and The Harmonised Methods of the European Honey Commission [11]. Samples were analyzed during the same time period to ensure uniform conditions and comparability. pH was measured by means of a potentiometric pH meter (CG822 SGH ) in a solution containing 10 g of honey in 75 ml of CO2 free distilled water [10]. |
| |
| The moisture content was determined based on the refractometric method. In general, the refractive index increases with the increase in the solid content. The refractive indices of honey samples were measured at ambient temperature using an Atago hand refractometer and the readings were further corrected for a standard temperature of 20°C by adding the correction factor of 0.00023/°C. Moisture content was determined in triplicate and the% moisture content values corresponding to the corrected refractive index values were calculated using Wedmore’s table [10]. |
| |
| Free acidity was determined as follows by the titrimetric method: 10 g honey samples were dissolved in 75 mL of CO2-free water in a 250 mL beaker. The electrode of the pH meter was immersed in the solution, stirred with a magnetic stirrer and tritated to pH 8.5 by adding 0.05 N NaOH solution [10]. |
| |
| Hydroxymethyl furfural (HMF) was detected using a technique based on the method described by Winkler [12]. Five grams of honey were dissolved, without heating, in oxygen free distilled water and transferred to a 125 ml graduated flask and diluted to volume with oxygen free distilled water. Two millilitres of honey solution was pipetted into two tubes and 5 ml of P-toluidine solution was added to each. Into one test tube, 1 ml of water was pipetted and into the other 1 ml of barbituric acid solution was added; both mixtures were then shaken. Absorbance was read using a spectrophotometer against a blank at a wave length of 550 nm. Calculation: Mg/100 g hydroxymethyl furfural = absorbance/test x 192 [13]. |
| |
| Preparation of inoculum suspension |
| |
| Aspergillus niger was kindly provided by the (Institute of Veterinary Sciences University Ibn-Khaldoun Tiaret, Algeria). Strains were maintained by subculture in specific media (SDA: Sabourad dextrose agar). Stock suspensions of A. niger were prepared from sporulating 7-day-old cultures grown on SDA at 28°C. Colonies were covered with 5 ml sterile distilled water and the surface scraped with a sterile loop. The mixture of conidia and hyphal fragments was filtered through an 8 mm sterile filter and collected in a sterile tube. This procedure removed the majority of the hyphae, producing inocula composed mainly of spores [13-15]. Turbidity of the final inocula was adjusted to 0.5 x 106–5.0 x106 spores ml-1, at a wavelength of 520 nm, and transmission adjusted to 70% in a spectrophotometer. |
| |
| Minimum Inhibitory Concentration measurement (MIC) |
| |
| Increased concentrations of honey (10-50 % vol/vol) were incorporated into media to test their efficiency against A.niger. Each plate with final volume of honey and media of 5 ml was inoculated and incubated at 37ºC for 5 days. The MIC was determined by finding the plates with the lowest concentration of honey on which the strain would not grow. All MIC values are expressed in % (vol/vol) were added to a range of honey concentrations lower than the MIC. |
| |
| Results |
| |
| The pH of the honey samples varied from 4.10 to 4.24 (Table 1). These values were within the pH range of 3.81–6.32 reported by [16,17]. The pH values of honey are of great importance during extraction and storage, since acidity can influence the texture, stability, and shelf life of honey [18]. |
| |
|
|
Table 1: Heat treatment, pH, Moistire, Free acidity and HMF and growth inhibition of C. albicans in different honey fractions. |
|
| |
| All the moisture values were under the allowed limit of 21% moisture content permitted by FSANZ [19]. The moisture content of the studied honey samples ranged from 15.65% to 15.83% (Table 1). |
| |
