Review Article Open Access
Resection and Ablation for Colorectal Liver Metastases
Keh M Ng, Terence C Chua* and David L MorrisHepatobiliary and Surgical Oncology Unit, Department of Surgery, University of New South Wales, St George Hospital, Kogarah, NSW 2217, Sydney, Australia
- *Corresponding Author:
- Terence Chua
Department of Surgery
University of New South Wales
St George Hospital, Kogarah, NSW 2217
Sydney, Australia
E-mail: terence.chua@unsw.edu.au
Received date: May 09, 2013; Accepted date: May 28, 2013; Published date: May 30, 2013
Citation: Ng KM, Chua TC, Morris DL (2013) Resection and Ablation for Colorectal Liver Metastases. J Gastroint Dig Syst 3:121. doi: 10.4172/2161-069X.1000121
Copyright: © 2013 Ng KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The surgical approach combining resection and ablation allows for a greater number of patients with colorectal liver metastases who would otherwise be considered to have unresectable disease to be given an opportunity to undergo a curative attempt surgery. Ablation results in local destruction of tumour and is often employed in lesions that are in locations which are not easily resectable. This may involve the use of radiofrequency ablation that causes thermal destruction or cryotherapy that freezes tumours. This review examined the literature reporting resection and ablation of colorectal liver metastases.
Keywords
Resection; Radiofrequency; Cryotherapy
Introduction
Surgical resection remains the gold standard treatment for CLMs, achieving 10-year survival rate of 22% in a selected group of patients [1]. However, only 20% of CLMs are amenable to resection at time of diagnosis. Despite the increased resectability in advanced CLMs with two-stage hepatectomy, approximately 20% of patients may fail to go through with the planned treatment [2]. The rate of resectability had also expanded over the years owing to the availability of better chemotherapy agents permitting improved local disease control and down staging of tumor burden allowing subsequent resection.
Post-operative hepatic functional reserve remains the main limitation to surgical resection, hence the attractive alternative of employing the adjunctive use of ablative therapies. There are multiple methods of percutaneous imaging-guided ablation. The simplest of these is alcohol injection but this has a role limited to small hepatocellular carcinoma. Cryotherapy and radiofrequency ablation will be discussed in detail. The role of these treatments are probably best limited to unresectable tumors or patients who are unfit for resection because we have been ultimately led to believe that ablation can ensure the long-term survival results of resection. The application of cryotherapy is not limited to lesion ablations but also may be used to treat suboptimal or involved margins of resection in patients precluded from extended resections. Long-term data following thermal ablation are scarce. Till date, the superiority of either thermal ablative method remains unclear and further sizeable comparative studies would be required to determine this.
Radiofrequency Ablation
Radiofrequency ablation (RFA) is currently the most frequently utilized thermal ablative modality. The radiofrequency energy conducted through the electrodes induces localized ionic agitation, which is converted into heat energy with an end-result of a zone of coagulation necrosis [3]. The clinical safety and efficacy of different approaches of RFA (open/laparoscopic/percutaneous) in eradication of hepatic malignancies had been demonstrated in early studies, extending curative treatment to non-resectable diseases [2,4,5]. RFA further gained popularity from its proven superiority over ethanol injection [6], and comparable results to surgical resection from randomized trials in treatment of hepatocellular carcinomas [7]. Its benefits in treatment of unresectable CRLMs was further supported by the EORTC trial comparing RFA plus systemic chemotherapy to systemic chemotherapy alone, whereby a prolonged median DFS by 7 months was observed in the combined treatment group albeit not translated into overall survival [8]. The present literature on RFA for the treatment of colorectal liver metastases, nonetheless, leaves limitations to interpretation, largely owing to the heterogeneity in study designs with majority of comparative trials being retrospective.
Delivery of RFA
RFA is better suited to percutaneous approach because of small probe diameter; however, better data exists for laparoscopy and open therapy. Although the approach of RFA was not shown to influence survival, there was a trend towards a higher rate of recurrence with percutaneous RFA (PcRFA) (15.1%) compared to open (6.7%) and laparoscopy (9%) in a study by Bleicher et al. of 153 patients treated for a total of 447 liver tumours [8]. In addition, several studies had consistently reported significantly higher complication rates with PcRFA relative to operative approaches [9,10]. Despite the perceived technical simplicity with PcRFA, the cooperation of patients’ respiratory effort following sedation poses difficulty in accurate probe placement [11]. The percutaneous approach as it’s minimally invasive, of course, avoids most of the morbidity of operative approaches resulting in shorter hospital stay [12].
The advantages of operative approaches translated by the eventual oncological outcome are undeniable as they allow accurate disease staging (undiagnosed on pre-operative imagings) via visualization, manual palpation and intraoperative ultrasonography (IOUS) [13-16]. In a study by Elias et al., surgical management was altered in 41.3% of patients with colorectal liver metastases (CLM) (total of 209) based on additional disease found intraoperatively - extrahepatic (16.2%), intrahepatic (30%), and both (4.9%) [16]. The subsequent analysis on 124 patients who could have had undergone PcRFA (<3 cm diameter, ≤ 3 lesions) revealed 21.7% with additional extrahepatic metastases [16]. Surgical access also protects adjacent structures from thermal injury and enables vascular occlusion to be performed; allowing control of hemorrhage and increasing the possibility of complete tumor ablation of lesions in close proximity to hepatic vasculature by minimizing the cooling effect [10,12,17-19] .
Image-controlled ablation is dependent on IOUS experience and on 3-D probe-placement expertise. Our senior author (DLM) has till date performed up to 2000 IOUS; and it’s still technically challenging. Regardless of the approach of RFA employed, treatment outcomes are indeed operator dependent. Experience with RFA determines complete ablation and post-operative morbidities, as demonstrated by Poon et al. [20]. On the other hand, Bale and colleagues demonstrated that experience did not influence treatment outcome of stereotactic radiofrequency ablation as the technology allows accurate multiple needle placements, however, with compromised complication rates of up to 17% [21,22].
Survival following Radiofrequency Ablation for Unresectable Colorectal Liver Metastases
Overall survival
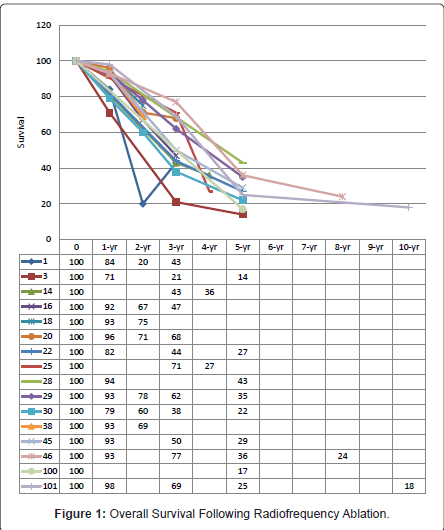
The heterogeneity in study designs with regards to inclusion criterion has rendered difficulty in interpretation of survival data within the current RFA literature. These data analysed a collective of tumour types, which differ in natural history. CLMs treated with RFA have poorer survival outcome compared to hepatocellular carcinomas and neuroendocrine metastases [10]. There is simply insufficient data on individual tumours. The best estimate of median, 1-, 2-, 3-, 4-, and 5-year survivals achieved with RFA in CLMs are 27-38 months [12,23,24], 71% - 94% [11,17,23-30], 60% - 78% [17,23-27,29], 21% - 72.7% [11,17,23-25,27,29,31-33], 27% - 36% [26,27], and 14% - 43% [11,12, 23,28,29,34,35], respectively (Figure 1).
Traditionally, unresectable hepatic disease was defined by multinodularity (>4 lesions), presence of extrahepatic disease, anatomical location of metastatic deposits, and presence of bilobar disease resulting in inadequate functional hepatic reserve post-resection. Recent advancements have led to the belief that these factors are merely of prognostic value. As early as 1996, incorporation of neoadjuvant systemic chemotherapy into treating initially unresectable CLMs has expanded curability with sequential surgical resection [36]. Most centres have, therefore, adopted an aggressive multimodal approach in treating CLMs; offering surgical resection alone or in combination with thermal ablation provided complete tumor extirpation can be achieved with sufficient functional post-operative hepatic parenchyma [9-11,15,17,20,23,25-27,31,35-44]. How RFA fits into the expanded unresectable criteria today is unclear. Several studies have also included patients who have refused surgical resection into their analysis along with those ineligible for resection.
Disease recurrence
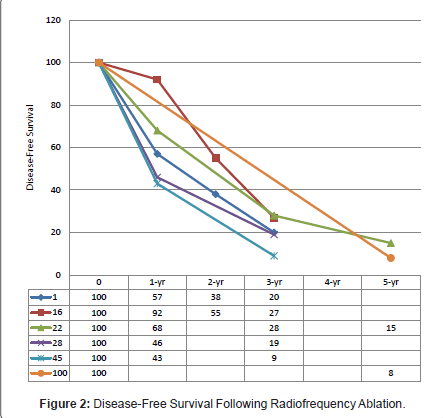
Disease recurrence following surgical interventions is a common phenomenon. Contrary to the belief, needle-track seeding following RFA is a sporadic event [23]. Data on recurrence within the available literature were variably reported, either by per patient or per lesion. The median, 1-, 2-, 3-, and 5-year disease-free survivals reported were 6 - 12 months [24,26,43], 43% - 92%, 38% - 55%, 8% - 34.1%, and 0% - 15% (Figure 2) [17,25,28,33,43,45]. The application of RFA influenced both overall and intrahepatic recurrence rates with most studies reporting poorer recurrence-free survival in CLMs treated with RFA alone [17,25,31]. Abdalla et al. observed an increased risk of recurrence in patients receiving RFA alone compared to RFA in combination with resection, and the occurrence of intrahepatic recurrence was 4 times more than resection alone [31]. Although statistically insignificant, Gleisner and colleagues demonstrated otherwise [33]. Treatment failure as depicted by local recurrence following any form of ablative therapy remains a concern. A wide range of local recurrence rates at RFA-treated sites had been reported, from as low as 2% up to 60% [9,11-15,17,19,23-29,32,35,37-39,42,43,45,46]. The majority of these were diagnosed within the first 12 months post-RFA, with virtually none observed after 18 months [24,26,43].
Prognostic Determinants
The following are significant prognostic determinants identified thus far. However, these factors are also related to the underlying biology of the tumor.
