| Research Article |
Open Access |
|
| Lotfi R*, Ragab N, Ghazala W |
| Department of Dermatology, Venereology and Andrology, Ain Shams University, Cairo, Egypt |
| *Corresponding authors: |
Ranya Lotfi
Department of Dermatology
Venereology and Andrology
Ain Shams University, 34 el Orouba Street
Cairo, Egypt
Tel: +201001550123
E-mail: rkuku2000@yahoo.com |
|
| |
| Received March 27, 2012; Published July 26, 2012 |
| |
| Citation: Lotfi R, Ragab N, Ghazala W (2012) Evaluation of Seminal Plasma Magnesium in Premature Ejaculation. 1: 156. doi:10.4172/scientificreports.156 |
| |
| Copyright: © 2012 Lotfi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Premature Ejaculation (PE) is the most common male sexual disorder worldwide. Until now, the exact cause of PE is not identified clearly. |
| |
| Aim of the work: To measure seminal magnesium level in men suffering from premature ejaculation and erectile dysfunction in an attempt to clarify its role in those disorders. |
| |
| Subjects and Methods: Seminal Mg was measured using atomic absorption spectrometer in 3 groups of patients; group (A) patients with premature Ejaculation (PE), group (B) patients with both PE and Erectile Dysfunction (ED), and group (C) healthy control men. |
| |
| Results: There was a highly significant difference in the mean seminal Mg level between the 3 groups, being highest in group (C) and lowest in (B). There was a highly significant negative correlation between semen Mg level and each of the age of patients and the duration of PE and ED. |
| |
| Conclusion: A decrease in the level of Mg in semen within a certain range is associated with PE, while further decrease in the level is associated with both PE and ED. This study strongly suggests a role of seminal magnesium in the pathogenesis of both sexual disorders. |
| |
| Keywords |
| |
| Seminal plasma; Magnesium; Premature ejaculation |
| |
| Abbreviations |
| |
| PE: Premature Ejaculation; ED: Erectile Dysfunction; Mg: Magnesium |
| |
| Introduction |
| |
| Premature ejaculation (PE), unlike erectile dysfunction (ED), affects men of all ages equally from 18 years old to the elderly. Both premature ejaculation and erectile dysfunction may coexist [1]. Many researchers reported that the pathogenesis of premature ejaculation is mostly due to psychological stress and anxiety [2], or due to organic diseases as pelvic congestion and chronic prostatitis [3]. Researchers reported that some trace elements as zinc, copper and selenium present in semen may play an important role in male sexuality [4]. Magnesium is one of the elements present in human seminal plasma. Seminal magnesium level (more than 70 mg/l) is much higher than in serum (17-24 mg/l) [5] which suggests that magnesium may play an important role in male sexuality. Magnesium (Mg) is the fourth most common cation in the body, and the second most common intracellular cation after potassium. It has a fundamental role as a co-factor in more than 300 enzymatic reactions involving energy metabolism and nucleic acid synthesis, and it is also involved in several processes including hormone receptor binding, gating of calcium channels, transmembrane ion flux and regulation of adenylate cyclase, muscle contraction, neuronal activity, control of vasomotor tone, cardiac excitability, and neurotransmitter release. In many of its actions it has been likened to a physiological calcium antagonist [6,7]. As calcium is one of the elements responsible for smooth muscle contraction of the vas deference and corpus cavernosum [8], magnesium may play a role in relaxation of smooth muscles of the male genital tract and the penis to delay the ejaculatory process and improve the erection. Therefore, and also because magnesium has such diverse biophysiologic and neuromuscular actions on different parts of the body, our objective was to measure seminal magnesium level in men suffering from premature ejaculation and erectile dysfunction in an attempt to elucidate its role in those disorders. |
| |
