| Research Article |
Open Access |
|
| Charles Mutai1*, Lucia Keter1, Lilian Ngeny1 and Pascaline Jeruto2 |
| 1Kenya Medical Research Institute, Centre for Traditional Medicine and Drug Research, PO Box 54840, Nairobi, Kenya |
| 2Department of Botany and Horticulture, Maseno University, P.O. Box 333, Maseno, Kenya |
| *Corresponding authors: |
Charles Mutai
Kenya Medical Research Institute
Centre for Traditional Medicine and Drug Research
PO Box 54840, Nairobi, Kenya
E-mail : CMutai@kemri.org |
|
| |
| Received March 01, 2012; Accepted June 25, 2012; Published July 02, 2012 |
| |
| Citation: Mutai C, Keter L, Ngeny L, Jeruto P (2012) Effects of Triterpenoids on Herpes Simplex Virus Type1 (Hsv-1) In Vitro. 1: 140. doi:10.4172/scientificreports.140 |
| |
| Copyright: © 2012 Mutai C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| Herpes simplex virus (HSV) infection is an ancient disease, which is mostly easily acquired. HSV remains a common cause of ulcerative mucocutaneous disease today in both healthy and immunocompromised hosts. Due to the increasing HIV infections, many immuno compromised persons are infected with HSV-1 and/or HSV-2. However, there is no effective treatment against the disease but only drugs to manage the infections. The aim of the study was to evaluate antiviral effects of triterpenoids. Five naturally occurring triterpenoids including lupenone, 3-oxo-20 (29)-lupen- 30al, lupeol, betulin and 3-(E)-trans coumaroylbetuline were tested on the infectivity of HSV-1 in vitro using the VERO cell monolayer. The activity was observed as inhibition of cytopathic effects on a VERO cell monolayer infected with a virus, using the end-point titration method. The antiviral activity was expressed as the maximal non-toxic dose (MNTD) as reduction factor (RF). Betulin was active at a concentration of 50ug/ml against the infectivity of HSV-1. Lupenone, lupeol, and 3-(E)-trans coumaroylbetulin had no effects on infectivity but at 100 ug/ml they all reduced intracellular replication of the HSV-1 virus. Lupeol, and 3-(E)-trans coumaroylbetulin disrupted 100% of the cell monolayer at a concentration of 50 μg/ml. In conclusion, these naturally occurring triterpenoids possess some anti-HSV-1 activity. Betulin showed a pronounced antiviral activity than the rest of the compounds tested. |
| |
| Introduction |
| |
| Herpes simplex viruses (both types, HSV-1 and -2) are common human pathogen which causes a broad spectrum of illness ranging from symptomatic infection to disseminated diseases, such as labial herpes, genital herpes, keratitis, and encephalitis [1-4]. HSV-1 infections are very common and mostly affect adult people [5]. |
| |
| The primary symptoms of HSV infection include a prodromal “flu-like” syndrome, with fever, headache, malaise, diffuse myalgias, followed by local symptoms consisting of genital itching, tenderness, dysuria, lesions, and painful papules over genital regions and ulceration [6,7]. Studies have shown that reactivation of latent HSV-1 can occur as a result of physical or emotional stress [8,9]. In Scotland, it found that 40% of genital herpes was due to HSV1. HSV-1 and HSV-2 are able to infect and reactivate in the same anatomic area, although the natural history of these infections is markedly different [10]. The HSV2 recurring more frequently than HSV1, so most clinical reactivations are likely to be due to HSV2 [10]. |
| |
| In immunosuppressed patients, the clinical manifestation of the disease exhibits different severity and several patients always encounter recurrent attacks [11,12]. In addition, HSV is involved in several ocular diseases [13-15]. For instance, herpetic stromal keratitis (HSK) is an immunopathological disease, which is one of the leading causes of blindness in the western world [16]. Therefore, anti-HSV molecules could be of great relevance for infectious epithelial keratitis, neurotrophic keratopathy, stromal keratitis and endotheliitis [17]. |
| |