Tumour size
Local recurrence is largely predicted by the maximal diameter of ablated metastatic deposits [14,32,39]. Although there is a wide range of RF devices available, the established literature on RFA is largely on monopolar RF device; its use limited to small lesions (<3 cm) to ensure optimal local tumor control [14,32,39,45,47]. Several studies have included treatment of larger lesions (>3 cm) with multiple overlapping ablation demonstrating a high rate of local recurrence owing to the inaccuracy of real-time imaging with ultrasound [13,39,48,49]. This is due to the presence of microbubbles obscuring the actual tumour margin during the process of RFA by creating an enlarged hyperechogenic area on sonographic images [49]. Kennedy et al. reported their laparoscopic experience a 3.6% local recurrence in lesions <3 cm, in contrast to 19.6% in lesions >3 cm [45]. In a study by Veltri, ablation of lesions larger than 4 cm with RFA not only doubles the rate of treatment failure but also significantly reduces median survival (23.2 vs. 36.2 months) [23].
Bipolar RF device has expanded treatment to lesions of 5 cm in diameter though early studies on its efficacy have been contradictory [50,51]. In a more recent study by Baldwin et al. on a small cohort with mean lesion size of 3.6 ± 1.3 cm, only 1 of 22 patients developed recurrence at the site of ablation [52].
Tumour margin
The aim of RFA is to induce coagulative necrosis of the target lesion with a margin of normal hepatic parenchyma. Ablated margins of less than 1 cm was associated with a 1.6 fold increased risk of local recurrence [17]. Similarly, another study demonstrated a trend towards higher local recurrence rate of 22.2% in CLM patients with post-ablation margin of less than 0.4 cm [48]. Therefore, a circumferential rim of 0.5 to 1.0 cm of ablation is required for best tumour control [9,35].
Proximity to vascular structures
Treatment failure is also determined by the anatomical location of metastatic lesions. Unlike small vessels, larger vessels (4 - 5 mm) are resistant to thermal injury induced by RFA. A rim of viable tumour adjacent to the vessel wall often persists following RFA as a result of convective cooling effect exerted by blood flow. Most studies analysing the manipulation of blood flow have found no difference in local tumour control. The effects of vascular occlusion with Pringle’s manoeuvre in surgical RFA have been controversial. Percutaneous portal or hepatic vein balloon occlusion has also been employed. This, however, did not demonstrate any benefit with regards to local tumour progression. In addition, one patient amongst the 10% who developed asymptomatic complete venous thrombosis, experienced left lobe atrophy owing to permanent left portal branch thrombosis [9,17,25,49,53].
Tumor number
The number of tumours ablated was associated with survival outcome. The median survival in unresectable CLMs with more than 3 lesions had a significantly lower median survival (29.7 months) compared to those with fewer than 3 lesions (41.3 months) [15]. This however, was reflective only in patients treated with RFA alone. Patients who were treated in RFA in combination with surgical resection were unaffected [31]. In general, patients who were treated with RFA and resection had better prognosis relative to those receiving RFA alone [13,14,16,31,32,53]. Even when cases with solitary disease were taken into consideration, the outcome was worse overall [31]. Similar pattern of survival was described in a study of 30 patients where 10 (33.3%) underwent RFA due to significant comorbidities precluding safe hepatic resection -5- year survival of 72% (surgically resected) vs. 18% (RFA) (p=0.006) [32].
RFA-Related Morbidity and Mortality
A wide range of morbidity rates following RFA for unresectable CLMs have been reported, ranging from 1.7% to 27% [13,15,22-24,26,30,37,47]. Morbidity following RFA is inversely proportional to the experience of the proceduralist [4]. Most studies have classified their morbidities into major and minor complications based on the Therapy-Oriented Severity Grading Systems (TOSGS) classification.
Of the major complications, abscess formation by far, has the highest prevalence; comprising of 7.5% within 308 tumours ablated in one study [14]. Major complications as a result of thermal injury include biliary stricture and leaks, and viscous perforation. Biliary injury frequently occurs in patients with bilioenteric anastomoses and biliary stents. The consequential severe morbidity following these thermal-induced complications has led to lesions within 1 cm range from the hepatic hilum, gallbladder and gastrointestinal structures a contraindication to RFA [15]. Cases of abdominal wall burn requiring surgical debridement have been reported. Other major complications related to RFA include haemorrhage and multiorgan failure [38]. Poon et al. described one multiorgan failure syndrome post-RFA, and similar to the cryoshock phenomenon was notioned to be related to systemic cytokine response [20]. Navarra et al. reported one case of perioperative multiorgan failure and one DIC. However, no further details were provided regarding these cases [15]. Further studies may be required to validate these possible incidents.
Minor complications happen more frequently after RFA. These include asymptomatic pulmonary complications (pneumothorax, pleural effusion), uncomplicated intra- and perihepatic haematomas, self-limiting hepatic failure, post-ablation syndrome, grounding pad burns, brachial plexopathy, and myoglobinuria [9,11,13,15,20,24,25,27,37, 47,54,55].
Mortality rates of RFA have been nil in many studies and very low in all - 0% to 3.4% [13,14,16,23,25,26,30,35,37-39,41,45,47,48,54,56]. The clinical safety and efficacy of RFA in oncological outcome along with its popularity has encouraged the ongoing exploration of its role in treatment of CLMs as an alternative to hepatic resection.
Cryotherapy
Introduction
Destroying tumours by freezing has a long history but it really only became practical in the liver using imaging-guided placements of vacuum-insulated coaxial probes. There are 2 types of available systems for clinical purpose - using liquid nitrogen (Erbe Tubingen Germany; Spembly, Andover, UK; Cryotech, Ripley, UK; Cryomedical Science Inc, MD, USA; Candela, Eindhoven, The Netherlands) [56-65] and argon gas (Endocare, CA USA) [62,66,67]. Some have reusable probes which very significantly reduces costs.
