Regression Model for Predicting Adult Female Aedes aegypti Based on Meteorological Variables: A Case Study of Jeddah, Saudi Arabia
Received: 26-Aug-2013 / Accepted Date: 16-Oct-2013 / Published Date: 22-Oct-2013 DOI: 10.4172/2157-7617.1000168
Abstract
Considerable interest exists in confirming that meteorological variables may play determinant roles in dengue vector abundance. The principle vector for dengue is Aedes aegypti. Dengue Fever has been considered the most important vector-borne viral disease in Jeddah, Saudi Arabia, and is susceptible to climate variability. The aim of this study is to describe the association between adult female Aedes aegypti mosquitoes and meteorological variables and to develop models for predicting the mosquito abundance using Pearson’s correlation and regression analyses. Our results show that mosquitoes have the highest correlation with temperature at lag 0 time and relative humidity at lag 5 weeks. The highest two correlations were found between the mosquitoes and minimum temperature (r=-0.57) and maximum relative humidity (r=0.46). Two models were created based on the regression analysis results. The first model shows that 86% of mosquito values were within the upper and lower limits of agreement. The second model shows that 94% of the values were within the limits of agreement. The study findings could contribute to the forecasting of mosquito abundance peaks and subsequently guide a plan for mosquito control operations ahead of time that would help to minimize the outbreak of dengue occurrence and prevent the spread of dengue infections.
Keywords: Aedes aegypti; Dengue fever; Meteorological variables; Regression; Temperature; Relative humidity
5535Introduction
Much attention has been paid by the media and scientific communities to the association between human health and climate [1,2]. Many researchers have investigated the impact of climate variability on the abundance of disease vectors, and many studies suggest that the geographic ranges of mosquito-borne diseases, such as dengue fever, may be expanding due to climatic change and impact [3,4].
Dengue fever has been considered by the World Health Organization to be one of the most important mosquito-borne diseases. It potentially affects 2.5 billion people, and the current estimated data suggest that 50 to 100 million dengue cases occur yearly in tropical and subtropical regions throughout the world [5]. Aedes aegypti is the main vector of dengue in many countries around the world [2]. It is a peridomestic, lives around human dwellings, is a day-biting mosquito, feeds preferentially on the blood of humans, is holometabolous, and undergoes complete metamorphosis through the egg, larval, and adult stages. Its development stages are usually influenced by climatic or meteorological variables such as temperature and relative humidity [6].
The effect of weather on insects in general and mosquitoes in particular has been investigated in many studies that have found that most insects respond to changes in meteorological conditions [2]. High rainfall was found to be a good indicator for increasing survival of the pupa-adult mosquito population and decreasing survival of larva [7]. Many studies have also investigated a role of weather in mosquito dynamics in terms of the spread of mosquito-borne diseases. The changes in the correlation between temperature, rainfall, and several arboviruses and their vectors were described in Rhode Island by Takeda [8]. The prevalence of the Ross River virus has been linked to sustained winter and spring rainfall, as these conditions allow for an initial amplification of the virus in the abundant floodwaters via Aedes mosquitoes and an ongoing risk from the Culex mosquitoes in ground pools, which lead in turn to an epidemic [9].
Public health officials would benefit if they could predict the weekly adult female Aedes aegypti population in advance based on meteorological variables to control mosquitoes and mosquito-borne diseases such as dengue fever. Aedes aegypti is currently the only dengue vector in Jeddah, Saudi Arabia [10,11] and is responsible for almost all dengue transmission. The region has experienced significant epidemic dengue activity since 2006, with more than 9,000 notified cases [11].
Determination of the amount of lag time between meteorological factors and Aedes mosquitoes abundance is necessary to develop prediction models. There has not been critical examination of the sources of variation in the association and lag structure (the magnitude of association between meteorological factors and adult Aedes mosquito at a later time) between weather and density of mosquitoes. The inconsistency of findings from different studies may be due in part to the interaction of meteorological factors affecting mosquito abundance and survival, and parasite maturation, key determinants of density and a disease transmission. Deposition of mosquito eggs, and their maturation into larvae and then into adults, requires aquatic breeding sites, time and is, therefore, dependent on meteorological factors and time [10-12].