| Materials and Methods |
| |
| Materials |
| |
| This prospective controlled study included 3 groups of patients. Group (A) included 20 patients aged 20 to 50 years complaining of premature ejaculation only without any other organic, sexual or psychological disorder, group (B) was added after finding that some patients of group (A) had superadded ED. It consisted of 10 male patients, aged 20 to 50 years, complaining of both premature ejaculation and erectile dysfunction for more than 6 months without any other organic, sexual or psychological disorder. Detailed history taking revealed that the patients complained of PE first before developing ED years after PE; group (C) included 30 healthy age-matched control men, not complaining or having history of PE or any organic, sexual or psychological disorder. Excluded from this study were patients with organic disorders, PE or ED of less than 6 months, intermittent PE or ED, or having an abnormal mental state or history of psychiatric disorders. The study was conducted in Ain Shams University Hospitals, Cairo, Egypt. An informed written consent was taken from all subjects before participating in this work. The study was approved by the ethical committee of Ain Shams University, Cairo, Egypt. |
| |
| Methods |
| |
| Semen was analysed in all subjects according to the WHO guidelines [9]. After 3-5 days of sexual abstinence, the semen obtained by masturbation was collected into a sterile acid-washed container, using no lubricant jelly. Specimens were centrifuged at 110 g for 10 min at 4°C within 30 min of sample collection. Aliquoted samples were stored at 80°C until they were assayed. Samples were sent to Balague center (Barcelona, Spain) to have the magnesium level measured there, using the atomic absorption spectrophotometry Perkin Elmer and Shimadzu, not available in our country at that time. |
| |
| Statistical Analysis |
| |
| Data were analyzed using the SPSS program version 15. Results are statistically analyzed using significance test, independent t-test, ANOVA test and Pearson's correlation test. A “P” value of 0.05 was chosen as the level of statistical significance. |
| |
| Results |
| |
| The normal level of Mg in seminal fluid is< 70 mg/l [5]. Results of seminal Mg level analysis in the 3 groups were as follows. |
| |
| Group (A) |
| |
| The 20 patients with PE only showed seminal Mg levels ranging from 41 mg/l to 69 mg/l with an average mean of 55.35 mg/l ± 8.9 and a highly significant decrease (p < 0.001) when compared to group C. |
| |
| |
| Group (B) |
| |
| In this group which includes 10 patients complaining of both PE and ED, the Mg level in semen showed a range of 9 mg/l to 46 mg/l with a mean of 26.5 mg/l ± 11.64, which was significantly lower when compared to patients of group A with PE only (p < 0.001). |
| |
| Group (C) |
| |
| This group of 30 healthy control subjects showed a magnesium level< 70 mg/l in semen with a range of 70 mg/l to 141 mg/l and an average mean of 103.73 mg/l ± 22.5 with a highly significant elevation of Mg level (p < 0.001) when compared to group A. |
| |
| The relation between seminal Mg level in the 3 groups is shown in table 1 which clarifies highest values of Mg in semen of healthy men without any sexual disorder (group C) and lower values of Mg in semen of patients with PE only (group A), while the lowest values of Mg in semen were found in patients with both PE and ED (group B). |
| |
| In this study there was a highly significant difference of the mean seminal Mg level among the 3 groups (Table 1). |
| |
|
|
Table 1: Comparison between the 3 groups regarding mean seminal Mg levels. |
|
| |
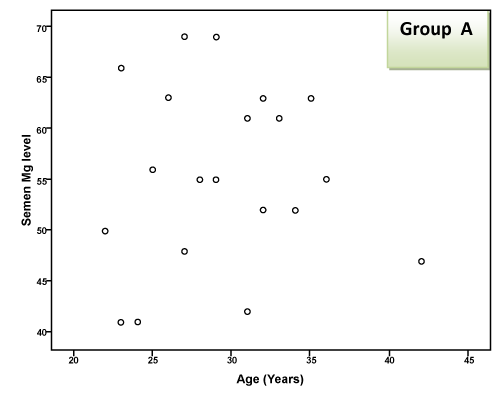
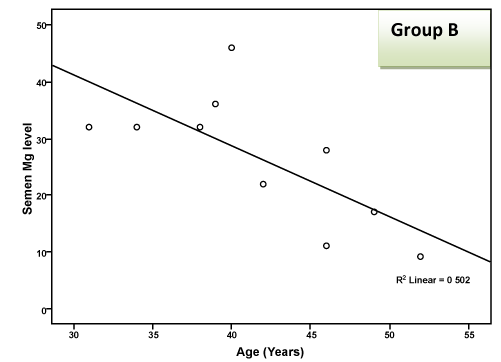
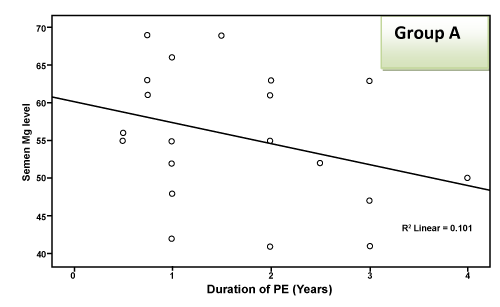
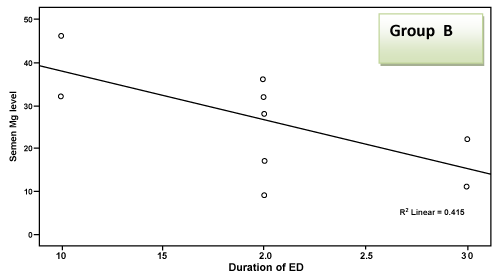