Method
The vast majority of literature on hepatic cryotherapy is from open surgery. It has also been used laparoscopically and percutaneously but RFA has the distinct advantage in the latter technique because of the smaller diameter probe. We routinely use spinal needle trial placement at surgery under intraoperative ultrasound control before finally introducing the probe or multiple probes. The use of ultrasound in monitoring the progress of cryoablation is easy owing to the distinctive ice ball appearance - hyperechoic rim with posterior acoustic shadowing. We essentially aim to achieve a 1 cm circumferential margin, confirmed with multiposition imaging, to ensure adequate coverage of tumour ablation. Incomplete ablation is no better than supportive treatment [68]. We do not monitor temperature of ablation site - temperature at the centre of the ice ball where the probe is located is of no real interests. Studies have demonstrated significant temperature discrepancies between the ablated centre and edge. For simplicity, we use the sonographic margin appearance as a surrogate.
After freezing, most systems have an active rewarming feature to remove the probe more rapidly and haemostatic material is packed into the probe track. Multiple probes can be used at the same time and, unlike RFA, will meld together rather than leaving small untreated areas which is a common predicament with multiple/overlapping application of RFA. A twin freeze thaw cycle is undoubtedly more effective but also has safety concerns [65] hence; we recommend just refreezing the edge (least well treated area) by allowing 1 cm to thaw then refreeze.
Perioperative risks
Apart from the risks of any surgery there are a few specific ones related to cryotherapy. Cryoshock is a rare but significant phenomenon related to cytokine release, presenting clinically as multiorgan failure syndrome and disseminated intravascular coagulation. Early elevated liver function (AST/ALT) and thrombocytopaenia warn of this syndrome. In practice, however, it is simply not a problem unless a very large volume (perhaps a third of liver), is frozen [66]. Unfortunately, treatment merely consists of supportive therapy. Other more treatable cryotherapy-related complications include hepatic abscess formation, haemorrhage, biliary injury and pleural effusion. Hepatic abscess is common (0.9% - 11.8%) but usually seen when combined colonic resection and liver cryotherapy are performed synchronously [57,61,64-66,69-72]. Bleeding from liver cracks can easily be controlled by pressure or suture [58,66,69]. The susceptibility of biliary tree to thermal injury may complicate cryo-treatment with biloma or bile fistula [57,58,63,64,66,69,71,72]. Pleural effusion is common, as it is after other liver surgeries, and may very often be treated conservatively [63,69-71,73].
Survival
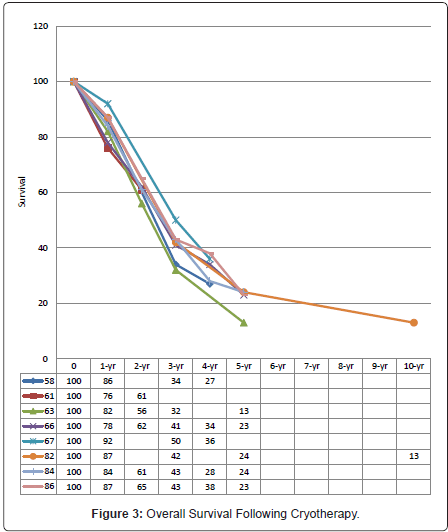
As for radiofrequency ablation, cryotherapy is either used alone or in combination with hepatic resection. Comparative studies on cryotherapy and surgical resection had demonstrated long-term survival in patients with CLMs with acceptable mortality rates (0% - 7%) [57-88]. Seifert et al. achieved 5-year survival rates of 26% in the hepatic cryotherapy group and 23% in the resection group; accompanied by mortality rates of 2% and 5%, respectively [59]. Similar survival results can be achieved when using cryotherapy as an adjunct to resection to treat non-resectable CLMs, with 5-year overall survival of 32% following resection alone, and 24% following cryotherapy and resection (p = 0.206) [80]. Application of hepatic cryotherapy has undoubtedly extended treatment to otherwise incurable CLMs. The curative potential with cryotherapy in CLMs had been further demonstrated by the 10-year survival of 13% [82]. The available literature on treatment of CLMs with hepatic cryotherapy reports median, 1-, 2-, 3-, 4-, 5-, and 10-year overall survivals of 18 - 33 months [56,57,59-61,63,64,66,70-73,77,78,80-83,85], 78% - 92% [56,58,61,63,66,70-72,80,82,83,86], 47% - 66% [61,63,66,70-72,80,83,86,87], 29% - 50% [56,58,60,63,66,72,80,82,86-88], 21% - 38% [58,66,67,72,80,86], 13% - 23% [56,60,63,66,72,74,80,82,84,87], and 13% [82], respectively (Figure 3).
Recurrence
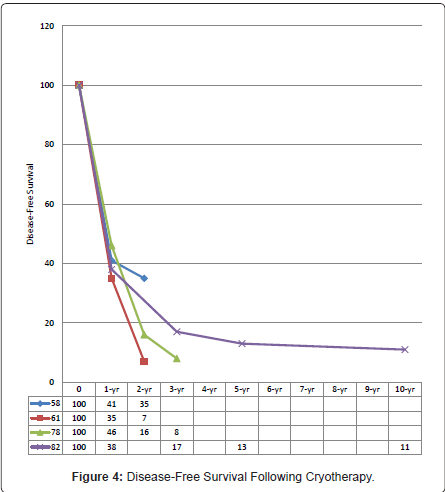
Margin clearance following surgical resection is essential for both recurrence-free and long-term survivals. In some centres, edge cryotherapy had been employed to suboptimal resection margins obtaining median and 5-year survivals of 39 months and 36% [69,82,88,89], respectively; resembling that of clear-margin resections [82,84,88-90]. The median, 1-, 2-, 3-, 5-, and 10-year disease-free survivals reported were 9 – 27 months [59,72,77,78,81,82,84,86,88], 38% - 41% [58,78,82,86], 16% - 35% [58,78,86], 8% - 17% [78,82,86], 12% - 13% [59,82], and 11% [82], respectively (Figure 4).