An understanding of dengue risk based on mosquito abundance and meteorological variables can be developed, applied sustainably, and measured prospectively. The development of this understanding is a priority for research of mosquito-borne diseases in general and dengue fever specifically [12]. If dengue risk factors could be included in a predictive model, the health authorities would then have quantitative measures that could be used to instigate advance mosquito control operations. This is an approach rarely used in dengue control [12].
In the present study, we examine the utility of adult mosquito index to determine how best to measure and predict mosquito risk. This can increase our ability to predict index ahead of time and would be useful for health authorities when planning control responses. To achieve this ability of prediction, we first clarify the relationship between adult female Aedes aegypti mosquito and meteorological variables.
The importance of such approach is explained in different studies. Wallis [13] found that mosquitoes are critically dependent on the climate for their survival and development. Also, climate circumscribes the distribution of mosquito-borne diseases such as dengue fever, while weather impacts the timing and intensity of outbreaks. Higher transmission of dengue generally occurs if temperature ranges from 14°C to 18°C at the lower end and 35°C to 40°C at the upper end [14].
Many studies [1,14-16] have found that development increases in warmer temperatures, raising the odds of disease transmission, while the reproduction rates and replication of disease are slower in cooler temperatures. High amounts of precipitation lead to increases in the number of breeding sites. As a factor in the life cycle of mosquitoes and in disease replication and transmission, humidity is often overlooked. Relative humidity is increased by rainfall, particularly following drought. Relative humidity strongly impacts the flight and the subsequent host-seeking behavior of mosquitoes [17,18].
This association between the meteorological variables and mosquitoes highlights the need to further investigate this relationship at a local ecological scale. Therefore, in this work, we characterize the link between weekly meteorological variables and weekly trapped mosquitoes. We develop a predictive tool for dengue risk factor to specifically determine the major meteorological influences that operate on a weekly basis and characterize the more proximal influences on mosquito collection.
Methods
The study area
Jeddah, Saudi Arabia (21°32’36.60’’N, 39°10’07.88’’E) has approximately 3.5 million residents (Figure 1). The climate is subtropical, with an annual average temperature of 29°C (84°F). The high is around 41°C (106°F) in June, and the low is 16°C (61°F) in January and February. The average rainfall in the study area is 60 mm. September and October are the driest months, while December and January are the wettest. Jeddah is the main sea port and travel hub for millions of Muslims who visit the two holy mosques in Makkah and Al Medinah.
Female Aedes aegypti data
Adult female Aedes aegypti daily counts (from 2007 to 2010) were provided by the mosquito laboratory of the Jeddah Municipality, which has systematically operated around 504 black hole UV traps since 2006. These traps are effective tools for attracting and collecting adult mosquitoes [10,19]. The black hole traps were distributed geographically based on population density and environmental factors (Figure 1). Mosquito laboratory staff are responsible for daily filtering and sorting of mosquitoes according to species, sex, date of collection, location coordinates, and number of mosquitoes for each trap using a dissecting microscope. Since the female Aedes aegypti mosquitoes is the main vector of transmitting dengue fever in Jeddah, this research and analysis are based on average weekly female specimens only.
Meteorological data
The Presidency of Meteorology and Environment (PME) provided the meteorological data that were used in this study. We have retrieved daily maximum temperature, daily minimum temperature, daily maximum relative humidity, and daily minimum relative humidity from the PME. We used the daily observations of these variables to calculate average weekly minimum and maximum temperatures and the average weekly minimum and maximum relative humidity for inclusion in regression and descriptive analyses. Because the rainfall usually occurs during a small number of weeks in November, December, or January, the data for rainfall were not used in this study. This study is instead dependent on temperature and relative humidity data.
Data analysis
Pearson’s correlation analysis was used to verify the associations between adult female Aedes aegypti and the meteorological variables. We used Pearson’s correlation because it is based on the assumption that both X and Y as variables follow a normal distribution; therefore, it reflects only the degree of linear relationship between two variables, which helped us to determine the highest correlations between the study variables in order to use them in the multiple regression analysis.
