| Research Article |
Open Access |
|
| Avinash Marwal, Anurag Sahu, Rajneesh Prajapati, DK Choudhary and R.K. Gaur* |
| Department of Science, faculty of Arts, Science and Commerce, Mody Institute of Technology and Science, Lakshmangarh, Rajasthan, India |
| *Corresponding authors: |
R.K. Gaur
Department of Science, faculty of Arts, Science and Commerce
Mody Institute of Technology and Science
Lakshmangarh, Sikar-332311
Rajasthan, India
E-mail: gaurrajarshi@hotmail.com |
|
| |
| Received Jan 26, 2012; Published July 20, 2012 |
| |
| Citation: Marwal A, Sahu A, Prajapati R, Choudhary DK, Gaur RK (2012) RNA Silencing Suppressor Encoded by Betasatellite DNA Associated With Croton Yellow Vein Mosaic Virus. 1: 153. doi:10.4172/scientificreports.153 |
| |
| Copyright: © 2012 Marwal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Summary |
| |
| A novel single stranded DNA of approximately 1350 nucleotides in length has been identified associated with infected yellow vein mosaic croton termed as DNA- β, requires the begmovirus for replication, encapsidation, insect transmission and movement in plants. It exhibit the properties of a satellite molecule having approximately 80 nucleotides conserved region which has been suggested to be important in trans-replication of DNA- β by the begmoviruses rep, possibly containing cryptic rep binding sites. In the present study βC1genes encoded by DNA betasatellite were targeted in transient assay for stable transformation to develop resistance against geminivirus. |
| |
| Geminivirus are a large diverse family of plant viruses that infect a broad variety of plants and cause significant crop losses worldwide. Their genome consists of either one or two single stranded (ss) DNA molecules of approximately equal size, which are encapsidated in geminate virus particles. The only high quality transgene-mediated resistance to geminiviruses is based on induction of sequencespecific degradation of a viral mRNA by a mechanism related to post-transcriptional gene silencing (PTGS), which is similar to RNA interference (RNAi) in other eukaryotes. There are two potential flaws with PTGS-mediated resistance. One is that many if not all plant viruses encode one or more proteins that suppress PTGS. Another is its specificity, since it is dependent on the sequence identity between the transgene and target RNA. Our results also suggest that RNA i technology plays an important role in the silencing of gene against begomovirus. |
| |
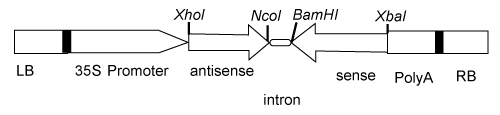
| A 750 nucleotides fragment corresponding to the DNA- β of Croton yellow vein mosaic virus (CYVMV) infected leaf (GenBank: HQ631429) was cloned in sense and anti-sense orientation with short introns. Primer designing was undertaken to amplify the full length DNA- β C1 region. The BamHI (5´-ATGGATCCACCACACAGACACCTTCAAAGG-3´ and XbaI GTATTCTAGATCTCTGTGAACTATATCTTCT-3´ restriction site was introduced in upstream and downstream for sense orientation and XhoI (5´-GTATCTCGAGTCTGTGAACTATATCTTCT-3´ and NcoI (TAAAAACCATGGAGACACCTTCAAACGACAAC-3´ as antisense orientation. The resulted amplicon were cloned in the pCambia 1300. The resulting binary construct was introduced into Agrobacterium tumefaciens LBA4404 by electroporation with a Gene Pulser apparatus (Bio-Rad). Seeds of T1 lines were grown on MS and two weeks old seedlings were infected with the infectious clones of croton yellow vein mosaic using Bio Rad particle delivery system as described [1]. The siRNA isolation and analysis was performed as described previously [2]. The DNA probe used for siRNA northern hybridization will correspond to the Nonanucleotide sequence within the CYVMV. |
| |
| DNA β is biological functional, being required for amplification of DNA -A to high levels for the efficient systemic spread of infection throughout susceptible plants and it is essential for the induction of symptoms in its natural hosts. The ORF of DNA- β are denoted on the virion (V) or complementary (C) strands [3]. In the present we approached transgeneic expression to develop resistance against distinct begomovirus. |
| |
| A transgene consisting of the promoter region of CYVMV DNA- β was designed to produce double stranded RNA (Figure 1). The Nicotiana benthamiana plants were transformed by Agrobacteium-mediated gene transfer and were tested for tested for siRNA expression. CYVMV-infected transgenic lines showed small RNAs of approximate sizes, 23 nt higher expression intensities. We choose to target DNA- β C1 by DNA- β for developing resistant as this β gene determine the virulence and suppress post transcription gene silencing, a manifestation of resistance gene mediated defense response. Results show that this β gene encodes virulence factor and suppresses host defense system. |
| |
|
|
Figure 1: Schematic diagram of the binary construct used for plant transformation. LB: Left border; RB: Right border. |
|
| |
| Dalmay et al. [4] have proposed a general model for both virus induced gene silencing and PTGS. They describe that dsRNAs generated by virus encoded replicases in VIGS and RdRP in PTGS are degraded to mRNA. Antisesnse transgenes could generate dsRNAs in two ways: when sense endogens is low, dsRNA arise by similar mechanism to that sense transgene process and when sense endogens is high, ds RNA arise by annealing of sense and antisense transcripts [5]. |
| |
| Our results of sense-antisense orientation derived form DNA-β confer immunity to the virus. Here we can easily conclude the relation of transgene transcription and immunity and that the transgene protein does not mediate the immunity. |
| |
| Acknowledgement |
| |
| The authors are also thankful to Department of Biotechnology (DBT), India and Department of Science and Technology, Rajasthan, India for financial support for the present studies. |
| |
| |
| References |
| |
- Klahre U, Crété P, Leuenberger SA, Iglesias VA, Meins F Jr (2002) High molecular weight RNAs and small interfering RNAs induce systemic posttranscriptional gene silencing in plants. Proc Natl Acad Sci U S A 99: 11981-11986.
- Hamilton A, Voinnet O, Chappell L, Baulcombe D (2002) Two classes of short interfering RNA in RNA silencing. EMBO J21: 4671-4679.
- Briddon RW, Mansoor S, Bedford ID, Pinner MS, Saunders K, et al. (2001) Identification of DNA components required for induction of cotton leaf curl disease. Virology 285: 234-243.
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC (2000) An RNA-dependent RNA polymerase gene in Arabidopsisis required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101: 543-553.
|
| |
| |