Approximately 80% of surgically treated CLMs will develop some form of recurrence; and the pattern of recurrence had been variably described [61,64,70,71,78,80,82,86]. Metastatic pulmonary disease was the most common initial site of extrahepatic recurrence observed. Whilst the extent and site of extrahepatic recurrence were similar following both hepatic cryotherapy and resection, this was not the case with intrahepatic recurrences. CLM patients treated with cryotherapy were noted to have higher rates of intrahepatic recurrences, partly due to disease recurrence at cryoablated sites [59]; the appearance of which may be detected as late as 39 months post-cryotherapy, and rarely up to 98 months [69,86]. Cryosite recurrence frequently occurs - rates of up to 44% have been reported by two different institutions [74,86]. The risk factors for local recurrence are similar to RFA’s - proximity to major vasculature related to the heat-sink effect, and size, as previously described [58,61,63,74,86].
Prognostic factors
Tumour number and size: More number of lesions is predictive of poorer disease-free survival following hepatic cryotherapy [63,71,72,78,80,86,87,89,91]. Nonetheless, the efficacy of this ablative modality is undeniable. The survival data on cryotherapy merely revolves around CLM patients with advanced disease, which is untreatable with the conventional surgical resection. Although the present literature on hepatic resection for CLM has improved over the years, with 5-year and 10-year survival of 58% and 22%, respectively; this represents only a selected group of patients. Several studies have compared surgical resection to cryotherapy (hepatic or edge) with or without resection in the presence of similar patient and tumour characteristics in between groups; and found no significant difference in survival rates obtained [58,91]. Furthermore, the prognostic value of tumour number becomes obsolete with the incorporation of perioperative chemotherapy, whether systemic of locoregional [67,87]. With the availability of more effective chemotherapy agents today, survival is expected to improve though more studies would be required to validate this hypothesis following hepatic cryotherapy.
While number is not a limitation to surgical intervention provided there is sufficient post-operative functional hepatic parenchyma, survival is inversely proportional to size [60,61,63,66,80,86,89]. All except one study have described better or equivalent survival outcome in patients undergoing synchronous hepatic resection and cryotherapy compared to cryotherapy alone [71,72,80]. Seifert and colleagues, who demonstrated otherwise, do not apply cryotherapy to lesions larger than 5 cm [59].
Serum CEA-level: The correlation of serum CEA-level with tumour burden has long been established. Nonetheless, no consistent cut-off value prognostic of outcomes has been identified, with preoperative serum CEA-levels above 5 ng/mL [72,80,86,87], 100 ng/mL [66,78] and 200 ng/mL [82] reflecting on worse survival. Decrement in CEA-level following cryotherapy may be observed over a period of 6 weeks to 3 months but when detected, indicates improved intrahepatic, local and overall recurrence-free survival [86,89-92].
Comparison of Ablative Modalities
The positive clinical and oncological outcome along with its “technical simplicity” has led to further investigation of RFA as a treatment alternative to surgical resection. Further studies are still required to validate the use of RFA as first-line treatment in colorectal hepatic metastases as most data within the literature express concerns regarding the significant risk of recurrence, both local tumour progression and new hepatic metastases. Even with the attempt to nullify the potential effects of tumour biology by comparing only solitary metastases, treatment efficacy of RFA with surgical resection have been variably described [67,88,93].
Of the available thermal ablative methods, RFA is widely favoured over cryotherapy, mainly due to its minimally invasive percutaneous approach. Relative to RFA, cryotherapy is associated with higher morbidities and longer hospital stays, as the open approach had been required for application of cryotherapy until recent years. These have been consistently demonstrated in the few comparative studies [59,73,94,95]. Also, there was a non-statistically significant trend towards poorer survival post-cryotherapy compared to RFA [94,95]. Nevertheless, Bilchik et al. [14] showed that the treatment efficacy of cryotherapy is superior to RFA, limiting treatment of RFA only to lesions smaller than 3 cm [59].
More recently, there has been an increase in the clinical application of microwave ablation (MWA) in treatment of hepatic malignancies. Its administration, like RFA, can be performed via the percutaneous, laparoscopic or open/laparotomic approach. Early studies comparing RFA and MWA in HCCs demonstrated comparable outcomes relating to local tumor control, long-term survival and treatment-related complications [96]. Unlike RFA, microwave energy is capable of propagating through tissues without a conductive medium resulting in relatively higher intratumoural temperatures allowing larger zone of ablations over a short treatment interval. While MWA has been designed to achieve larger area of necrosis, treatment of larger lesions is associated with a higher rate of complications [97]. Overall, the occurrence of complications post-MWA is similar to that of RFA [97-101].
Conclusion
Although surgical resection remains the goal of modern cancer therapy that achieves a cure, ablation provides a non-surgical but curative option. It may be used as an alternate local therapy for patients who are considered non-surgical candidates and may be used in combination with resection as a local option to expand the boundaries of resection for patients with multiple metastases. The efficacy of both radiofrequency ablation and cryotherapy are largely user dependent and its availability varies with each centre depending on local expertise.
