| Research Article |
Open Access |
|
| Hardik Pathak1*, Kamna Bhatnagar1 and P Jaroli2 |
| 1Department of Biotechnology, Mahatma Gandhi Institute of Applied Sciences, India |
| 2Department of Zoology, University of Rajasthan, Jaipur, India |
| *Corresponding author: |
Hardik Pathak
Head Department of Biotechnology
Mahatma Gandhi Institute of applied Sciences
JECRC Campus, Tonk Road, India
Tel: +919602453825
E-mail: hardikaeshu@gmail.com |
|
| |
| Received June 09, 2011; Published June 23, 2012 |
| |
| Citation: Pathak H, Bhatnagar K, Jaroli P (2012) Serratia-The 4T Engine oil degrader. 1: 117. doi:10.4172/scientificreports.117 |
| |
| Copyright: © 2012 Pathak H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| |
| Abstract |
| |
| In the last few years, large numbers of ecosystems have changed by the significant influence of human activity. As a result, many people have become aware of the need to protect different ecosystems as well as to evaluate the damage caused by contamination. Microorganisms used in all experiments were isolated by selective enrichment technique. MSM broth was used in enrichment technique supplemented with 1% v/v hydrocarbon substrate (4T engine oil). Bacterial inoculation of biometric flask (containing 48 ml minimal salt medium) consisted of 1 ml of a 48 hours culture. Hydrocarbon substrate (4T engine oil) was then added to 1% v/v. The GC/MS analysis was performed using a MS 5973 spectrometer coupled to 9 Hewlett Packard model 6890. I5 isolate microorganisms degraded around 64% of 4T engine oil. Gas chromatography showed significant differences in the composition of hydrocarbons at the end of the experiments. After all biochemical tests Serratia scored as positive for glucose and negative for lactose fermentation. |
| |
| Keywords |
| |
| Serratia; GC-MS; Spectrophotometer; MSM |
| |
| Introduction |
| |
| During the previous years, the frequency and risk of oil pollution has led to extensive research. Most of the petroleum goes in the ecosystem via leakage of coastal oil refineries. This fact evoked the interest of scientists to investigate the oil distribution system and its fate in the environment, especially the marine environment. Approximately five million tons of crude oil and refined oil enter the environment each year as a result of anthropogenic sources such as oil spills (1). Past analysis of reported oil spills indicate that most of the oil comes from tankers, barges and other vessels as well from land pipeline spills. Extensive changes in marine, as well as terrestrial ecosystems resulting from the grounding of the Exxon Valdez (1989). The Nahodka oil spill, the Erica spill (1999) and the Prestige spill (2002), have recently increased the attention of environmentalists, chemists, biotechnologists and engineers [2,3]. The recent spill of more than 200,000 barrels of crude oil from the oil tanker Exxon Valdez in Prince William Sound Alaska [4], as well as smaller spills in Texas, Rhode Island, and the Delaware Bay, have refocused attention on the problem of hydrocarbon contamination in the environment. It is estimated that the annual global input of petroleum is between 1.7 and 8.8 million metric tons, the majority of which is derived from anthropogenic sources [4]. |
| |
| PAHs present as natural constituents in fossils fuels, are formed during the incomplete combustion of organic material and are therefore present in relatively high concentration in products of fossil fuels refining [5-11]. Petroleum refining and transport activities are major contributors to localized loadings to PAHs into the environment. Such loadings may occur through discharge of industrial effluents and through accidental release of raw and refined products. However, PAH released into the environment may originate from many sources including gasoline and diesel fuel combustion [11] and tobacco smoke PAHs are detected in air [11,12], soil and sediment [13- 17), surface water groundwater, and road runoff [18-21) are dispersed from the atmosphere to vegetation [22] and contaminate foods [23-25]. Anthropogenic and natural sources of PAHs in combination with global transport phenomena result in their world wide distribution. Hence, the need to develop practical bioremediation strategies for heavily impacted sites is evident [26]. PAHs concentration in the environment vary widely, depending on the proximity of the contaminated site to the production source, the level of industrial development and the mode of PAH transports. Soil and sediment PAH concentration at contaminated and uncontaminated sites ranging from 1 μ/kg to over 300 g/kg have been reported [27-30]. |