| The moisture level of the analyzed fractions was consistent with the previous reported values for some Algerian honeys for which the corresponding values ranged from 18.7% to 21.8% [20] In Codex Alimentarius [21]) and EU [22] . Council directives the maximum M content value of pure floral honey is given as 23% for heather honeys and not more than 20% in general. The water content of honey depends on various factors, for example: the harvesting season, the degree of maturity reached in the hive, and environmental factors [23]. Furthermore, the water content value is also of great importance because it is considered to be a useful parameter for describing moistness and viscosity of honey. |
| |
| Free acidity of all four samples fell within the permitted range proposed by [21] of no more than 50 milliequiv acid/kg. The free acidity of honey samples in this study ranged from 33.08 to 38.36 meq kg-1 respectively (Table 1). High free acidity values may indicate the fermentation of honey sugar by yeasts. It is well known that during fermentation, glucose and fructose are converted into carbon dioxide and alcohol. Alcohol is further hydrolysed in the presence of oxygen and converted to acetic acid, which contributes to the level of free acidity in honey. |
| |
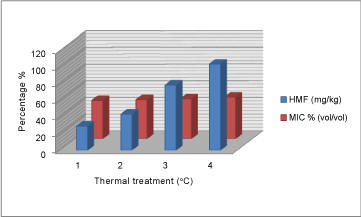
| The HMF content is widely recognized as a parameter of honey samples freshness, because it is absent in fresh honeys and tends to increase during processing and/or aging of the product. Several factors influence the levels of HMF, such as temperature and time of heating, storage conditions, pH and floral source, thus it provides an indication of overheating and storage in poor conditions [24]. HMF shows values between 28.8 and 43.29 kg-1, fractions 3 and 4 with values between 78.32 and 103.44 mg kg-1 exceeded the limits set by European Community legislation [21] due to overheating. |
| |
| The different level of value for the four fractions of honey showed antifungal activity against A.niger to varying degrees (Table 1). |
| |
| HMF value of unheated honey (28.8 mg/kg) showed the highest inhibitory effect on yeast growth compared to heated fractions (Figure 3). Similarly, free acidity value of unheated honey (33.8 meq kg-1) showed the highest inhibitory effect on mould growth compared to treated fractions by heat (Figure 2). Furthermore, pH value of honey 4.24 showed the highest inhibitory effect on yeast growth compared to heated fractions (Figure 1). |
| |
| |
|
|
Figure 1: Variation of pH / MIC %(vol/vol) |
|
| |
|
|
Figure 2: Variation of Free acidity / MIC % (vol/vol) |
|
| |
|
|
Figure 3: Variation of HMF / MIC % (vol/vol) |
|
| |
| Discussion |
| |
| Aspergillus niger could be implicated in ophthalmic and some coetaneous problems [25,26].The resistance of A. niger to conventional antifungal is also a common problem [27,28]. Therefore, there is an urgent need for new antifungal agents for the efficient management of mould infections. Research on the active constituents of natural or traditional medicines, which are a potential source for new drugs, is drawing more attention. Honeys have long been recognized for their antimicrobial activity against bacteria, molds and yeasts with unique properties that render it bacteriostatic and bactericidal. The high osmotic pressure, low water activity, low pH, low redox potential of honey, hydrogen peroxide and other phytochemical factors might contribute to the honey antimicrobial nature. Their relative importance depends on the sensitivity of the species and the level of additional factors in any honey [6,29]. |
| |
| Honey has been used since ancient times for the treatment of several diseases. Although several in vitro studies have demonstrated the antibacterial activity of honey [30], limited numbers of studies have examined the activity of honey against fungi. |
| |
| Ahmed et al. [32] found that the MIC of four varieties of honey against A. niger ranged between 53 and 57% (vol/vol). According to Boukraa et al. [33], the minimum concentration of honey in Yeast Peptone Glucose Agar Media (YPGA) required to inhibit A. niger was 46% (vol/vol). |