| A major advance in antiviral therapy has been the use of effective anti-herpes drugs, such as acyclovir, ganciclovir, valaciclovir, penciclovir, famciclovir (a prodrug of penciclovir and vidarabine). Among these acyclovir is the most commonly used drug for treatment of HSV infections, followed by penciclovir/famciclovir, acyclovir and related nucleoside analogues. However, the appearance of acyclovir and related nucleoside analogues-resistant HSV strains has become evident in immunosuppressed patients, such as organ transplant recipients and patients with acquired immunodeficiency syndrome. A study has shown resistance to acyclovir and related nucleoside analogues can occur following mutation in either HSV thymidine kinase (TK) or DNA polymerase [18]. Moreover, it should be considered that these drugs are very expensive and several patients with frequent attacks may not be able to afford the cost of long-term treatment [19]. Therefore, new antiviral agents exhibiting different mechanisms of action and affordable are urgently needed. |
| |
| Interestly, the uses of traditional natural products have increased [20-23]. It has been suggested that aqueous and ethanolic extracts from plants used in allopathic medicine are potential sources of antiviral and antitumor agents [23,24]. Furthermore, the selection of crude plant extracts for screening programs has the potential of being more successful in its initial steps than the screening of pure compounds isolated from natural products [21,25]. Therefore, Acacia mellifera stem barks, which are commonly used in treatment of pneumonia, malaria, primary infection of syphilis, and stomachaches were analyzed in this study. The dichloromethane extract was fractionated and purified through HPLC to give six pure compounds. These compounds were examined for anti-HSV activity according to method described by [23]. |
| |
| Materials and Methods |
| |
| Isolation of compounds |
| |
| Grounded stem bark of Acacia mellifera (2.25 kg) was extracted at room temperature, successively with dichloromethane (CH2Cl2) and methanol (MeOH) for 24 hours each. The crude extract was evaporated under reduced pressure in vacuo to yield CH2Cl2 extract and MeOH extract. The crude extract of dichloromethane (CH2Cl2) was initially fractionated using silica gel column chromatography. Elution was started with n-hexane, and the polarity of solvents was increased in a stepwise manner in the following sequence: n-hexane– ethyl acetate, followed by ethyl acetate, then ethyl acetate–methanol and finally methanol. Fractions of 50 ml each were collected and examined on silica TLC plate. Identical fractions were pooled together. Further purification was done by using HPLC. The structures of pure compounds were elucidated using 1HNMR and 13CNMR. |
| |
| Antiviral assays |
| |
| End-point titration technique (EPTT) - The technique described by [23] with a few modifications, was used. Briefly, confluent monolayer Vero cells were grown in 96-well flat-bottomed plates. Two-fold dilutions of the extracts in maintenance medium, identical to growth medium except for FBS, which was 2%, were added 1 h before viral infection. Cells were infected with 0.1 ml of serial ten-fold dilutions of the previously titrated virus suspension and incubated again at 37°C in humidified 5% CO2 atmosphere for 48 h. Controls consisted of infected cells with HSV-1 serial ten-fold dilutions in the absence of the extracts, treated non-infected and untreated non-infected cells. Furthermore all tests were compared with positive controls (acyclovir and 3-methylquercetin) tested simultaneously under identical conditions. The antiviral activity is expressed as the maximal non-toxic dose (MNTD) of the test extract needed to obtain the reduction virus titer. The reduction in virus titer was determined as the reduction factor (RF) of the virus titer, i.e. the ratio of the virus titer in the absence over virus titer in the presence of the extract. Three assays were carried out in duplicate with at least 5 concentrations. The results are expressed as the mean obtained from 3 different assays. The RF value of Acyclovir and 3-methylquercetin was 104 at MNTD of 0.1 μg/ml. Therefore, the extracts with RF values of 1x102 to 1x104 indicated a pronounced antiviral activity. |
| |
| Results and Discussion |
| |
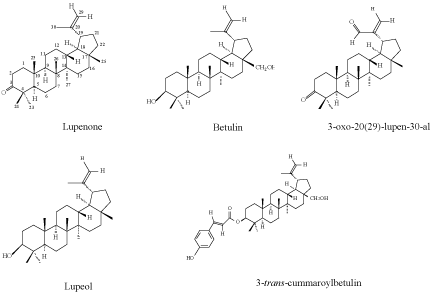