There is now a substantial body of data to demonstrate its safety and efficacy and future directions should be aimed at improving the usability of these technologies, reduce its invasiveness to improve recovery times and continued investigations in combination with other multi-modality options for which will be the only way forward to improve the outcomes of patients with metastatic disease.
References
-
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230: 309-318.
- Buscarini L, Rossi S, Fornari F, Di Stasi M, Buscarini E (1995) Laparoscopic ablation of liver adenoma by radiofrequency electrocauthery. Gastrointest Endosc 41: 68-70.
- Cosman ER, Nashold BS, Ovelman-Levitt J (1984) Theoretical aspects of radiofrequency lesions in the dorsal root entry zone. Neurosurgery 15: 945-950.
- Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, et al. (1996) Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol 167: 759-768.
- Elias D, Debaere T, Muttillo I, Cavalcanti A, Coyle C, et al. (1998) Intraoperative use of radiofrequency treatment allows an increase in the rate of curative liver resection. J Surg Oncol 67: 190-191.
- Shiina S, Teratani T, Obi S, Sato S, Tateishi R, et al. (2005) A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 129: 122-130.
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, et al. (2006) A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 243: 321-328.
- Tait IS, Yong SM, Cuschieri SA (2002) Laparoscopic in situ ablation of liver cancer with cryotherapy and radiofrequency ablation. Br J Surg 89: 1613-1619.
- Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, et al. (2003) Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol 10: 52-58.
- Amersi FF, McElrath-Garza A, Ahmad A, Zogakis T, Allegra DP, et al. (2006) Long-term survival after radiofrequency ablation of complex unresectable liver tumors. Arch Surg 141: 581-587.
- Gillams AR, Lees WR (2004) Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur Radiol 14: 2261-2267.
- Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC (2009) Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg 13: 486-491.
- Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, et al. (2000) Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 7: 593-600.
- Bilchik AJ, Wood TF, Allegra D, Tsioulias GJ, Chung M, et al. (2000) Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg 135: 657-662.
- Navarra G, Ayav A, Weber JC, Jensen SL, Smadga C, et al. (2005) Short- and-long term results of intraoperative radiofrequency ablation of liver metastases. Int J Colorectal Dis 20: 521-528.
- Elias D, Sideris L, Pocard M, de Baere T, Dromain C, et al. (2005) Incidence of unsuspected and treatable metastatic disease associated with operable colorectal liver metastases discovered only at laparotomy (and not treated when performing percutaneous radiofrequency ablation). Ann Surg Oncol. 12: 298-302.
- Elias D, Baton O, Sideris L, Boige V, Malka D, et al. (2005) Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol 90: 36-42.
- Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, et al. (2005) Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 242: 158-171.
- Berber E, Siperstein A (2008) Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol 15: 2757-2764.
- Poon RT, Ng KK, Lam CM, Ai V, Yuen J, et al. (2004) Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 239: 441-449.
- Widmann G, Schullian P, Haidu M, Bale R (2012) Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol 35: 570-580.
- Bale R, Widmann G, Schullian P, Haidu M, Pall G, et al. (2012) Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol 22: 930-937.
- Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, et al. (2008) Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol 31: 948-956.
- Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, et al. (2001) Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 221: 159-166.
- Fioole B, Jansen MC, van Duijnhoven FH, van Hillegersberg R, van Gulik TM, et al. (2006) Combining partial liver resection and local ablation of liver tumours: a preliminary Dutch experience. World J Surg Oncol 4: 46.
- Joosten J, Jager G, Oyen W, Wobbes T, Ruers T (2005) Cryosurgery and radiofrequency ablation for unresectable colorectal liver metastases. Eur J Surg Oncol 31: 1152-1159.
- Jakobs TF, Hoffmann RT, Trumm C, Reiser MF, Helmberger TK (2006) Radiofrequency ablation of colorectal liver metastases: mid-term results in 68 patients. Anticancer Res 26: 671-680.
- Evrard S, Rivoire M, Arnaud J, Lermite E, Bellera C, et al. (2012) Unresectable colorectal cancer liver metastases treated by intraoperative radiofrequency ablation with or without resection. Br J Surg 99: 558-565.
- Veltri A, Guarnieri T, Gazzera C, Busso M, Solitro F, et al. (2012) Long-term outcome of radiofrequency thermal ablation (RFA) of liver metastases from colorectal cancer (CRC): size as the leading prognostic factor for survival. Radiol Med 117: 1139-1151.
- Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I (2003) Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 90: 1240-1243.
- Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, et al. (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239: 818-825.
- Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, et al. (2006) Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 141: 460-466.
- Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, et al. (2008) Colorectal liver metastases. Recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 143: 1204-1212.
- Siperstein AE, Berber E, Ballem N, Parikh RT (2007) Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 246: 559-565.
- Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, et al. (1999) Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 230: 1-8.
- Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, et al. (1996) Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 224: 509-520.
- Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, et al. (1999) Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 178: 592-599.
- Bowles BJ, Machi J, Limm WM, Severino R, Oishi AJ, et al. (2001) Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg 136: 864-869.
- Oshowo A, Gillams AR, Lees WR, Taylor I (2003) Radiofrequency ablation extends the scope of surgery in colorectal liver metastases. Eur J Surg Oncol 29: 244-247.
- Berber E, Pelley R, Siperstein AE (2005) Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol 23: 1358-1364.
- Topal B, Hompes D, Aerts R, Fieuws S, Thijs M, et al. (2007) Morbidity and mortality of laparoscopic vs. open radiofrequency ablation for hepatic malignancies. Eur J Surg Oncol 33: 603-607.