| |
| The inhibitory effect of honey on A. niger is not correlated with the decrease in pH or the increase in free acidity as seen in table 1. In general, bacteria grow optimally in the pH range 6.0–8.0, yeasts 4.5–6.0 and filamentous fungi 3.5-4.0 [34]. Koc et al. [31] assessed the in vitro antifungal activity of four Turkish honey samples from different botanical origin against Trichosporon spp. and Candida spp. and concluded that multifloral honey had the highest antifungal activity and it probably contains the highest total phenolic content. According to Brudzynski and Miotto [35], heat-treatment resulted in about a two•fold increase in phenolic content for all tested honeys (n =2 2) compared to the control. Hydrogen peroxide is known to have antimicrobial properties and much evidence exists to suggest that it is this compound which confers antimicrobial activity to honey [36-38]. Hydrogen peroxide at low concentration, far beneath, the apparent threshold of its biological activity, can affect the fungus development [39]. H2O2 produced by honey glucose oxidase is heat-sensitive (White) as our results showed a decrease in antifungal activity after heat treatment, it can be concluded that the antifungal activity was not due to the phenolic but to H2O2, or in a synergistic effect. |
| |
| Collectively, our findings indicate that different levels of parameters physical-chemical properties of honey to different temperatures showed inhibitory activity against A.niger with variable degrees. |
| |
| Acknowledgements |
| |
| Authors thank Staff of Tiaret University for providing material. |
| |
| |
| References |
| |
- Melo e Silva F, de Paula JE, Espindola LS (2009) Evaluation of the antifungal potential of Brazilian Cerrado medicinal plants. Mycoses 52: 511âÃâ¬Ãâ517
- G´omez-Caravaca AM, G´omez-Romero M, Arr´aez-Rom´an D, Segura-Carretero A, Fern´andez-Guti´errez A (2006) Advances in the analysis of phenolic compounds in products derived from bees. J Pharmac Bio Anal 41: 1220-1234.
- Gheldof N, Wang XH, Engeseth NJ (2002) Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem 50: 5870-5877.
- Nagai T, Inoue R, Kanamori N, Suzuki N, Nagashima T (2006) Characterization of honey from different floral sources. Its functional properties and effects of honey species on storage of meat. Food Chem 97: 256âÃâ¬Ãâ262.
- Ferreres F, Garciaviguera C, Tomaslorente F, Tomasbarberan FA (1993) Hesperetin C a marker of the floral origin of citrus honey. J Sci Food Agric 61: 121-123.
- Molan PC (1992) The antibacterial activity of honey: the nature of the antibacterial activity. Bee World 73: 5-28.
- Corbella E, Cozzolino D (2006) Classification of the floral origin of Uruguayan honeys by chemical and physical characteristics combined with chemometrics. Food Sci Tech 39: 534-539.
- Klára B, Michaela D, Ivana Bo, Lenka V (2011) Impact of Microwave Heating on Hydroxymethylfurfural Content in Czech Honeys. Czech J Food Sci 29: 328-336.
- Bodganov S, Martin P, Lu¨llmann C (1997) Harmonised methods of the European Honey Commission. Apidologie 1-59.
- AOAC (1990) Official methods of analysis. In K Helrich (Ed.) (15th ed.). Arlington, VA, USA: Association of official Analytical Chemists, Inc.
- Winkler C (1955) Beitrag Zur Nachweis und Zur Bestimmung Von Kunsthoning. Zeitschr. Lebensm. Unters. Forsch 102: 161-167.
- Auerbach F, Borries G (1924) Auerbach and Borries equation. Z Nahr Genssm 22: 353-358.
- Petrikkou E, Rodri¢guez-Tudela JL, Cuenca-Estrella M, Go¢mez A, Molleja A, et al. (2001) Inoculum standardization for antifungal susceptibility testing of filamentous fungi pathogenic for humans. J Clin Microbiol 39: 1345-1347.
- Santos DA, Hamdan JS (2005) Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J Clin Microbiol 43: 1917-1920.
- Santos DA, Barros MES, Hamdan JS (2006) Establishing a method of inoculum preparation for susceptibility testing of Trichophyton rubrum and Trichophyton mentagrophytes. J Clin Microbiol 44: 98-101.