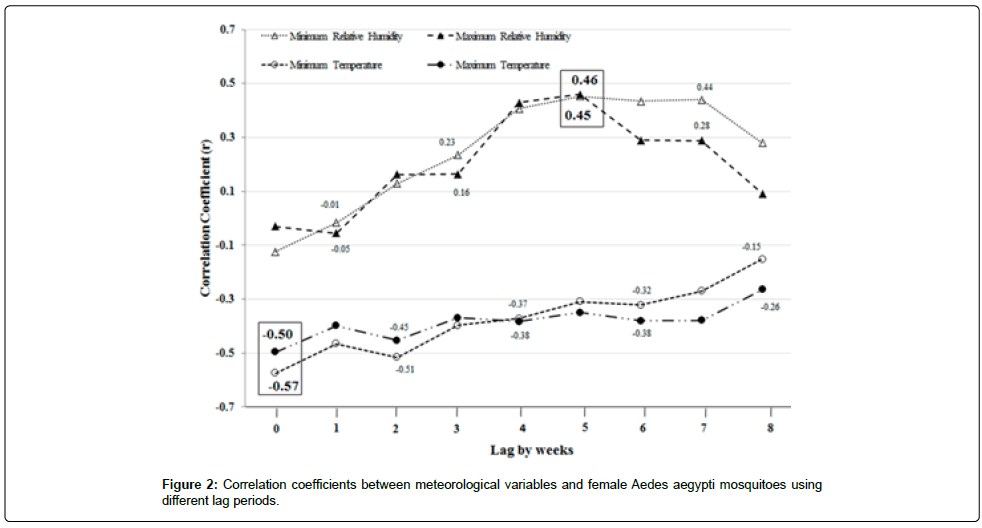
Excel 2007 was used to perform the correlation analysis and multiple regression analysis. Before we undertook the correlation analysis, we calculated the weekly average number of adult Aedes aegypti mosquitoes based on the weekly sum of mosquitoes of each trap, weekly average minimum and maximum temperatures, and weekly average minimum and maximum relative humidity from 2007 to 2010. We then calculated the correlations between average weekly mosquitoes and the weekly values of the selected meteorological variables at different weekly lags (0, 1, 2, 3, 4, 5, 6, 7 and 8) to avoid multicollinearity before running the multiple regression analysis (Figure 2). Variables at lag time that did not seem logically essential to our model were removed to reduce or eliminate multicollinearity. Also, to reduce the impact of collinearity, we increased the sample size in the analysis and used 4-year daily data to get narrower confidence intervals despite multicollinearity.
The highest correlation between temperatures and mosquitoes were found at lag 0 week (r=-0.50 for maximum temperature and r=- 0.57 for minimum temperature). The highest correlation between the relative humidity and mosquitoes were found at lag 5 weeks (r=0.46 for maximum relative humidity and r=0.45 for minimum relative humidity). It is important to mention the result of the correlation analysis to make the purpose of selecting these lags clear (Figure 2).
We ran the multiple regression analysis using the four variables at these time lags to create the first model. Then we selected the two meteorological variables (minimum temperature lag 0 week and maximum relative humidity lag 5 weeks) that had the highest correlation with mosquitoes (r=-0.57 and r=0.46, respectively) to create the second model since they could better explain the variance in average weekly mosquitoes. However, in the multiple regressions, we used the average of the meteorological variables and mosquitoes from 2007 to 2009 and then predicted the weekly mosquito values for 2010 based on the result of this analysis.
To validate models 1 and 2, we used the actual weekly female Aedes aegypti data from 2010 and performed correlation models between predicted and actual values of the models. We then plotted the difference between each pair of the predicted and actual weekly values (Y-axis) versus the average of each pair (X-axis) to assess the agreement between these values because the correlation alone might not be adequate for the assessment. Altman and Bland originally introduced this method in 1983 [20].
