Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
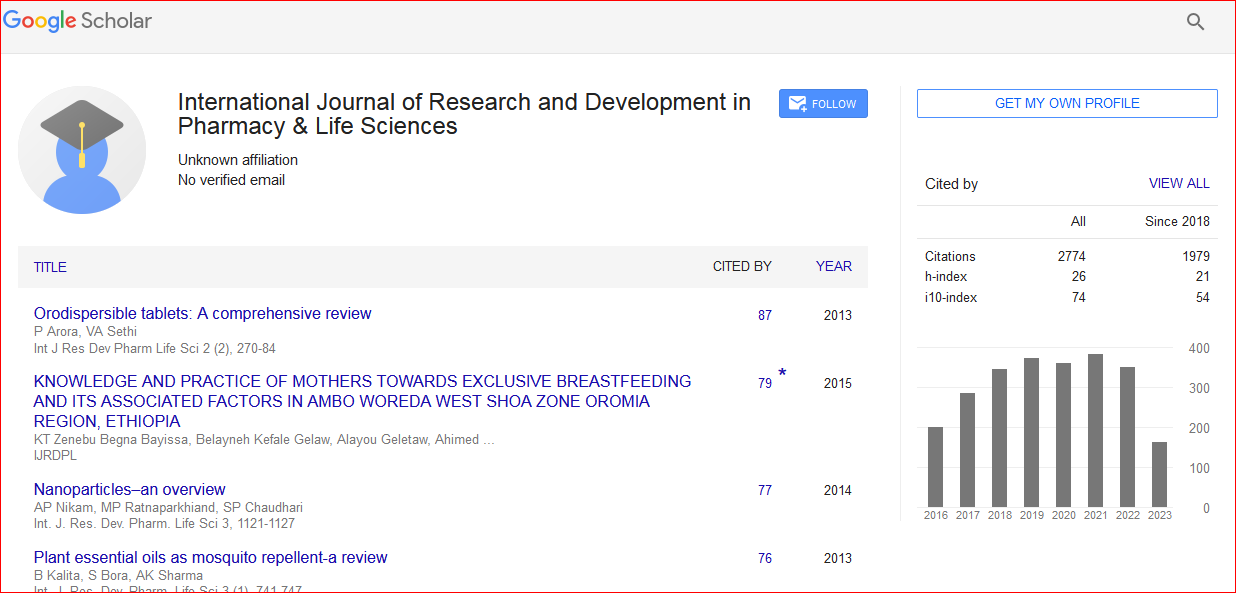
Google Scholar citation report
Citations : 3790
International Journal of Research and Development in Pharmacy & Life Sciences peer review process verified at publons
Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Clinical Trial Design
Clinical trial design is the structured process of planning and organizing a clinical study to evaluate the safety, efficacy, and optimal use of new treatments or interventions.
It involves defining the study's objectives, selecting appropriate methodologies, and determining key aspects such as participant selection, randomization, control groups and endpoints. A well-designed trial ensures rigorous data collection, minimizes biases and adheres to ethical standards. It supports regulatory approval and guides clinical practice.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi