Review Article Open Access
Reviews: Serum Anticholinergic Activity: A Biomarker for Rapid Progression of Alzheimer's Disease
| Koji Hori1*, Kimiko Konishi1,2, Hiroi Tomioka1, Masayuku Tani3, Genshin Minegishi4, Hiroaki Tanaka4, Ryo Akita1, Sachiko Yokoyama1, Tomonori Oshio1 and Mitsugu Hachisu5 | ||
| 1Department of Psychiatry, Showa University Northern Yokohama Hospital, Kanagawa, Japan | ||
| 2Tokyo Metropolitan Tobu Medical Center for Persons with Developmental/ Multiple Disabilities, Tokyo, Japan | ||
| 3Department of Psychiatry, Showa University Karasuyama Hospital, Tokyo, Japan | ||
| 4Department of Psychiatry, Showa University Fujigaoka Hospital, Kanagawa, Japan | ||
| 5Department of Clinical Psychopharmacy, School of Pharmaceutical Sciences, Showa University, Tokyo, Japan | ||
| Corresponding Author : | Koji Hori Department of Psychiatry Showa University Northern Yokohama Hospital 35-1 Chigasakichuo, Tsuzukiku Yokohama-City, Kanagawa 224-8503, Japan Tel: +81-45-949-7000 Fax: +81-45-949-7927 E-mail: kojihori@med.showa-u.ac.jp |
|
| Received October 28, 2011; Accepted February 27, 2012; Published March 01, 2012 | ||
| Citation: Hori K, Konishi K, Tomioka H, Tani M, Minegishi G (2012) Reviews: Serum Anticholinergic Activity: A Biomarker for Rapid Progression of Alzheimer’s Disease. Autacoids S4:001. doi: 10.4172/2161-0479.S4-001 | ||
| Copyright: © 2012 Hori K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Autacoids and Hormones
Abstract
We have previously reported the possibilities that anticholinergic activity (AA) appeared endogenously in Alzheimer’s disease (AD) and accelerated AD pathology. In this article we reviewed the reasons why AA endogenously appeared in AD and accelerated AD pathology and that the serum anticholinergic activity (SAA) was suitable as one of the biological marker of AD. We argued firstly that an acethycholine (Ach) played a role to maintain an endogenous anti-inflammatory pathway in the brain and the peripheral tissue. Secondarily, the deficiency of Ach in the brain might trigger the inflammatory process in AD patients by way of the suppression of cholinergic anti-inflammatory pathway and AA might be generated by inflammatory process. At third, AA disturbed not only memory functions but also accelerated AD pathology (increasing of amyloidgenic), and at fourth, we proposed a possibility that the SAA was useful as a marker of rapid progression of AD pathology in the moderate stage. SAA was considered to appear at moderate stage of AD when the symptoms rapidly were progress and an inflammation was occurred in the brain. The activities of N-methyl-D-aspartate (NMDA) receptors were accelerated and it progressed an inflammation in the brain. Memantine should be prescribed to AD patients with positive SAA in order to suppress inflammation by antagonizing the NMDA receptor. Therefore, when SAA was detected in AD, it should be considered to prescribe memantine. Finally, we speculated that down regulation of Ach and up regulation of NMDA receptor were involved in the pathology (increasing of amyloid) of AD disease and commented that cholinesterase inhibitors in mild stage and NMDA receptor antagonist in moderate stage in AD should be prescribed to prevent appearance of AA as well as to ameliorate symptoms by way of the up-regulation of Ach and the suppression of inflammation process.
| Keywords | |
| Alzheimer’s disease; Serum anticholinergic activity; Inflammation; N-methyl-d-aspartate receptor; Anti-inflammatory pathway; Biomarker | |
| Abbreviation | |
| AD: Alzheimer’s Disease; SAA: Serum Anticholinergic Activity; NMDA: N-Methyl-D-Aspartate Receptor | |
| Introduction | |
| We have reported the relationships between serum anticholinergic activity (SAA), a marker for anticholinergic burden, and clinical symptoms in Alzheimer’s disease (AD) [1,2]. The articles described that SAA correlated with a number of prescribed psychotrophic medications, a severity of dementia, a reduction of global cognitive dysfunction and a severity of psychotic symptoms. In the case of psychotic symptoms, patients with higher SAA value show higher rate of delusion and diurnal rhythm disturbance [1]. In cognitive function in AD assessed with Mini-Mental State Examination (MMSE), there were the relationships between SAA and global cognitive disturbances as well as memory disturbances (registration and recall) [2]. From these results we pointed out that the possibilities that AA might accelerate the AD pathology and the SAA was endogenously generated in AD [1,2]. In this article we reviewed the role of AA to progress AD symptoms and discussed the reasons why AA was detected in AD. Moreover, we also proposed that SAA was possibly useful as a biological marker for rapid deterioration of AD symptoms. | |
| Anticholinergic activity appears endogenously in Alzheimer’s disease and accelerates Alzheimer’s pathology (amyloidgenic process) | |
| Needless to say, to prescribe psychotropic medicine with AA have to be paid attention because of an adverse effects of worsening of cognition, extra pyramidal symptoms and psychotic symptoms in elderly patients, especially on AD or Lewy body disease patients [3]. SAA was measured by receptor-binding assay using muscarinic receptors in the forebrains excised from rat and this assay measures inhibition of quinuclidinyl benzilate, L-[benzilic-4, 4’ 3H]( 3H-QNB)) binding to rat brain muscarinic acetylcholine receptors [4]. Therefore, it is included all substances those have capacity to bind to muscarinic receptors. The substances those appear in the serum or in the brain and related to positive SAA or AA are not identified. Therefore, it is important to identify these substances, especially in AD. Although those include agonist and antagonist on muscarinic receptors, generally speaking, all most of those have antagonistic actions. SAA is regarded as the peripheral marker for the burden of AA in the central nervous system because SAA is correlated with the severity of delirium [5-10] and SAA was correlated with the AA in the cerebrospinal fluid [11,12]. In generally, SAA is considered mainly to come from prescribed medicines, especially with potent AA and complex medication regimen [13]. | |
| According to the cognitive functions, patients with schizophrenia, elderly people at their homes or nursing homes were investigated in view of the relationships between cognitive disturbances and anticholinergic burden [14-22]. Beside the correlation between SAA and MMSE score (global cognitive function), there was correlation between SAA and memory disturbances, especially in demented patients with low central cholinergic activity [16,18]. In the BPSD, there was correlation between SAA and delusion or hallucination. Moreover, Cummings [23] and Lemstra et al. [24] described that the central cholinergic deficiency was characterized by neuropsychiatric symptoms rather than by cognitive dysfunctions. | |
| In this context, we reported a positive relationship between SAA and clinical symptoms of AD patients [1,2]. Among 76 AD patients, 26 AD patients were positive for SAA [SAA (+)], and the remaining 50 patients were negative [SAA (-)]. The SAA (+) group showed a significantly higher numbers of prescribed psychotrophic medications, significantly lower total score of the MMSE, especially registration and recall domain. Moreover, SAA (+) group showed significantly higher scores of the Functional Assessment Staging (FAST), and the items of paranoid and delusional ideation, hallucinations and diurnal rhythm disturbances of the Behavioral Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD). By means of logistic regression analysis, the items of paranoid and delusional ideation, and diurnal rhythm disturbances in BEHAVE-AD were positively correlated with SAA. We considered at first that prescribed psychotorphic medicines were one of main origins of positive SAA, because there was a correlation between number of prescribed psychotorphic medicines and the SAA value in AD patients. Our paper also described that delusions and diurnal rhythm disturbance i.e., behavioral and psychotic symptoms of dementia (BPSD), were more strongly affected by SAA than the cognitive function. These results were consistent with previous reports. However, in these two articles, we found two novel findings. First, we also considered that there might be cyclic relationships among three factors of BPSD, the prescription of psychotropic medicines and SAA. The main reason for the appearance of AA is the prescription of psychotropic medicines and AA induces BPSD. Moreover, in general we prescribe psychotropic medicines for the clinical psychiatric symptoms