- Park IJ, Kim HC, Yu CS, Kim PN, Won HJ, et al. (2008) Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol 15: 227-232.
- McKay A, Fradette K, Lipschitz J (2009) Long-term outcomes following hepatic resection and radiofrequency ablation of colorectal liver metastases. HPB Surg 2009: 346863.
- Pawlik TM, Abdalla EK, Ellis LM, Vauthey JN, Curley SA (2006) Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg 10: 240-248.
- Kennedy TJ, Cassera MA, Khajanchee YS, Diwan TS, Hammill CW, et al. (2013) Laparoscopic radiofrequency ablation for the management of colorectal liver metastases: 10-year experience. J Surg Oncol 107: 324-328.
- Van Tilborg AA, Meijerink MR, Sietses C, Van Waesberghe JH, Mackintosh MO, et al. (2011) Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol 84: 556-565.
- Gillams AR, Lees WR (2009) Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 19: 1206-1213.
- Liu CH, Arellano RS, Uppot RN, Samir AE, Gervais DA, et al. (2010) Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol 20: 877-885.
- Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK (2000) Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 88: 2452-2463.
- Ritz JP, Lehmann KS, Reissfelder C, Albrecht T, Frericks B, et al. (2006) Bipolar radiofrequency ablation of liver metastases during laparotomy. First clinical experiences with a new multipolar ablation concept. Int J Colorectal Dis 21: 25-32.
- Meijerink MR, van den Tol P, van Tilborg AA, van Waesberghe JH, Meijer S, et al. (2011) Radiofrequency ablation of large size liver tumours using novel plan-parallel expandable bipolar electrodes: initial clinical experience. Eur J Radiol 77: 167-171.
- Baldwin K, Katz SC, Rubin A, Somasundar P (2012) Bipolar radiofrequency ablation of liver tumors: technical experience and interval follow-up in 22 patients with 33 ablations. J Surg Oncol 106: 905-910.
- de Baere T, Deschamps F, Briggs P, Dromain C, Boige V, et al. (2008) Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology 248: 1056-1066.
- Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA (2003) Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 10: 1059-1069.
- Eisele RM, Neumann U, Neuhaus P, Schumacher G (2009) Open surgical is superior to percutaneous access for radiofrequency ablation of hepatic metastases. World J Surg 33: 804-811.
- Brooks AJ, Wang F, Alfredson M, Yan TD, Morris DL (2005) Synchronous liver resection and cryotherapy for colorectal metastases: survival analysis. Surgeon 3: 265-268.
- Paganini AM, Rotundo A, Barchetti L, Lezoche E (2007) Cryosurgical ablation of hepatic colorectal metastases. Surg Oncol 16: S137-140.
- Bageacu S, Kaczmarek D, Lacroix M, Dubois J, Forest J, et al. (2007) Cryosurgery for resectable and unresectable hepatic metastases from colorectal cancer. Eur J Surg Oncol 33: 590-596.
- Seifert JK, Springer A, Baier P, Junginger T (2005) Liver resection or cryotherapy for colorectal liver metastases: a prospective case control study. Int J Colorectal Dis 20: 507-520.
- Seifert JK, Junginger T (2004) Prognostic factors for cryotherapy of colorectal liver metastases. Eur J Surg Oncol 30: 34-40.
- Ruers TJ, Joosten J, Jager GJ, Wobbes T (2001) Long-term results of treating hepatic colorectal metastases with cryosurgery. Br J Surg 88: 844-849.
- Cha C, Lee FT Jr, Rikkers LF, Niederhuber JE, Nguyen BT, et al. (2001) Rationale for the combination of cryoablation with surgical resection of hepatic tumors. J Gastrointest Surg 5: 206-213.
- Seifert JK, Morris DL (1998) Prognostic factors after cryotherapy for hepatic metastases from colorectal cancer. Ann Surg 228: 201-208.
- Weaver ML, Ashton JG, Zemel R (1998) Treatment of colorectal liver metastases by cryotherapy. Semin Surg Oncol 14: 163-170.
- Dilley AV, Dy DY, Warlters A, Copeland S, Gillies AE, et al. (1993) Laboratory and animal model evaluation of the Cryotech LCS 2000 in hepatic cryotherapy. Cryobiology 30: 74-85.
- Xu KC, Niu LZ, He WB, Hu YZ, Zuo JS (2008) Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol 14: 1430-1436.
- Rivoire M, De Cian F, Meeus P, Négrier S, Sebban H, et al. (2002) Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer 95: 2283-2292.
- Shafir M, Shapiro R, Sung M, Warner R, Sicular A, et al. (1996) Cryoablation of unresectable malignant liver tumors. Am J Surg 171: 27-31.
- Gruenberger T, Jourdan JL, Zhao J, King J, Morris DL (2001) Reduction in recurrence risk for involved or inadequate margins with edge cryotherapy after liver resection for colorectal metastases. Arch Surg 136: 1154-1157.
- Seifert JK, Achenbach T, Heintz A, Böttger TC, Junginger T (2000) Cryotherapy for liver metastases. Int J Colorectal Dis 15: 161-166.
- Wallace JR, Christians KK, Pitt HA, Quebbeman EJ (1999) Cryotherapy extends the indications for treatment of colorectal liver metastases. Surgery 126: 766-772.