- Gerard D, Karen HJ, Daniel K, Thomas FW, Martin PG (2005) Preliminary contribution to the characterisation of artisanal honey produced on the island of Ireland by palynological and physico-chemical data. Food Chem 91: 347-354.
- Amina C, Abderrahmane R, Gian LM, Paola F (2011) Physicochemical properties of some honeys produced from different plants in Morocco. Arab J Chem.
- Terrab A, DÃez MJ, Heredia FJ (2003) Palynological, physicochemical and colour characterization of Moroccan honeys: III. Other unifloral honey types. Int J Food Sci Technol 38: 395-402.
- Food Standard Australia New Zealand (FSANZ) (2006) Standard 2.8.2: Honey,update 2006. Accessed March 2012.
- Singh N, Bath PK (1997) Quality evaluation of different types of Indian honey. Food Chem 58: 129-133.
- CODEX STAN (2001) Codex Alimentarius Commission Standards 12-1981, Rev.1 (1987), Rev.2.
- EU Council Directive 2001/110/CE relating to honey (2002a) Official Journal of the European Communities 10: 47-52.
- Acquarone C, Buera P, Elizalde B (2007) Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chemistry 101: 695-703.
- Fallico B, Zappalã M, Arena E (2004) Effects of heating process on chemical composition and HMF levels in Sicilian monofloral honeys. Food Chem 85: 305-313.
- Denning DW (1998) Invasive aspergillosis. Clin Infect Dis 26: 781-803.
- Xuguang S, Zhixin W, Zhiqun W, Shiyun L, Ran L (2007) Ocular fungal isolates and antifungal susceptibility in Northern China. Am J Ophthalmol 143: 131-133.
- Perea S, Patterson TF (2002) Antifungal resistance in pathogenic fungi. Clin Infect Dis 35: 1073-1080.
- Kaya AD, Kiraz N (2007) In vitro susceptibilities of Aspergillus spp. causing otomycosis to amphotericin B, voriconazole and itraconazole. Mycoses 50: 447-450.
- Mollan PC (1992) The antibacterial activity of honey: 2 Variation in the potency of the antibacterial activity. Bee World 73: 59-76.
- Finola MS, Lasagno MC, Marioli JM (2007) Microbial and chemical characterization of honeys from central Argentina. Food Chem 100: 1649-1653.
- Koc AN, Silic S, Ercal BD, Kasap F, Hormet-Oz HT, Buldu HM (2009) Antifungal activity of Turkish honey against Candida spp. and Trichosporon spp: an in vitro evaluation. Med Mycol 47: 707-712.
- Ahmed M, Djebli N, Aissat S, Aggad H, Boucif A (2011) Antifungal Activity of a Combination of Algeria Honey and Starch of Ginger Against Aspergillus niger. Int J Microbiol Res 2: 263-266.
- Laid B, Hama B, Moussa A (2008) Synergistic action of starch and honey against Aspergillus niger in correlation with Diastase Number. Mycoses 51: 520-522.
- Banwart GJ (1989) Basic Food Microbiology. New York, Van Nostrand Reinhold.
- Brudzynski K, Miotto D (2011) Honey melanoidins: Analysis of the compositions of the high molecular weight melanoidins exhibiting radical-scavenging activity. Food Chem 127: 1023-1030.
- Adcock D (1962) The effect of catalase on the inhibine and peroxide values of various honeys. J Apic Res 1: 38-40.
- White JW, Subers MH, Schepartz AI (1963) The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in honey glucoseoxidase system. Biochimica Biophysica Acta 73: 57-70.
- Molan P (1992) The antibacterial activity of honey 1. The nature of the antibacterial activity. Bee World 1: 5-28.
- Averyanov AA, Lapikova VP, Pasechnik TD, Kuznetsov VV, Baker CJ (2007) Suppression of early stages of fungus development by hydrogen peroxide at low concentrations. Plant Pathol J 6: 242-247.
|
| |
| |