of agitation and psychosis in AD [25,26]. Therefore, relationship among prescription of psychotropic medications, SAA and BPSD (especially hallucination and diurnal rhythm disturbance) might be cyclic. We termed the cyclic relationships as “vicious cycle of anti-cholinergic activity in AD (VCAA)”. Then we commented that there might be also a possibility that the SAA was endogenously generated [1] because we had not prescribed psychotropic medications except antidementia agents for AD patients who were not show psychotic symptoms. In fact we found some patients show positive SAA in spite of no medications. Second, we considered that AA not only disturbed cognitive functions but also accelerated the process of Alzheimer’s disease by amyloidgenic pathology, which is irreversible. In fact, patients with positive SAA showed memory disturbances [2], those are related to AD symptoms [27]. Moreover, these patients showed not only disturbed higher cognitive function but also higher severe FAST scores. | |
| Lowering cholinergic activity progresses an inflammatory processes in the brain | |
| Why and how does the AA endogenously generate in AD? We speculate that lowering cholinergic activity generated anticholinergic substances by way of inflammatory processes. AD is well known as a disease with reduction of cholinergic neuronal activity in the brain by the degeneration of cholinergic neurons [28]. The deficiencies of cholinergic neuronal activities worsen not only the clinical symptoms in AD but also the reduction of activity in anti-inflammatory system which is called “the cholinergic anti-inflammatory pathway” [29-34]. For examples, Pavlov et al. [31] reported that muscarinic acetylcholine receptors agonists inhibited the overproduction of serum TNF (tumor necrotic factor), a cytokine. Marbley et al. [33] reported that activation of cholinergic system reduced mortality and organ failure in mice induced by ricin, a proinflammatory toxin, which activated a stressactivated protein kinase which causes systemic inflammation. On the other hand, acetylcholine (Ach) reduces this systemic inflammation by way of the activation of cholinergic anti-inflammatory pathway [32]. Therefore, there may be possibility that neuronal immuno-reactions (inflammation) are induced by the reduction of Ach neuronal activity in the brain of AD patients. | |
| Anticholinergic active substance is generated by inflammatory process | |
| There may be also the possibility that AA appears endogenously by way of inflammation process. Flacker and Lipsitz [7] reported that SAA disappeared without the changes of medications in patients in accordance with the amelioration of acute physical illnesses and they pointed out that SAA might reflect a nonspecific stress response to illness in elderly people. Moreover, Nazarov et al. [35] reported that C-reactive protein (CRP), which increases at inflammation state, might show AA itself, with capturing Ach or binding to a part of Ach receptor. They discussed the cross talk between Ach and inflammatory pathways. However, there is only one article that commented the relationship between AA in peripheral tissue and CRP. Tsuruta et al. [36] reported that plasma AA were related with delirium in critically ill and injured patients. There Horiuchi et al. [37] reported that endogenously and immunologically active peptide, apelin, inhibited cholinergic activity by way of binding to the Ach receptor. In fact, many reports published as new concept that AD was caused by the neuronal inflammation [38-41] or that endogenous ligands binding to Ach receptors in the AD brain were generated [42-44]. Indeed, an endogenous ligand binding to muscarinic receptor in the AD brain has been detected with higher concentration than in the non-demented elderly control and is supposed to be a low molecular substance of 100-1000 Da which has been catalyzed by oxidation [42,44]. | |
| Plaschke et al. [45] reported a correlation between cortisol levels and SAA. They commented that stress was causal main factor for SAA and that SAA was appeared not only by extrinsic factors, e.g. medications or physical illnesses, but also by intrinsic factors e.g. stress. Therefore we consider that even if AA comes from prescribed medications, the intrinsic factor also may be attributed to the generation of AA. Therefore, the anticholinergic load in each patient is not inferable proper by AA of individual medication alone. We consider that anticholinergic burdens are not estimated by AA in each medicines i.e., using Anticholinergic Drug Scale [46], Anticholinergic Risk Scale [47] etc. We should measure SAA in order to estimate the anticholinergic load in each patient [48]. | |
| Recently it is pointed out that neuropsychiatric disorders such as schizophrenia or bipolar disorder are attributable to hyperinflammatory state or conditions by the appearance of AA. In fact, there are some reports that AA is detected in sera in schizophrenic patients [49] and patients with Sojgren syndrome [50]. These diseases show psychiatric symptoms, such as delusions, hallucinations and diurnal rhythm disturbances, similar to elderly patients who show high SAA [5-10]. The central cholinergic deficiency is characterized clinically by neuropsychiatric symptoms rather than by cognitive impairments [23,24]. Therefore, relatively younger patients with high SAA have tendency to show neuropsychiatric symptoms as is elder patients are tended to show delusion [51] or delusion and diurnal rhythm disturbances [1] rather than cognitive impairments. | |