- Yan TD, Padang R, Morris DL (2006) Longterm results and prognostic indicators after cryotherapy and hepatic arterial chemotherapy with or without resection for colorectal liver metastases in 224 patients: longterm survival can be achieved in patients with multiple bilateral liver metastases. J Am Coll Surg 202: 100-111.
- Stubbs RS, Alwan MH, Booth MW (1998) Hepatic cryotherapy and subsequent hepatic arterial chemotherapy for colorectal metastases to the liver. HPB Surg 11: 97-104.
- Mala T, Edwin B, Mathisen Ø, Tillung T, Fosse E, et al. (2004) Cryoablation of colorectal liver metastases: minimally invasive tumour control. Scand J Gastroenterol 39: 571-578.
- Crews KA, Kuhn JA, McCarty TM, Fisher TL, Goldstein RM, et al. (1997) Cryosurgical ablation of hepatic tumors. Am J Surg 174: 614-617.
- Johnson LB, Krebs TL, Van Echo D, Plotkin JS, Njoku M, et al. (1997) Cytoablative therapy with combined resection and cryosurgery for limited bilobar hepatic colorectal metastases. Am J Surg 174: 610-613.
- Weaver ML, Atkinson D, Zemel R (1995) Hepatic cryosurgery in treating colorectal metastases. Cancer 76: 210-214.
- Seifert JK, Morris DL (1999) Indicators of recurrence following cryotherapy for hepatic metastases from colorectal cancer. Br J Surg 86: 234-240.
- Seifert JK, Morris DL (1999) Repeat hepatic cryotherapy for recurrent metastases from colorectal cancer. Surgery 125: 233-235.
- Niu R, Yan TD, Zhu JC, Black D, Chu F, et al. (2007) Recurrence and survival outcomes after hepatic resection with or without cryotherapy for liver metastases from colorectal carcinoma. Ann Surg Oncol 14: 2078-2087.
- Chung MH, Ye W, Ramming KP, Bilchik AJ (2001) Repeat hepatic cryotherapy for metastatic colorectal cancer. J Gastrointest Surg 5: 287-293.
- Ng KM, Chua TC, Saxena A, Zhao J, Chu F, et al. (2012) Two decades of experience with hepatic cryotherapy for advanced colorectal metastases. Ann Surg Oncol 19: 1276-1283.
- Hewitt PM, Dwerryhouse SJ, Zhao J, Morris DL (1998) Multiple bilobar liver metastases: cryotherapy for residual lesions after liver resection. J Surg Oncol 67: 112-116.
- Hou RM, Chu F, Zhao J, Morris DL (2007) The effects of surgical margin and edge cryotherapy after liver resection for colorectal cancer metastases. HPB (Oxford) 9: 201-207.
- Gruenberger T, Jourdan JL, Zhao J, King J, Morris DL (2000) Echogenicity of liver metastases is an independent prognostic factor after potentially curative treatment. Arch Surg 135: 1285-1290.
- Yan TD, Nunn DR, Morris DL (2006) Recurrence after complete cryoablation of colorectal liver metastases: analysis of prognostic features. Am Surg 72: 382-390.
- Yan DB, Clingan P, Morris DL (2003) Hepatic cryotherapy and regional chemotherapy with or without resection for liver metastases from colorectal carcinoma: how many are too many? Cancer 98: 320-330.
- Dwerryhouse SJ, Seifert JK, McCall JL, Iqbal J, Ross WB, et al. (1998) Hepatic resection with cryotherapy to involved or inadequate resection margin (edge freeze) for metastases from colorectal cancer. Br J Surg 85: 185-187.
- Seifert JK, Junginger T, Morris DL (1998) A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb 43: 141-154.
- Yan TD, Padang R, Xia H, Zhao J, Li J, et al. (2006) Management of involved or close resection margins in 120 patients with colorectal liver metastases: edge cryotherapy can achieve long-term survival. Am J Surg 191: 735-742.
- Finlay IG, Seifert JK, Stewart GJ, Morris DL (2000) Resection with cryotherapy of colorectal hepatic metastases has the same survival as hepatic resection alone. Eur J Surg Oncol 26: 199-202.
- Hocking RA, Morris DL (1998) Patterns of serum CEA fall after hepatic arterial chemotherapy as sole therapy and combined with cryotherapy for colorectal metastases. Aust N Z J Surg 68: 722-724.
- Hur H, Ko YT, Min BS, Kim KS, Choi JS, et al. (2009) Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 197: 728-736.
- Stella M, Mithieux F, Meeus P, Kaemmerlen P, Lafon C, et al. (2006) Transpleurodiaphragmatic cryosurgical ablation for recurrent unresectable colorectal liver metastases. J Surg Oncol 93: 268-272.
- Tait IS, Yong SM, Cuschieri SA (2002) Laparoscopic in situ ablation of liver cancer with cryotherapy and radiofrequency ablation. Br J Surg 89: 1613-1619.
- Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, et al. (2005) Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 40: 1054-1060.
- Livraghi T, Meloni F, Solbiati L, Zanus G; Collaborative Italian Group using AMICA system (2012) Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 35: 868-874.
- Liang P, Wang Y, Yu X, Dong B (2009) Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology 251: 933-940.
- Martin RC, Scoggins CR, McMasters KM (2010) Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 17: 171-178.
- Agcaoglu O, Aliyev S, Karabulut K, El-Gazzaz G, Aucejo F, et al. (2013) Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: an analysis of 395 patients. World J Surg 37: 1333-1339.
- Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, et al. (2012) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265: 958-968.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15220
- [From(publication date):
June-2013 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10539
- PDF downloads : 4681