Results
Regression analyses
Only minimum (MinTemp) and maximum temperatures (MaxTemp) of lag 0 week and minimum (MinRH) and maximum (MaxRH) relative humidity of lag 5 weeks were used in the first regression model (Table 1). Others were excluded due to their lack of significant correlation with mosquito numbers (Figure 2). A decrease in average weekly minimum temperature of lag 0 week (the current week) prior to the mosquito collection week was the most significant entomological indicator for the rise in weekly abundance of adult female Aedes aegypti. This was followed by an increase in weekly minimum and maximum relative humidity 35 days (5 weeks) prior to the mosquito collection week. The average maximum temperature from the current week (lag 0) of collecting the mosquitoes seemed to have an effect on the increase of the average weekly amount of mosquito, but to a lesser extent when compared to maximum and minimum relative humidity and in a different direction compared to the minimum temperature.
| Variable | Coefficients | Standard Error | P-value | Cl95 |
|---|---|---|---|---|
| MaxRH Lag 5 week | 1.34 | 0.67 | 0.05 | 0.00 – 2.69 |
| MinRH Lag 5 week | 1.38 | 0.52 | 0.01 | 0.33 – 2.42 |
| MaxTemp Lag 0 week | 0.83 | 2.10 | 0.69 | -3.39 – 5.04 |
| MinTemp Lag 0 week | -3.43 | 2.39 | 0.16 | -8.23 – 1.37 |
Table 1: Meteorological Variables With the Most Significant Correlations With Average Number of Adult Female Aedes aegypti (Model 1).
For the second model, only two meteorological variables were used in the analysis. These variables were average weekly minimum temperature of lag 0 week (current week) and the maximum relative humidity of lag 5 weeks. They were included in the analysis since they were significantly related to the average number of mosquitoes (Table 2 and Figure 2).
| Variable | Coefficients | Standard Error | P-value | Cl95% |
|---|---|---|---|---|
| MinTemp Lag 0 week | -3.43 | 2.39 | 0.16 | -8.23 – 1.37 |
| MaxRH Lag 5 week | 1.34 | 0.67 | 0.05 | 0.00 – 2.69 |
Table 2: Significant Meteorological Variables With the Highest Correlation With Average Number of Adult Female Aedes aegypti Mosquitoes (Model 2).
Validation of models 1 and 2
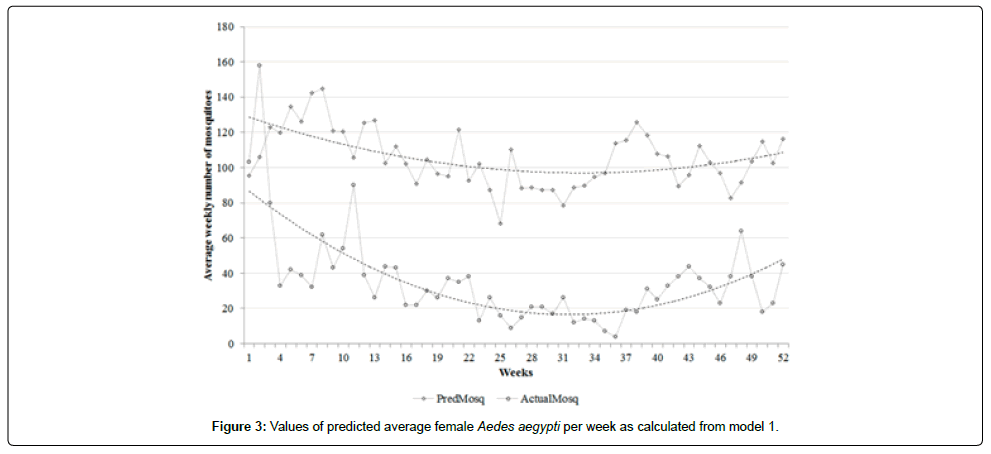
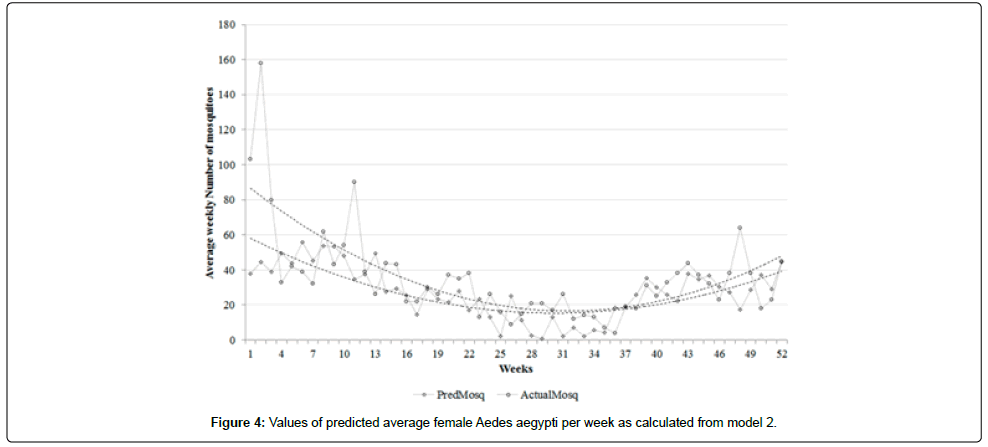
A correlation coefficient of 0.60 was observed between the actual and predicted average mosquito numbers (resulting from model 1), albeit with different scales-lower in actual mosquito numbers and higher in the predicted values (Figure 3). A higher correlation coefficient of 0.69 was observed between actual and predicted values (resulting from model 2) with narrower bands (Figure 4). Model 2 predicted values that were more accurate in terms of showing the trends of mosquito abundance over weeks compared to model 1 (Figures 3 and 4).