| In case of bipolar patients, it is hypothesized that AA contribute to manic switch in bipolar patients [52]. Dysfunction of dopaminergic system is found out in bipolar patients [53] and the manic switch is thought to relate to rapid up-regulation of dopaminergic system [54]. Therefore, in steady state (remitted state); the hyper-dopaminergic activity is inhibited by the up-regulation of Ach system [55]. In those states, when AA acts on the bipolar patients, Ach system is downregulated and dopaminergic system is up-regulated, then manic switch is induced in bipolar patients. Therefore, use of tricyclic antidepressants, sleep deprivations and exogenous steroid hormone should be prohibited [52]. However, even if these treatments (use of tricyclic antidepressants, sleep deprivations, exogenous steroid) are stopped, the manic switch will be occurred, because AA endogenously appears in bipolar patients. | |
| Moreover, there was reported that the prevalence of Parkinson’s disease (PD) was increased with the increased numbers of concurrent use of medications, i.e., polypharmacy increased the risk of PD [56]. We also speculate that there might be the possibility that AA accelerated the pathology of PD. We consider that in PD or LBD AA also appears endogenously and accelerates PD pathology (increasing LB) [57]. | |
| Anticholinergic activity accelerates AD pathology (amyloidgenic process) | |
| The relationship AA and progression of AD pathology or cognitive dysfunction is already reviewed [2]. The previous articles demonstrated that memory domains were more confidently vulnerable by AA in AD patients [2,16,18]. Therefore, we supposed that memory functions were more highly dependent on the cholinergic function than other cognitive domains of MMSE. In generally, it is considered that AA by medications is disappeared by the interruption of medications, and then cognitive impairments by AA would be principally reversible [58]. However, the recovery of cognitive impairment takes longer time and sometimes it is partial, even though the prescribed medications are completely quitted [59]. Therefore we considered that AA mainly disturbed the memory domain, which is mostly characteristic to AD [27] with irreversible manner. In fact, Perry et al. [60] reported that amyloid plaque densities increased to more than 2.5-fold by treatment of antimuscarinic medications as long as more than 2 years, when compared with untreatment or short term (less than 2 years) treatment. Lu and Tune [61] commented that the chronic exposure of medications with AA accelerated the clinical course of AD. Moreover, Fisher [62] and Jones et al. [63] reported that muscarinic 1 receptor agonist processed amyloid precursor protein to nonamyloid protein (α processing), preventing the amyloidosis. Therefore, antagonistic action of muscarinic 1 receptor (i.e., AA) induces to process amyloid precursor protein to amyloid protein. | |
| From these results, we consider that AA directly worsens the cognitive functions especially memory domain as well as exacerbates AD pathology through increasing amyloid plaques (amyloidgenic process), though how AA increases amyloid plaques remains to be elucidated. However, it is a fact that a long-term exposure of anticholinergic medications may irreversibly change the AD pathology. | |
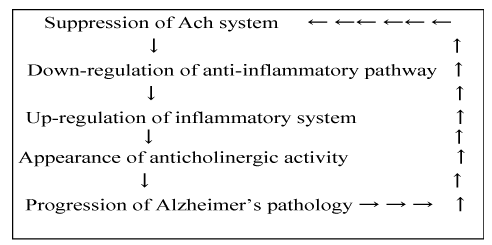
| Therefore, we speculate that AA is generated endogenously through the activation of the inflammatory processes, which is caused by the down-regulation of Ach activity (Figure 1). These processes more accelerate the cholinergic neurons deficiency and the symptoms worsen more rapidly. We refer these process “endogenous anticholinergic cascades in Alzheimer’s disease”. We also refer this accelerate progression of AD by endogenous AA as “Alzheimer’s disease is progress by the mechanism of its own”. | |
| Serum anticholinergic activity: possibility of a biomarker for rapid progression of Alzheimer’s symptoms | |
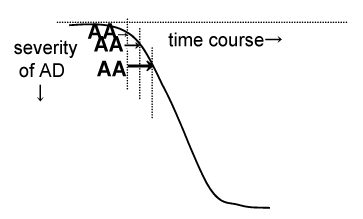
| As previously noted, a level of Ach gradually decreases from early stage of AD, then the Ach level goes down to sub-threshold level to suppress “a cholinergic anti-inflammatory activity” and the neuronal inflammatory pathway is activated in the brain at the moderate stage. The activation of neuronal inflammation pathway generates AA endogenously. In summary, the deficiencies of cholinergic activities cause anticholinergic activities by way of inflammatory processes. Because AD is well known as a disease with degeneration of cholinergic neuronal activity in the brain, the deficiencies of cholinergic neuronal activities cause the reduction of anti-inflammatory activity which is called “the cholinergic anti-inflammatory pathway” and then it changes to up-regulation of inflammatory process. Moreover, AA is thought to appear by way of inflammation process. Therefore, AA appears endogenously in AD. We refer this “endogenous anticholinergic cascade in Alzheimer’s disease”. Of course, the more AD pathology is progress, the lower cholinergic neuronal activity is accelerated (Figure 2). Therefore, these relations are considered cyclic relationships (Figure 1). The increased amyloidogenic pathplogies more deteriorate Ach system, suggesting vicious cycle in progression of AD. | |