| |
| Bioremediation is defined as use of biological processes to degrade, breakdown, transform, and/or essentially remove contaminants or impairments of quality from soil and water. Bioremediation is a natural process which relies on bacteria, fungi, and plants to alter contaminants as these organisms carry out their normal life functions. Metabolic processes of these organisms are capable of using chemical contaminants as an energy source, rendering the contaminants harmless or less toxic products in most cases. This investigation focuses on the general processes of bioremediation with in the soil environment and highlighting the biodegradation of petroleum hydrocarbons. |
| |
| Materials and Methods |
| |
| Isolation of Microorganisms |
| |
| 4 T engine oil (mainly composed of medium chain length of hydrocarbon) obtained from Bharat petrol pump. The entire chemical was purchased from MERCK. MSM (Minimal Salt Media) media containing 1 g K 2 HPO4, 1g NH 4 NO3, 1 g KH 2 PO4, 0.02 g CaCl2, 0.05 g FeCl3, 0.2 g MgSO4 was used for serial dilutions to determine viable cell counts. For isolation and enumeration of total viable cells MSM agar plates were used. Microorganisms used in all experiments were isolated by selective enrichment technique. MSM broth was used in enrichment technique supplemented with 1% v/v hydrocarbon substrate (4T engine oil). The hydrocarbon substrate used in enrichment methods was a mixture of hydrocarbons. After one week of incubation on rotary shaker at 27°C and 200 rpm, 10 ml of sample from primary enrichment was transferred to a fresh MSM broth. Unless otherwise stated, after 2nd enrichment 1ml of medium was plated after appropriate dilution on MSM agar plate and incubated at 27°C. After 48 hour incubation, pure colonies were isolated by using single colony isolation method. Isolated colonies were stored at 4°C and re-plated at MSM agar plates at 3 weeks interval. Bacterial isolates not used in biodegradable experiments were mixed with 40% glycerol and stored at 70°C for future use. The initial number of total viable cells in the original sample (before enrichment) was determined by serial dilution agar plating procedure. |
| |
| Biodegradation of 4T Engine Oil in a Biometric System |
| |
| Bacterial inoculation of biometric flask (containing 48 ml minimal salt medium) consisted of 1 ml of a 48 hours culture. Hydrocarbon substrate (4Tengine oil) was then added to 1% v/v. The sidearm of biometric flask was fitted with 10 ml of 0.1 M KOH flask and was incubated at 27°C, non-shaking. Sample for measuring carbon dioxide were taken by syringe in scheduled time periods from a side arm of the flask. Evolution of carbon dioxide during microbial utilization of 4T engine oil and organic waste was determined by colorimetric titration (Table 1). The amount of trapped CO2 in KOH was accurately titrated after addition of 1 ml of saturated barium chloride and 0.1 ml of phenolphthalein with 0.05 M HCl until colorless solution. The control sample contained 10 ml of fresh KOH with 1 ml of barium chloride and 1 ml of phenolphthalein. The volume of HCl needed for neutralization of the experimental KOH was subtracted from the amount of HCl needed for neutralization of the un-exposed KOH. The difference in milliliters was converted into micromoles of evolved carbon dioxide during microbial degradation of hydrocarbons. ( Figure 3) |
| |
|
|
Table 1: Chemical Analysis of 4T Engine Oil. |
|
| |
| Hydrocarbon Analysis |
| |
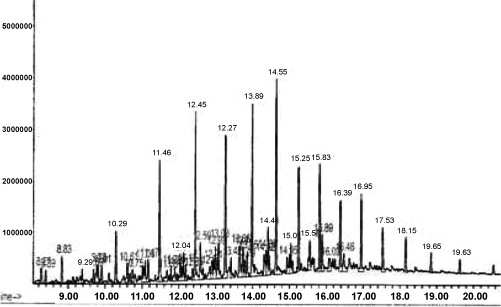
| Sets of test tubes experiments were designed in order to quantitatively analyze the microbial hydrocarbon degradation at GCMS (Figure 1). Tube containing cultures of bacteria and hydrocarbon were incubated during the same time period and in the same experimental conditions as biometric flask. Each test tube contained 4 ml of sterilized minimal salt medium, 80 μl of microbial inoculums and 50 μl of hydrocarbon substrate. The sample extraction was carried out by mixing the entire volume of one set tube (approximately 5 ml) with 1 ml of hexane (petroleum fraction). This mixture was emulsified by shaking and allowed to resettle for five minutes. The top layer was recovered and transformed to a clear vial for further use. Mass spectra as well the retention times of standard mixture of hydrocarbons were used to quantify each analyte. |
| |
|
|
Figure 1: showing GC-MS analysis of 4T engine oil. |