The limits of agreement were calculated from d ± 1.96s, where d is the mean of difference between each pair of predicted and actual values, and s is the standard deviation of the difference between these pairs [20]. The calculation results showed that the upper and lower limits of agreements of model 1 were 15 and 99, respectively, and the upper and lower limits of agreements of model 2 were -53 and 37, respectively.
Figure 5a shows that 86% (45 out of 52) of datasets of model 1 are within the upper and lower limits of agreement, indicating a strong concordance between the predicted and actual average of weekly adult female Aedes aegypti mosquitoes. In contrast, Figure 5b shows that 94% (49 out of 52) of datasets of model 2 are within the limits of agreements, indicating the strongest concordance between the values.
Figure 5: Altman [20] agreement plot showing the difference between each pair of average predicted and actual weekly female Aedes aegypti (horizontal axis) plotted against the difference between predicted and actual weekly female Aedes aegypti (vertical axis). Values between the dashed lines (lightly shaded regions) are within the limits of agreement [Note: This is an assessment for results of model 1(a) and model 2(b)].
Discussion
Significant relationships were revealed between minimum and maximum relative humidity and average adult female Aedes aegypti with a lag of 5 weeks. Other significant associations were found between minimum and maximum temperatures and adult female Aedes aegypti with no lag. The coefficient of maximum relative humidity, minimum relative humidity, and maximum temperature was positive, indicating that a decrease in vector abundance could be forecasted by a decrease in these variables. On the other hand, the coefficient for minimum temperature was negative, indicating that a decrease in vector abundance could be forecasted by an increase in weekly minimum temperature. This result adds significantly to our understanding of female Aedes aegypti changes. It shows that mosquitoes are more abundant in times of lower temperature. That result is different than the findings of Azil [21], who determined that female Aedes aegypti are more abundant in times of higher temperature and relative humidity. In Jeddah, the average minimum temperature is usually around 28°C, any increase in that averages to around 35°C can leads to decrease in the abundance of adult Aedes mosquitoes because that reduces the humidity and kill larva. Any decrease in that average to around 18°C can lead to increase in the mosquito abundance because it increases the humidity and offers the best conditions for mosquito to survive.
Model 2 explains more immediate effects of weather on vector abundance, and for this reason, we can select it as the better model compared to model 1. It was found to be sensitive in predicting sharp increases (e.g, from week 1 to week 2 and from week 51 to week 52) and the reduction of mosquito numbers in some weeks (e.g, week 2 to week 3 and week 43 to week 44). This model is able to explain the weekly trends of mosquito numbers and that was observable from around week 1 to week 52.
The validation stage of model 2 shows that the weather-based predictive modeling can be used to predict the weekly increases and decreases in vector abundance, which would allow mosquito control measures to be initiated before the sharp increases happens. An easy method is presented to forecast the weekly abundance of mosquitoes, and only the forecasted minimum temperature and maximum relative humidity, which can be provided by Saudi PME, are needed. Also based on this model, mosquito control measures could be intensified prior to the detected mosquito abundance increase in traps.