| There are no biological markers for AD until now. However, based on a growing body of evidences concerning the pathophisiology of AD, many candidate biomarkers are evaluated in AD. These are beta amyloid in plasma or cerebrospinal fluid (CSF), total or phosphorylated tau protein in plasma or CSF, Apolipoprotein E, Cholesterol, antioxidant, interleukin-6, etc [64]. There are three putative biomarkers, i.e., a diagnostic marker, a classification marker and a prognostic marker [65]. Among them, especially CSF beta and CSF phosphorylated tau are mostly evaluated [64-67]. However, until now candidate markers are almost diagnostic markers and classification markers, i.e., those discriminate AD from normal aging or other demented diseases. These only a prognostic marker is alpha-1-antichymotrypsin. The AD pathology is slowly progressed during mild stage, while it is progress after at the moderate stage [68]. At moderate stage, behavioral and psychological symptoms (BPSD) are increased [69,70] and BPSD in AD becomes complicated [71]. These induce institutionalizations. Therefore, it important to know the prognosis of diseases. We propose a possibility that the SAA value is useful marker if the deterioration of AD pathology is rapidly progressed at the moderate stage. If SAA is positive, this will suggest the rapid progression of AD. The faults of evaluating SAA are methodolical issue, i.e., plasma proteins bind [3H] QNB [72] and the extent which peripheral anticholinergic activity reflects AA in central nervous system (not all studies supported the relationships between SAA and cognitive dysfunction or clinical manifestation). However, as mentioned before SAA was correlated with the AA in the cerebrospinal fluid [11,12], AD patients are vulnerable to AA and memory functions and behavioral symptoms are especially vulnerable to anticholinergic burden. Therefore we consider that SAA might be a candidate biological marker for AD (a prognostic marker). Longitudinal study are needed to elucidate the endogenous appearance of AA in amnesic patients. | |
| The medications for the prevention of AA appearance in Alzheimer’s disease | |
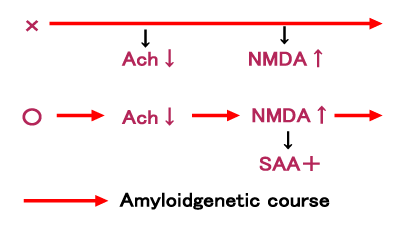
| On the other hand, there are several reports that activation of NMDA receptor enhances the inflammatory process in AD brain [73- 75] and that activation of nicotinic cholinergic receptor inhibits the inflammatory system [76,77]. From these results we consider that the down-regulation of cholinergic neuronal activity and an up-regulation of NMDA receptor activity are not the secondary events form caused by the amyliodgenic process but the primary events in AD pathology (Figure 3), antidementia agents of cholinesterase inhibitors (ChEIs) and NMDA receptor antagonist works not only as symptomatic treatment but also as disease modifying treatment. Indeed, ChEIs and NMDA antagonist, memantine are proven to protect neuronal cell death caused by amyloid toxicities [78-81]. As a reduction of Ach neuronal activity and a hypoactivity of nicotinic receptor are reported in AD [82], ChEIs should be prescribed for maintaining the Ach neuronal activities because the enhancement of Ach activity prevents the appearance of AA. Memantine, an open channel uncompetitive NMDA receptor antagonist [83], is used for SAA positive AD patients to inhibit hyperactive NMDA receptor system. We consider that SAA might be also a marker for the hyperactivity of NMDA receptor system and progress the AD disease. Therefore, it should be considered that when SAA is detected in AD, it is a timing to prescribe memantine. | |
| Elevation of SAA is observed not only in AD but also in schizophrenia, bipolar disorder and Sojgren syndrome etc., and we speculate that these neuropsychiatric disorders are also caused by neuronal inflammation. Therefore, the antidementic agents such as NMDA antagonist and ChEIs are effective to these neuropsychiatric disorders regarding to prevent neuronal inflammation related AA and that antidementia agents might to be used these neuropsychiatric disorders in near future. In fact, there are two reports. One is that galantamine was effective to schizophrenic patients those were responding to clozapine but not tolerated because of side effect [84] and the other is that memantine was effective to obsessive-compulsive disorder patients [85]. | |
References
- Hori K, Konishi K, Watanabe K, Uchida H, Tsuboi T, et al. (2011) Influence of anticholinergic activity in serum on clinical symptoms of Alzheimer's disease. Neuropsychobiology 63: 147-153.
- Konishi K, Hori K, Uchida H, Watanabe K, Tominaga I, et al. (2010) Adverse effects of anticholinergic activity on cognitive functions in Alzheimer's disease. Psychogeriatrics 10: 34-38.
- Salzman C (1982) A primer on geriatric psychopharmacology. Am J Psychiatry 139: 67-74.
- Tune L, Coyle JT (1980) Serum levels of anticholinergic drugs in treatment of acute extrapyramidal side effects. Arch Gen Psychiatry 37: 293-297.
- Rockwood K (1989) Acute confusion in elderly medical patients. J Am Geriatr Soc 37: 150-154.