| There was a highly significant negative correlation (p < 0.001) between semen Mg level and age of patients in groups A & B together (effect of group B) and a significant negative correlation in group B alone, but this correlation was not significant in group A due to small sample size and narrow range of ages. This negative correlation means that the more the age of the patient, the less the level of Mg in semen as shown in Table 2 and Figure 1 and 2. There was a highly significant negative correlation (p < 0.001) between semen Mg level and duration of PE in groups A & B together, which means that the more the duration of suffering from PE, the less the level of Mg in semen (Table 2, Figure 3, 4). This negative correlation was not significant for either group alone due to small sample size and narrow range of duration; hence it is significant when both groups are added together. There was a significant negative correlation between semen Mg level and duration of ED in group B, meaning that the more the duration of suffering from ED, the less the level of Mg found in semen (Table 2, Figure 5). |
| |
|
|
Table 2: Correlation between semen Mg level and each of age, duration of PE and duration of ED in group A and group B. |
|
| |
|
|
Figure 1: Correlation between semen Mg level (mg/l) and age in group A. |
|
| |
|
|
Figure 2: Correlation between semen Mg level and age in group B. |
|
| |
|
|
Figure 3: Correlation between semen Mg level and duration of PE in group A. |
|
| |
|
|
Figure 4: Correlation between semen Mg level and duration of PE in group B. |
|
| |
|
|
Figure 5: Correlation between semen Mg level and duration of ED in group B. |
|
| |
| Discussion |
| |
| Until now, the exact cause of PE is not identified clearly and many theories have tried to explain this sexual disorder from many aspects. Some studies postulate a role of seminal magnesium in the pathogenesis of PE [10-12]. |
| |
| In this study we tried to put a light spot on the role of magnesium level in semen of men suffering from PE. Three groups were included in the study, and a highly significant difference in the mean seminal Mg level was found among them, being highest in group C that consisted of healthy men without any sexual, organic or psychological disorder, followed by group A (patients complaining of PE only), and lowest values were found in group B (patients complaining of both PE and ED). |
| |
| We also found that the older the age of patients, the less the level of Mg in the semen. In addition we found that the longer the duration of suffering from PE or ED, the less the level of Mg found in semen. A decrease in the level of Mg in semen within a certain range is associated with PE, while further decrease in this level is associated with both PE then ED. All these statistically significant data strongly suggest a role of seminal magnesium in the pathogenesis of both sexual disorders. |
| |
| Omu et al. in 2001 [10] analyzed the level of magnesium in three groups of men with normal semen parameters, oligoathenozoospermia and premature ejaculation and showed that there was a significant decrease in level of magnesium in semen of the group of men with PE, thus, it was concluded that magnesium is probably involved in sperm transport and PE. They explained this through a relation between electrical activity of the vas deferens and extracellular magnesium concentration as they proved that extracellular Mg2+ depletion enhanced the contractile response of the smooth muscles of vas deferens to electrical stimulation while increasing extracellular Mg2+ concentration inhibited the contractions [13]. |
| |
| In accordance with our study, Nikoobakht et al. [11] and Aloosh et al. [12] measured magnesium level in semen and plasma of healthy men and men with PE. The results showed a significant decrease of magnesium in semen of men with PE when compared to control cases, but there were no much difference regarding serum magnesium levels in both groups. |
| |
| In premature ejaculation, low seminal magnesium with normal serum magnesium level may be due to a defect in the active transport system that transports magnesium from blood to semen or the presence of a magnesiumdiminishing factor like chelating agents in the semen of the patients or a previous hypomagnesaemia caused by low consumption of magnesium that might contribute to the decline in seminal plasma magnesium levels [11,12]. |