|
| |
| The GC/MS analysis were performed using a MS 5973 spectrometer coupled to 9 Hewlett Packard model 6890, with a column ULBON HR-1 which is equivalent to Ov-1 fused silica capillary (.25 mm x 50 mm) with thickness of 0 .25 micron; 1 ml/min; pressure 18.5 psi and split ratio 20%.The initial temperature was 70°C kept for 5 minutes with a temperature range of 14°C per minute and final temperature for 280°C kept for 10 minutes with total running time 30 minutes. The solvent used in analysis was hexane (petroleum fraction). Soil samples were collected from coastal area of Mumbai in the month of July, 2005. Samples were collected from Sai Motor Garage nearby Mumbai High; as such samples were highly polluted with engine oil. |
| |
| Cell Counts and Characterization of Petroleum Degrading |
| |
| Microorganisms |
| |
| Prior to the screening of hydrocarbon degrading microorganisms, the microbial populations were estimated in each original sample. Appreciable numbers of bacteria up to 1010 Colony Forming Units (CFU) have been found. |
| |
| Indigenous organisms isolated in this study were selected by enrichment culturing technique. As the results in table 2 indicates a significant increase in hydrocarbon degrading microorganisms in PCS– 1, after the first and second week of enrichment in 4T engine oil. In PCS I original sample the CFU was 1.3×1010 before adding the substrate. As the substrate 4T engine oil was added to the sample, the CFU was 8.3×1010 after Ist enrichment, and again increased when 2nd enrichment was performed (9.4×1010). In second sample PCS II, the CFU was 1.6 × 109 before enrichment culture. After 2nd and 3rd enrichment it increased up to 6.4 × 109 and 7.3 × 109 respectively. Due to high diversity of microorganisms after second enrichment 15 bacterial isolates were collected from the samples. All these isolate were tested for their ability to utilize various hydrocarbons. Five bacterial strains were found to be the best degrader of 4T engine oil. |
| |
| |
|
|
Table 2: Isolation and Identification of Microbial Strain. |
|
| |
| The type of enrichment substrate significantly affected the microbial population. It is observer that the large and more complex the structure of hydrocarbon, the more slowly is oxidized. This may depends on the type of organisms involved and the medium, in which it was developed. For this reason, the longer enrichment period as well as fresh medium and decantation of toxic co-metabolites apparently enhanced the proliferation of bacteria capable of utilization more complex compounds in investigated samples collected from coastal area Mumbai. |
| |
| The morphology and type of bacterial colonies were also investigated in cell counts experiments. One of the objectives of this study was to isolate as many culturable strains as possible in standardized culture condition. For this reason, a first screening of strain was done after gram staining and microscopic examination for bacteria to eliminate apparently similar strains. As the results exhibited original sample were characterized with a very high diversity of microorganisms. The metabolic diversity of microorganisms in the natural environments is an important factor in the biodegradation of hydrocarbon. Extensive degradation of petroleum pollutants is generally accomplished by mixed microbial population, rather than single microbial species. |
| |
| Even though enrichment culturing selected only those indigenous microorganisms that have been especially acclimated to degrade hydrocarbons, it was necessary to characterize the biodegradation potential for individual isolates. For this reason, each isolated microbial strain was submitted to a preliminary batch flask test experiment for detailed investigation of hydrocarbon utilization. The structure of a compound is important in determining its biodegradability. Generally, straight chain alkanes are degradable more readily than branched alkanes. In order to investigate the capabilities of isolates to utilize both chemical structures, liner as well as branched, 4T engine oil was used as a substrate. The growth was monitored regularly by measuring optical density of experimental medium. Among all the isolated microbial strains, biodegradation potential was recorded ranging from 44% to 91% degradation of 4T engine oil (Table 3). Considering the fact that some of these microorganisms have been not exposed to hydrocarbons at all or only in very low concentration, it is very possible that the adaptation process might