- Mach JR jr, Dysken MW, Kuskowski M, Richelson E, Holden L, et al. (1995) Serum anticholinergic activity in hospitalized older persons with delirium: a preliminary study. J Am Geriatr Soc 43: 491-495.
- Flacker JM, Lipsitz LA (1999) Serum anticholinergic activity changes with acute illness in elderly medical patients. J Gerontol A Biol Sci Med Sci 54: 12-16.
- Mussi C, Ferrari R, Ascari S, Salvioli G (1999) Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol 12: 82-86.
- Trzepacz PT (2000) Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry 5: 132-148.
- Tune LE (2000) Serum anticholinergic activity levels and delirium in the elderly. Semin Clin Neuropsychiatry 5: 149-153.
- Miller PS, Richardson JS, Jyu CA, Lemay JS, Hiscock M, et al. (1988) Association of low serum anticholinergic levels and cognitive impairment in elderly presurgical patients. Am J Psychiatry 145: 342-345.
- Plaschke K, Thomas C, Engelhardt R, Teschendorf P, Hestermann U, et al. (2007) Significant correlation between plasma and CSF anticholinergic activity in presurgical patients. Neurosci Lett 417: 16-20.
- Tune L, Carr S, Hoag E, Cooper T (1992) Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium. Am J Psychiatry 149: 1393-1394.
- Tune LE, Strauss ME, Lew MF, Breitlinger E, Coyle JT (1982) Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry 139: 1460-1462.
- Richardson JS, Miller PS, Lemay JS, Jyu CA, Neil SG, et al. (1985) Mental dysfunction and the blockade of muscarinic receptors in the brains of the normal elderly. Prog Neuropsychopharmacol Biol Psychiatry 9: 651-654.
- Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, et al. (1987) Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. Arch Gen Psychiatry 44: 418-426.
- Rovner BW, David A, Lucas-Blaustein MJ, Conklin B, Filipp L, et al. (1988) Self-care capacity and anticholinergic drug levels in nursing home patients. Am J Psychiatry 145: 107-109.
- Thienhaus OJ, Allen A, Bennett JA, Chopra YM, Zemlan FP (1990) Anticholinergic serum levels and cognitive performance. Eur Arch Psychiatry Clin Neurosci 240: 28-33.
- Tollefson GD, Montague-Clouse J, Lancaster SP (1991) The relationship of serum anticholinergic activity to mental status performance in an elderly nursing home population. J Neuropsychiatry Clin Neurosci 3: 314-319.
- Remillard AJ (1994) A pilot project to assess the association of anticholinergic symptoms with anticholinergic serum levels in the elderly. Pharmacotherapy 14: 482-487.
- de Leon J, Odom-White A, Josiassen RC, Diaz FJ, Cooper TB, et al. (2003) Serum antimuscarinic activity during clozapine treatment. J Clin Psychopharmacol 23: 336-341.
- Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, et al. (2003) Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry 60: 198-203.
- Cummings JL (2000) Cholinesterase inhibitors: A new class of psychotropic compounds. Am J Psychiatry 157: 4-15.
- Lemstra AW, Eikelenboom P, van Gool WA (2003) The cholinergic deficiency syndrome and its therapeutic implications. Gerontology 49: 55-60.
- Chien IC, Hsu JH, Bih SH, Lin CH, Chou YJ, et al. (2008) Prevalence, correlates, and disease patterns of antipsychotic use in Taiwan. Psychiatry Clin Neurosci 62: 677-684.
- Wood-Mitchell A, James IA, Waterworth A, Swann A, Ballard C (2008) Factors influencing the prescribing of medications by old age psychiatrists for behavioural and psychological symptoms of dementia: a qualitative study. Age Ageing 37: 547-552.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939-944.
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, et al. (1982) Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 215: 1237-1239.
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, et al. (2002) Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med 195: 781-788.
- Sonkusare SK, Kaul CL, Ramarao P (2005) Dementia of Alzheimer's disease and other neurodegenerative disorders--memantine, a new hope. Pharmacol Res 51: 1-17.
- Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, et al. (2006) Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A 103: 5219-5223.
- Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, et al. (2009) The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology 56: 254-263.
- Mabley JG, Pacher P, Szabo C (2009) Activation of the cholinergic antiinflammatory pathway reduces ricin-induced mortality and organ failure in mice. Mol Med 15: 166-172.
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, et al. (2009) Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain 132: 2464-2477.
- Nazarov PG, Krylova IB, Evdokimova NR, Nezhinskaya GI, Butyugov AA (2007) C-reactive protein: a pentraxin with anti-acetylcholine activity. Life Sci 80: 2337-2341.