| As far as studies on isolated compounds, different research groups have recently reported some compounds, which exhibit potent antiviral activity against HSV-1 [23,26,27] has also reported and explained that extracts with RFs of the virus titer of > 1x103 show relevant antiviral activity. Therefore looking at the results of triterpenoids tested against the HSV-1 virus in Table 1, betulin exhibited the moderately anti- HSV-1 activity at 50 μg/ml and lower anti-HSV-1 activity between 1-10 μg/m. But lupenone, 3-oxo-20 (29)-lupen-30al, lupeol and 3-(E)-trans coumaroylbetulin lacked anti-HSV-1 activity. Although, Madureira et al. (2003) had reported lupenone isolated from the whole plant of Euphorbia segetalis, exhibited strong viral plaque inhibitory effect against HSV-1 and -2 [28] ,it was not so with our studies (Figure 1). |
| |
|
|
Table 1: Results of the antiviral activity screening of triterpenoids. |
|
| |
|
|
| |
| The lupenone, 3-oxo-20 (29)-lupen-30al, lupeol and 3-(E)-trans coumaroylbetulin all affected the cell monolayer Vero cells. Two compounds; lupeol and 3-(E)-trans coumaroylbetulin disrupted 100% of the cell monolayer at concentrations of 50 μg/ml and precipitate in the growth medium at 100 μg/ml. These results agrees with the previous reports that found lupeol to be a major bioactive fraction with growthdisrupting effects against some insectual pests in some plants [29]. The 3-oxo-20 (29)-lupen-30-al has also been reported to be cytotoxicity to KB cell growth and the reason was due the carbonyl at C-3 [30]. |
| |
| Betulin and 3-(E)-trans coumaroylbetulin are related in their structures. The only different come when the hydroxyl group at position three of betulin is substituted Coumaroyl moiety at C-3 in 3-(E)-trans coumaroylbetulin. This small structural different seems to reduce betulin activity against HSV-1 and simultaneously increases it toxicity against the cell line. |
| |
| |
| Our conclusion is that crude from plants employed in ethnomedicine can exhibit antiviral activity against HSV-1. Accordingly, medicinal plants can be a source for the isolation of pure compounds for example triterpenoids acting against HSV-1. Many bioactive anti-HSV molecules responsible for the activity of plant extracts have been identified, isolated, synthesized and tested [23,31]. About seven compounds with antiviral activity derived from 3-methylether and kamferol have been isolated from E.gramtii [32,33]. Therefore possibility of this active compound here, which will be of advantage because in lower and higher concentrations, the pure compounds extract have a reasonable activity hence it can be used at all concentration because it is not very toxic. |
| |
| |
| References |
| |
- Vlietinck AJ, Van Hoof L, Totté J, Lasure A, Vanden Berghe D, et al. (1995) Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmacol 46: 31-47.
- Kalinyak JE, Fleagle G, Docherty JJ (1977) Incidence and distribution of herpes simplex virus types 1 and 2 from genital lesions in college women. J Med Virol 3: 175-181.
- Corey L, Adams HG, Brown ZA, Holmes KK (1983) Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med 98: 958-972.
- Johnson RE, Nahmias AJ, Magder LS, Lee FK, Brooks CA, (1989) A Seroepidemiologic survey of the prevalence of herpes simplex virus type 2 infection in the United States. N Engl J Med 321: 7-12.
- Yoosook C, Chantratita W, Rimdusit P, (1989) Recovery frequencies of herpes simplex virus Types 1 and 2 from symptomatic and asymptomatic genital herpes cases and antiviral sensitivities of isolates. J Med Assoc Thai 72: 572-576.
- Whitley RJ, Kimberlin DW, Roizman B (1998) Herpes simplex viruses. Clin Infect Dis 26: 541-553.
- Hardin TC (1996) Sexually transmitted diseases In: Herfindal ET, Gourley DR, Textbook of Therapeutics-Drug and Disease Management, Williams and Wilkins, Baltimore: 1389-1404.
- Pereira FA (1999) Herpes simplex: Evolving concepts. J Am Acad Dermato 35: 503-520.
- Blondeau JM, Aoki FY, Glavin GB (1993) Stress-induced reactivation of latent herpes simplex virus infection in the rat lumbar dorsal root ganglia. J Psychosom Res 37: 843-849.
- Roizman B, Sears AE (1996) Herpes simplex virus and their replication. In: Fields BN, Knipe DM, Howley PM, Chanock RM, Hirsh MS, et al. Editors, Fields' virology, Lippincott-Raven, Philadelphia: 2231-2295.