play an important role in degradation process. There is high degree of variability in the ability of a microbial community to adapt to compounds and chemicals that they were never exposed to before. Adaptation depends on many factors, such as the induction or de-repression of enzymes specific for the degradation pathway of a particular compound or an adaptation of existing catabolic enzymes to the degradation of novel compounds. Serratia was scored as positive for glucose and negative for lactose fermentation. However, most of the Enterobacteriaceae member are Gelatinase negative, a representative form this taxonomy showed positive result which indicates that this bacteria is not able to use malonate. It is also characterized by production of red pigment at 25°C [31]. In spite of the fact that Serratia in not considered to be a main representative of hydrocarbon degraders, this bacteria was isolated from oil polluted soil and groundwater contaminated with gasoline. It showed good crude oil and gasoline degradability [32, 33]. |
| |
|
|
Table 3: Degradation of 4T Engine Oil by Microbial Strains on MSM Broth. |
|
| |
| Biodegradation of 4T Engine Oil in Biometric Flask Evolution of Carbon Dioxide |
| |
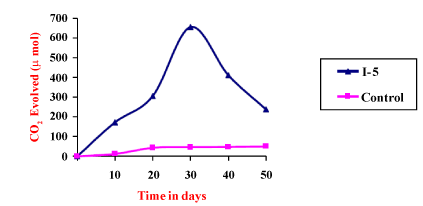
| Effective method to study the degradation of hydrocarbon by microorganisms is to measure the amount of carbon dioxide evolve during utilization of organic compound. Respiratory activities of isolated microorganisms were measured during growth on addition of 4T engine oil in a biometric flask. The cumulative amounts of CO2 evolved during mineralization of 4T engine oil were measured. The I5 bacterial isolates exhibited maximum CO2 production (656 μmol) in 27 days. I5 isolate microorganisms degraded around 64% of 4T engine oil. Gas chromatography showed significant differences in the composition of hydrocarbons at the end of the experiments. |
| |
| Among the identified strains I5 had following characteristics. It was Gram negative, motile and Red pigmented. It exhibited positive for Gelatin hydrolysis, Voges-Proskauer, Citrate utilization, Lysine Decarboxylase. It was negative for Sulphate production, Indole, Methyl red, Arginine dehydrolase, Phenylalanine deaminase, Glucose utilization. It produced red pigment at 25°C. It was identified as Serratia Sp. (Table 4) (Figure 2). |
| |
|
|
Table 4: Evolution of CO2 during 4T engine oil degradation by I5 Microbial Strain. |
|
| |
|
|
Figure 2: Degradation of 4T engine oil by I5 Strain. |
|
| |
|
|
Figure 3: GC analysis of 4T engine oil after addition of I5 isolate after 50 days of incubation at 27 ± 2°C without shaking. |
|
| |
| Acknowledgements |
| |
| We wish to thank Prof. C. K. Ojha, Director Academics, M.G.I.A.S, for his encouragement and support. |
| |
| |
| References |
| |
- Hinchee E R, Kitte A J (1995) Applied Bioremediation of Petroleum Hydrocarbons. Battelle Press, Columbus, OH (United States).
- Braddock J F, Ruth M L, Catterall P H (1995) Enhancement and Inhibition of Microbial Activity in Hydrocarbon- Contaminated Arctic Soils: Implications for Nutrient-Amended Bioremediation. Environ Sci Technol 31: 2078-2084.
- Tazakib K, Asadab R, Kogurec K (2004) Bioremediation of coastal areas 5 years after the Nakhodka oil spill in the Sea of Japan: isolation and characterization of hydrocarbon degrading bacteria. Environment International 30: 911-922.
- Hagar R (1989) Huge cargo of North Slope oil spilled. Oil Gas J 87: 26-27.
- Nestler F H N (1974) Characterization of wood preserving coal tar creosote by gas liquid chromatography. Anal Chem 46: 46-53.
- Lee S D and Grant L (1981) Health and ecological assessment of polynuclear aromatic hydrocarbons. Pathotox Publishers, Inc.,Park Forest South, Ill.
- Bos RP, Theuws JL, Leijdekkers CM, Henderson PT (1984) The presence of the mutagenic polycyclic aromatic hydrocarbons benzo-[a]pyrene and benz[a]anthracene in creosote P1. Mutat Res 130:153-158.
- Nishioka M, Chang HY, Lee M (1986) Structural characteristics of polycyclic aromatic hydrocarbon isomers in coal tars and combustion products. Environ Sci Technol 20: 1023-1027.
- Wang Z, Fingas M, Shu YY, Sigouin L, Landriault M, et al. (1999) Quantitative characterization of PAHs in burn residue and soot samples and differentiation of pyrogenic PAHs from petrogenic PAHs The 1994 mobile burn study. Environ Sci Technol 33: 3100-3109.
- Deschênes L, Lafrance P, Villeneuve JP, Samson R (1996) Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl.Microbiol.Biotechnol 46: 638-646.
- Wang Z, Fingas M, Li K (1994) Fractionation of a light crude oil and identification and quantitation of aliphatic, aromatic, and biomarker compounds by GC-FID and GC-MS, Part II. J Chromatogr Sci 32: 367-382.
- Koeber R, Bayona JM, Niessner R (1999) Determination of benzo[a]pyrene diones in air particulate matter with liquid chromatography mass spectrometry. Environ Sci Technol 33: 1552-1558.
- Heitkamp M A , Cerniglia C E (1989) Polycyclic aromatic hydrocarbon degradation by a Mycobacterium sp. in microcosms containing sediment and water from a pristine ecosystem. Appl Environ Microbiol 55: 1968 - 1973.
- Zeng E Y, Vista C L (1997) Organic pollutants in the coastal environment off San Diego, California. 1. Source identification and assessment by compositional indices of polycyclic aromatic hydrocarbons. Environ Toxicol Chem 16: 179-188.
- Lamoureux EM, Brownawell BJ (1999) Chemical and biological availability of sediment sorbed hydrophobic organic contaminants. Environ Toxicol Chem 18: 1733-1741.
- Ohkouchi N, Kawamura K, Kawahata H (1999) Distributions of three to seven ring polynuclear aromatic hydrocarbons on the deep sea floor in the central Pacific. Environ Sci Technol 33: 3086- 3090.
- Pathak H, Jain PK, Jaroli DP, Lowry M (2008) Technical Note: Degradation of Phenanthrene and Anthracene by Pseudomonas Strain, Isolated From Coastal Area .Bioremediation Journal 12: 111-116.
- Boxall A B, Maltby L (1997) The effects of motorway runoff on freshwater ecosystems. Toxicant conformation. Arch Environ Contam Toxicol 33: 9-16.
- Holman HYN, Tsang YW, Holman WR (1999) Mineralization of sparsely water soluble polycyclic aromatic hydrocarbons in a water table fluctuation zone. Environ Sci Technol 33: 1819- 1824.
- Martens D, Maguhn J, Spitzauer P, Kettrup A (1997) Occurrence and distribution of polycyclic aromatic hydrocarbons (PAHs) in an agricultural ecosystem. Fresenius J Anal Chem 359: 546-554.
- Pitt R, Field R, Lalor M, Brown M (1995) Urban storm water toxic pollutants: assessment, sources, and treat ability. Water. Environ Res 67: 260-275.
- Wagrowski DM, Hites RA (1997) Polycyclic aromatic hydrocarbon accumulation in urban, suburban and rural vegetation. Environ Sci Tech nol 31: 279-282.
- Lee S D and Grant L (1981) Health and ecological assessment of polynuclear aromatic hydrocarbons. Pathotox Publishers, Inc., Park Forest South, Ill.
- Edwards N T (1983) Polycyclic aromatic hydrocarbons (PAHs) in the terrestrial environment. A review. J Environ Qual 12: 427-441.
- Sims RC, Overcash MR (1983) Fate of polynuclear aromatic compounds (PNAs) in soil-plant systems. Residue Rev 88: 1-68.
- Harayama S (1997) Polycyclic aromatic hydrocarbon bioremediation design. Curr Opin Biotechnol 8: 268-273.
- Blumer M (1976) Polycyclic aromatic hydrocarbons in nature. Sci Am 234: 35-45.
- Jones K C, Stratford J A, Waterhouse K S, Vogt NB (1989) Organic contaminants in Welsh soils: polynuclear aromatic hydrocarbons. Environ. Sci. Technol. 23: 540-550.
- Otte MP, Gagnon J, Comeau Y, Matte N, Greer CW, Samson R (1994) Activation of an indigenous microbial consortium for bioaugmentation of pentachlorophenol/ creosote contaminated soils. Appl Microbiol Biotechnol 40: 926-932
- Wilson SC, Jones KC (1993) Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs) review. Environ Pollut 81: 229-249.
- Oppong D, King VM, Zhou X, Bowen JA (2000) Cultural and biochemical diversity of pink-pigmented bacteria isolated from paper mill slimes. J of Industrial Micro Biotech 25: 74-80.
- Ijah U J J (1998) Studies on relative capabilities of bacterial and yeast isolates from tropical soil in degrading crude oil. Waste Manag 18: 293-299
- Malatova Katarina (2005) Isolation and Characterization of hydrocarbon degrading bacteria from environmental habitats in western New York State. Ph.D. thesis.
|
| |
| |