- Tsuruta R, Girard TD, Wesley EE, Fujimoto K, Ono T, et al. (2008) Associations between markers of inflammation and cholinergic blockers and delirium in intensive vcare unit patients: a pilot study. Bull Yamaguchi Med School 55: 34-42.
- Horiuchi Y, Fujii T, Kamimura Y, Kawashima K (2003) The endogenous, immunologically active peptide apelin inhibits lymphocytic cholinergic activity during immunological responses. J Neuroimmunol 144: 46-52.
- McGeer PL, McGeer EG (1995) The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev 21: 195-218.
- Hynd MR, Scott HL, Dodd PR (2004) Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int 45: 583-595.
- Guerreiro RJ, Santana I, Brás JM, Santiago B, Paiva A, et al. (2007) Peripheral inflammatory cytokines as biomarkers in Alzheimer's disease and mild cognitive impairment. Neurodegener Dis 4: 406-412.
- Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, et al. (2010) Neuroinflammation - an early event in both the history and pathogenesis of Alzheimer's disease. Neurodegener Dis 7: 38-41.
- Frey WH 2nd, Emory CR, Wiebenga ME, Saxena S, Cardelli D, et al. (1994) Inhibitor of antagonist binding to the muscarinic receptor is elevated in Alzheimer's brain. Brain Res 655: 153-160.
- Fang YI, Suzuki T, Momose K (1996) Partial purification of an endogenous inhibitor of muscarinic ligand binding. Biochem Mol Biol Int 38: 501-507.
- Venters HD jr, Bonilla LE, Jensen T, Garner HP, Bordayo EZ, et al. (1997) Heme from Alzheimer's brain inhibits muscarinic receptor binding via thiyl radical generation. Brain Res 764: 93-100.
- Plaschke K, Kopitz J, Mattern J, Martin E, Teschendorf P (2010) Increased cortisol levels and anticholinergic activity in cognitively unimpaired patients. J Neuropsychiatry Clin Neurosci 22: 433-441.
- Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR (2006) The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol 46: 1481-1486.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE (2008) The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 168: 508-513.
- Hori K, Funaba Y, Konishi K, Moriyasu M, Hirata K, et al. (2005) Assessment of pharmacological toxicity using serum anticholinergic activity in a patient with dementia. Psychiatry Clin Neurosci 59: 508-510.
- Borda T, Gomez R, Berría MI, Sterin-Borda L (2004) Antibodies against astrocyte M1 and M2 muscarinic cholinoceptor from schizophrenic patients' sera. Glia 45: 144-154.
- Orman B, Sterin-Borda L, Pita ADC, Reina S, Borda E (2007) Anti-brain cholinergic auto antibodies from primary Sjogren syndrome sera modify simultaneously cerebral nitric oxide and prostaglandin biosynthesis. Int Immunopharmacol 7: 1535-1543.
- Mulsant BH, Gharabawi GM, Bossie CA, Mao L, Martinez RA, et al. (2004) Correlates of anticholinergic activity in patients with dementia and psychosis treated with risperidone or olanzapine. J Clin Psychiatry 65: 1708-1714.
- Koszewska I, Rybakowski JK (2009) Antidepressant-induced mood conversions in bipolar disorder: a retrospective study of tricyclic versus non-tricyclic antidepressant drugs. Neuropsychobiology 59: 12-16.
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH (2007) Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32: 1888-1902.
- Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, et al. (2010) The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry 71: 1488-1501.
- Rybakowski JK, Koszewska I, Puzynski S (2010) Anticholinergic mechanisms: a forgotten cause of the switch process in bipolar disorder. J Clin Psychiatry 71: 1698-1699.
- Lai SW, Su LT, Lin CH, Tsai CH, Sung FC, et al. (2011) Polypharmacy increases the risk of Parkinson's disease in older people in Taiwan: a population-based study. Psychogeriatrics 11: 150-156.
- Konishi K, Hori K, Tomioka H, Tani M, Akita R, et al. (2012) Is anticholinergic activity related with Parkinson’s disease ? Psychogeriatrics 12.
- Salzman C, Fisher J, Nobel K, Glassman R, Wolfson A, et al. (1992) Cognitive impairment following benzodiabepine discontinuation in elderly nursing home residents. Int J Geriatr Psychiatry 7: 89-93.
- Tönne U, Hiltunen AJ, Vikander B, Engelbrektsson K, Bergman H, et al. (1995) Neuropsychological changes during steady-state drug use, withdrawal and abstinence in primary benzodiazepine-dependent patients. Acta Psychiatr Scand 91: 299-304.
- Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH (2003) Increased Alzheimer pathology in Parkinson's disease related to antimuscarinic drugs. Ann Neurol 54: 235-238.
- Lu CJ, Tune LE (2003) Chronic exposure to anticholinergic medications adversely affects the course of Alzheimer disease. Am J Geriatr Psychiatry 11: 458-461.
- Fisher A (2000) Therapeutic strategies in Alzheimer's disease: M1 muscarinic agonists. Jpn J Pharmacol 84: 101-112.
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, et al. (2008) Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci 28: 10422-10433.
- Hampel H, Mitchell A, Blennow K, Frank RA, Brettschneider S, et al. (2004) Core biological marker candidates of Alzheimer's disease - perspectives for diagnosis, prediction of outcome and reflection of biological activity. J Neural Transm 111: 247-272.
- Schneider P, Hampel H, Buerger K (2009) Biological marker candidates of Alzheimer's disease in blood, plasma, and serum. CNS Neurosci Ther 15: 358-374.
- Blennow K, Vanmechelen E (2003) CSF markers for pathogenic processes in Alzheimer's disease: diagnostic implications and use in clinical neurochemistry. Brain Res Bull 61: 235-242.
- Andreasen N, Sjögren M, Blennow K (2003) CSF markers for Alzheimer's disease: total tau, phospho-tau and Abeta42. World J Biol Psychiatry 4: 147-155.
- Ji M, Xiong C, Grundman M (2003) Hypothesis testing of a change point during cognitive decline among Alzheimer's disease patients. J Alzheimers Dis 5: 375-382.
- Teri L, Larson EB, Reifler BV (1988) Behavioral disturbance in dementia of the Alzheimer's type. J Am Geriatr Soc 36: 1-6.
- Hope T, Keene J, Gedling K, Cooper S, Fairburn C, et al. (1997) Behaviour changes in dementia. 1: Point of entry data of a prospective study. Int J Geriatr Psychiatry 12: 1062-1073.
- Hori K, Konishi K, Tomioka H, Tani M, Minegishi G, et al. (2012) Mood symptoms are related to psychotic symptoms in severe Alzheimer's disease. J Addict Res Ther 2.
- Cox EA, Kwatra SG, Shetty S, Kwatra MM (2009) Flaws in the serum anticholinergic activity assay: implications for the study of delirium. J Am Geriatr Soc 57: 1707-1708.
- Liu J, Gao X, Wu Y (2008) N-Methyl-D-Aspartate receptors mediate excitotoxicity in amyloid beta induced synaptic patholpgy of Alzheimer’s disease. Neuroembryol Aging 5: 134-143.
- Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS (2008) Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum 58: 3439-3449.
- Takada Y, Yonezawa A, Kume T, Katsuki H, Kaneko S, et al. (2003) Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons. J Pharmacol Exp Ther 306: 772-777.
- Nyakas C, Granic I, Halmy LG, Banerjee P, Luiten PG (2011) The basal forebrain cholinergic system in aging and dementia. Rescuing cholinergic neurons from neurotoxic amyloid-ß42 with memantine. Behav Brain Res 221: 594-603.
- Toyohara J, Hashimoto K (2010) a7 Nicotinic Receptor Agonists: Potential Therapeutic Drugs for Treatment of Cognitive Impairments in Schizophrenia and Alzheimer's Disease. Open Med Chem J 4: 37-56.
- Parsons CG, Danysz W, Quack G (1999) Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology 38: 735-767.
- Kimura M, Komatsu H, Ogura H, Sawada K (2005) Comparison o donepezil and mementine for protective effect against amyloid-beta (1-42) toxicity in rat septal neurons. Neurosci Lett 391: 17-21.
- Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Niidome T, et al. (2006) Neuroprotective effects of galanthamine and tacrine against glutamate neurotoxicity. Eur J Pharmacol 549: 19-26.
- Bailey JA, Lahiri DK (2010) A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer's disease. J Neurochem 112: 843-853.
- Shimohama S, Taniguchi T, Fujiwara M, Kameyama M (1986) Changes in nicotinic and muscarinic cholinergic receptors in Alzheimer-type dementia. J Neurochem 46: 288-293.
- Parsons CG, Danysz W, Quack G (1999) Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology 38: 735-767.
- Allen TB, McEvoy JP (2002) Galantamine for treatment-resistant schizophrenia. Am J Psychiatry 159: 1244-1245.
- Stewart SE, Jenike EA, Hezel DM, Stack DE, Dodman NH, et al. (2010) A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol 30: 34-39.
Tables and Figures at a glance
 |
 |
 |
||
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14841
- [From(publication date):
May-2012 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10228
- PDF downloads : 4613