| |
| Low magnesium level might manifest as uncontrolled contractility of the male genital tract, to cause premature emission and ejaculation [12]. This is explained by fact that low magnesium level stimulates angiotensininduced aldosterone synthesis and thromboxane-A2 over-production by phospholipase-A2 activation. Engagement of thromboxane-A2 results in Ca2+ influx [14,15]. Elevated calcium in endothelial cells promotes phosphodiesterases and decreases G-cyclase activity [16,17] resulting in decreased NO production and release from the endothelium [14]. This decrease in Nitric Oxide (NO) production, which is a vascular smooth muscle relaxing factor, leads to contraction of smooth muscles of genital tract causing rapid emission and premature ejaculation [11,12]. It also leads to contraction of cavernosal smooth muscles causing a state of erectile dysfunction. |
| |
| On different systems of the human body, Mg acts through many biophysiologic and neuromuscular mechanisms [7]. These actions have not been studied yet in relation to the potential role they play in the pathogenesis of PE and ED, so further studies should be conducted to clarify this role. |
| |
| To best of our knowledge, this is the first study done to compare between the level of Mg in the semen of men with PE alone and that of patients having a later, additional ED and to correlate them with the age of patients and duration of each of PE and ED. We hypothesize that low seminal Mg has a pathological role in PE and with a further decrease of Mg also leads to an additional ED. This might be explained by the fact that the common pathological factor for both sexual disorders is the level of NO that leads to PE when it decreases & to PE and ED together when diminished more. This is also supported by the fact that in group B, PE occurred first before ED that took place with lowest Mg levels found in this group. |
| |
| As nitric oxide is released from the nerve and endothelial cells mainly in the corpora cavernosa of the penis [18], it may act first on the nearby smooth muscles of the corpora causing relaxation and erection, thus, when nitric oxide decreases most of its amount will be consumed for having an erection and so ejaculation will be affected and compromised as only small amounts of NO will reach the distant smooth muscles of the genital tract. This will lead to increased contractions of smooth muscles of the genital tract lacking the relaxant activity that NO provides, causing a state of PE. Further decrease of nitric oxide will eventually also affects the cavernosal smooth muscles, and/or endothelial blood vessels of the corpora, so that ED may insue. |
| |
| It was suggested that chronic prostatitis frequently occurs in men with PE [19]. Then it was found that Mg is significantly decreased in chronic prostatitis patients, proposing Mg as a marker of prostatitis [20]. This provides an indirect link between magnesium and prostate function in PE. |
| |
| Low magnesium level also causes an increase in thromboxane A2 (TXA2) [21] which causes vasoconstriction of corporal blood vessels and thus, a state of ED. TXA2 is an important mediator of intrapenile oxidative stress in relation to the erectile process [22]. Low magnesium level also leads to a decrease in prostaglandin I2 (PGI2) [21] which are a vasodilator and thus leading to a contraction of corporal blood vessels and ED. So, further controlled studies should be conducted to evaluate the role of Mg in the pathogenesis of PE and ED and to explain further possible connecting factors for both sexual disorders, using bigger sample size and wider range of ages. |
| |
| |
| References |
| |
- Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP (2004) AUA guideline on the pharmacologic management of premature ejaculation. J Urol 172: 290-294.