Pre-emptive mosquito control could be targeted toward a considerable number of potential mosquito breeding habitats. Media campaigns could be used at the stage of prediction to inform the public that dengue vector was on the rise. This can help minimize the likelihood of dengue risk. The predictive model developed in this study could partly inform decision makers for determining when to initiate these control measures.
Different studies [21,22] have used similar analyses of meteorological factors and mosquitoes in Argentina and Australia. In 2008, Estallo [22] used a combination of 13 meteorological variables and satellite-derived indicators to create predictive models of the Breteau and House indices. These predictive models were found to be of potential use in predicting larval indices when time lags of these variables were included in the analysis. This study shows that a 15-day time lag (approximately 2 weeks) for humidity was related strongly to the mosquito abundance. These same results were found by Azil [21], who constructed models based on temperature with time lag 0 week and relative humidity of lag 2 weeks. They discovered that mosquito abundance was strongly associated with a 14-day time lag for humidity.
For humidity and temperature, our study shows different results. We found that 5-week time lags were strongly associated with the mosquito abundance, and we found a similar result showing temperature to be significantly associated with mosquito abundance at lag 0 week. Study of the development of predictive tools for pre-emptive dengue vector control showed that long-term meteorological factors have a significant relationship with the abundance of adult Aedes mosquitoes, particularly when mean minimum temperature is lagged at 6 months and mean daily temperature is lagged at 4 months [21].
The Aedes mosquito survival rate can increase at higher temperatures (not more than 38°C) [23], and our study indicates the weekly temperatures and relative humidity that are suitable for survival of adult Aedes mosquitoes and transmission of dengue. Similar results have been presented by Githeko [14], who found that temperature ranges from 14°C to 18°C at the lower end and 35°C to 40°C at the upper end could lead to higher dengue transmission.
The main reason underlying these differences was that most of the previous studies were based on seasonal or yearly data while our study was based on average weekly data that were derived from daily datasets, which are more reliable for describing associations.
We did not include the rainfall variable as part of the predictive models presented here because of limitations of rainfall data (approximately 2 to 3 weeks a year), and because rainfall is usually highly collinear with temperature and humidity variables and it was not as strongly predictive of the mosquito abundance [21,24].
The predictive models developed in this study successfully showed the presence of mosquitoes in most of the study weeks and also the fluctuations in vector abundance. Some plausible reasons could explain these fluctuations and presence. The number of adult mosquitoes could be fluctuating due to the use of insecticide spraying in the air that leads to the death of adult mosquitoes, while the eggs and larvae remain protected from the danger of insecticides. This later leads to increases in the mosquitoes that transmit dengue. Also, some areas of the study site have limited access to the water supply, leading residents to use water storage containers [25] and ground-level water storage tanks. Many studies have shown that containers and tanks provide suitable places for larvae and pupae to live. Bisset [26] found that out of 1,000 samples, the immature stages of Aedes were found in 70 containers and the pupae of this species were seen in 52 containers. They also found that 74.1% of the pupae that were collected were in the ground-level water storage tanks and that 19% were in miscellaneous small containers.
The models presented here explain most of the variation in the mean number of adult female Aedes aegypti per week. Other factors, such as human population growth rate and carrying capacity (e.g., breeding container density) for dengue vectors should also be considered [27]. This consideration can help further understanding on how other factors contribute to the fluctuation and magnitude of vector abundance [28]. Khormi [11] found that the variations in annual meteorological variables indicated that certain factors other than biological characteristics of adult Aedes mosquitoes determine dengue fever transmission. These other factors have a significant influence on dengue transmission and its vector and include human population factors such as abundance, social status, population immunity, and economic status.
Conclusion
This study has developed regression models that can be used to predict the weekly abundance and trends of adult female Aedes aegypti mosquitoes. It is clear that minimum temperature at lag 0 time and maximum relative humidity at lag 5 weeks have the highest association with the weekly abundance and distribution of the mosquitoes.