- Sucato G, Wald A, Wakabayashi E, Vieira J, Corey L, (1998) Evidence of latencey and reactivation of both herpes simplex virus (HSV)-1 and HSV-2 in the genital region. J Infect Dis 177: 1069-1072.
- Reeves WC, Corey L, Adams HG, Vontve, LA, Holmes KK, (1981) Risk of recurrence after first episodes of genital herpes: relation to HSV type and antibody response. N Engl J Med 305: 315-319.
- Whitley RJ, Roizman B (1997) Herpes simplex viruses. In: Richman DD, Whitley RJ, Hayden FG Editors, Clinical Virology, Churchill Livingstone, New York: 380-382.
- Liesegang TJ (2001) Herpes simplex virus epidemiology and ocular importance. Cornea 20: 1-13.
- Wilhelmus KR (2000) The treatment of herpes simplex virus epithelial keratitis. Trans Am Ophthalmol. Soc 98: 505-532.
- Souza PM, Holland EJ, Hunag AJ, (2003) Bilaterail herpetic keratoconjunctivitis. Ophthalmology 110: 493-496.
- Liesegang TJ (2001) Herpes simplex virus epidemiology and ocular importance. Cornea 20: 1-13.
- Wilhelmus KR (2000) The treatment of herpes simplex virus epithelial keratitis. Trans Am Ophthalmol. Soc 98: 505-532.
- Souza PM, Holland EJ, Hunag AJ, (2003) Bilaterail herpetic keratoconjunctivitis. Ophthalmology 110: 493-496.
- Hammer SM, Inouye RT (1997) Antiviral agents. In: Richman DD, Whitley RJ, Hayden FG Jr, Editors, Clinical Virology, Churchill Livingstone, New York: 186-201.
- Kurokawa M, Ochiai H, Nagasaka K, Neki M, Xu H,et al. (1993) Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice.Antiviral Res 22: 175-188.
- Cordell GA (1995) Changing strategies in natural products chemistry. Phytochemistry40: 1585-1612.
- Taylor RS, Manandhar NP, Hudson JB, Towers GH, (1996) Antiviral activities of nepalese medicinal plants.J Ethnopharmacol52: 157-163.
- Chung TH, Kim JC, Kim MK, Choi SC, Kim SL,et al. (1995) Investigation of korean plant extracts for potential phytotherapeutic agents against B-virus Hepatitis. Phytotherapy Research 9: 429-434.
- Kusumoto IT, Nakabayashi T, Kida H, Miyashiro H, Hattori M, (1995) Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1(HIV-1) protease. Phytotherapy Research 9: 180-184.
- Thompson KD, Dragar C, (2004) Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytother Res 18: 551-555.
- Alche LE, Barquero AA, Sanjuan NA, Coto CE, (2002) An antiviral principle present in a purified fraction from Melia azedarach L. leaf aqueous extract retains herpes simplex virus type 1 propagation. Phytother Res 16: 348-352.
- Madureira AM, Ascenso JR, Valdeira L, Duarte A, Frade JP, et al. (2003) Evaluation of the antiviral and antimicrobial activities of triterpenes isolated from Euphorbia segetalis. Nat Prod Res 17: 375-380.
- González-Coloma, A., Reina, m., Farga, B.M., Gutiérrez, C., Balboa, B.C. 1999. Bioactive Lupeol from Senecio heritieri structure – activity relationship. Conference of the International Society of Chemical Ecology, Marseille; Abstract p. 44.
- Kupchan SM, Altland HW, (1973) Tumor inhibitor. Structural requirements for tumor-inhibitory activity a mong benzylisoquinoline alkaloids and related synthetic compounds. J Med Chem16: 913-917.
- Unander DW, Webster GL, Blumberg BS, (1995) Usage and bio-assays in Phyllanthus (Euphorbiaceae). IV. Clustering of antiviral uses and other effects. J Ethnopharmacol 45: 1-18.
- Vlietinck AJ, Vanden Berghe DA, Van Hoof LM, Vrijsen R, Boeye A, (1986) Antiviral activity of 3-methoxyflavones. Prog Clin Biol Res 213: 537-540.
- Van Hoof L, Vanden Berghe DA, Hatfield GM, Vlietinck AJ (1984) Plant antiviral agents. V. 3-methoxyflavones as potent inhibitors of viral induced block of cell synthesis. Planta Med50: 513-517.
|
| |
| |