- American Psychiatric Association (2004) The Diagnostic and Statistical Manual of Mental Disorders. (4th Edn) American Psychiatric Association, Washington, DC.
- Waldinger MD (2005) Lifelong premature ejaculation: de?nition, serotonergic neurotransmission and drug treatment. World J Urol 23: 102-110
- Yuyan L, Junqing W, Wei Y, Weijin Z, Ersheng G (2008) Are serum zinc and copper levels related to semen quality? Fertil Steril 89: 1008-1011.
- Bartis CA, Ashwood (2001) ER Mineral and bone metabolism. In Enders DB, Rude RK (eds). Tietz Fundamentals of Clinical Chemistry. (5th Edn) WB Saunders, Philadelphia.
- Fawcett WJ, Haxby EJ, Male DA (1999) Magnesium: physiology and pharmacology. Br J Anaesth 83: 302-320.
- Romani AM, Maguire ME (2002) Hormonal regulation of Mg2+ transport and homeostasis in eukaryotic cells. Bio Metals 15: 271-283.
- Berridege MJ (2008) Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047-5061.
- Cooper TG, Elizabeth N, Sigrid von E, Auger J, Gordon BH, et al. (2009) World Health Organization reference values for human semen characteristics. Hum Reprod Update 1-15.
- Omu AE, Al-Bader AA, Dashti H, Oriowo MA (2001) Magnesium in human semen: possible role in premature ejaculation. Arch Androl 46: 59-66.
- Nikoobakht M, Aloosh M, Hasani M (2005) Seminal plasma magnesium and premature ejaculation: a case-control study. Urol J Spring; 2: 102-105.
- Aloosh M, Hassani M, Nikoobakht M (2006) Seminal plasma magnesium and premature ejaculation: a case-control study. BJU Int 98: 402-404.
- Omu AE, Al-Bader AA, Dashti H, Oriowo MA (2001) Effect of extracellular mg concentration on electrically induced contractions of rat vas deferens in vitro. Arch androl 46: 159-167.
- Ryzen E, Rude RK (1990) Low intracellular magnesium in patients with acute pancreatitis and hypocalcemia. West J Med 152: 145-148.
- Liang CZ, Zhang XJ, Hao ZY, Shi HQ, Wang KX (2004) Prevalence of sexual dysfunction in Chinese men with chronic prostatitis. BJU Int 93: 568-570.
- Kanmura Y, Itoh T, Kuriyama H (1987) Mechanism of vasoconstriction induced by 9,11-epithio-11,12 methanothromboxane A2 in the rabbit coronary artery. Circ Res 60: 402-409.
- Baltrons MA, Saadoun S, Agulló L, García A (1997) Regulation by calcium of the nitric oxide/cyclic GMP system in cerebellar granule cells and astroglia in culture. J Neurosci Res 49: 333-41.
- Burnett Arthur L (2006) The role of nitric oxide in erectile dysfunction implications for medical therapy: J Clin Hypertens (Greenwich) 8: 53-62.
- Screponi E, Carosa E, Di Stasi SM, Pepe M, Carruba G, et al. (2001) Prevalence of chronic prostatitis in men with premature ejaculation. Urology 58: 198-202.
- Edorh AP, Tachev K, Hadou T, Gbeassor M, Sanni A, et al. (2003) Magnesium content in seminal fluid as an indicator of chronic prostatitis. Cell Mol Biol (Noisy-le-grand) 29: 419-423.
- Wiese JG, Shlipak MG, Browner WS (2000) The alcohol hangover. Ann Intern Med 132: 897-902.
- Koupparis AJ, Jeremy JY, Muzaffar S, Persad R, Shukla N (2005) Sildenafil inhibits the formation of superoxide and the expression of gp47NAD[P]H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int 96: 423-427.
|
| |
| |