The results of the models presented here support the view that a few meteorological variables could explain much of the weekly fluctuations of the mosquito abundance. The study findings could contribute to forecasting mosquito abundance peaks and subsequently guide the development of a plan for mosquito control operations ahead of time, which would help minimize the outbreak of dengue occurrence and prevent the spread of dengue infections.
We were able to produce highly accurate descriptive models of female Aedes aegypti by using regression and correlation methods to control which lag times of the variables were included in the models. This approach of modeling is even more powerful because, like studies that require previous and current mosquito counts and forecasted meteorological variables, the variables identified here are readily available in the study area, allowing for the modeling method to be applied in mosquito control and management. The selection of the highest correlated variables at different lag times to predict the mosquito abundance is the first step towards developing predictive models that could be used to predict potential population spikes of important vector species in the future and thwart an outbreak by preparing mosquito control efforts.
To improve monitoring and forecasting of mosquito abundance in each district or sub-district of Jeddah, we suggest that every trap that is used to capture adult mosquitoes must have devices for measuring temperature and relative humidity to give a better understanding of the climatic conditions in each area. This can be used later to create a regression model based on temperature and relative humidity for each of Jeddah districts or sub-districts to predict mosquito values and be used as parameters for modeling dengue incidences and mosquito risks to monitor and control these risks successfully.
Acknowledgement
We deeply thank KACST Geographic Information System Technical Innovation Center for financial support under grand no. (GISTIC-13-04). Also, we wish to thank Jeddah Municipality and the Presidency of Meteorology and Environment for providing mosquito and meteorological data.
References
- Hopp MJ, Foley JA (2001) Global-scale relationships between climate and the dengue fever vector, Aedes aegypti. Clim Change 48: 441-463.
- Jackson EK (1995) Climate change and global infectious disease threats. Med J Aust 163: 570-574.
- Martens WJM, Jetten TH, Focks DA (1997) Sensitivity of malaria, schistosomiasis, and dengue to global warming. Clim Change 35: 145-156.
- Pinheiro FP, Chuit R (1998) Emergence of dengue hemorrhagic fever in the Americas. Infect Med 15: 244-251.
- Bliss AR, Gill JM (1993) The effects of freezing on the larvae of Aedes aegypti. Amer J Trop Med Hyg 13: 583-588.
- Chadee D, Shivnauth B, Rawlins S, Chen A (2007) Climate mosquito indices and the epidemiology of dengue fever in Trinidad (2002-2004). Ann Trop Med Parasitol 101: 69-77.
- Takeda T, Whitehouse CA, Brewer M, Gettman AD, Mather TN (2003) Arbovirus surveillance in Rhode Island assessing potential ecologic and climatic correlates. J Am Mosq Control Assoc 19:179-189.
- Woodruff RE, Guest CS, Garner MG, Becker N, Lindesay J, et al. (2002) Predicting Ross River virus epidemics from regional weather data. Epidemiology 13: 384-393.
- Aburas HM (2007) ABURAS Index: A statistically developed index for dengue-transmitting vector population prediction. Proceedings of World Academy of Science, Engineering and Technology 23: 151-154.
- Khormi HM, Kumar L, Elzahrany R (2011) Describing and analyzing the association between meteorological variables and adult Aedes aegyptimosquitoes. J Food Agri Environ 9: 954-959.
- Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R (2008) Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med 5: e68.
- Wallis RC (2005) A GIS model for predicting potential “high risk†areas of West Nile virus by identifying ideal mosquito breeding habitats. M.Sc. thesis, Environmental Science, Mississippi State University, Mississippi, USA.
- Githeko AK, Lindsay SW, Confalonieri UE, Patz JA (2000) Climate change and vector-borne diseases: A regional analysis. Bull World Health Organ 78: 1136-1147.
- Dye C, Reiter P (2000) Climate chance and malaria-temperatures without fevers? Science 289: 1697-1698.
- Monath TP, Tsai TF (1987) St. Louis encephalitis-lessons from the last decade. Amer J of Trop Med Hyg 37: S40-S59.
- Khormi MH, Kumar L (2011) Examples of using spatial information technologies for mapping and modelling mosquito-borne diseases based on environmental, climatic, socio-economic factors and different spatial statistics, temporal risk indices and spatial analysis: A review. J Food Agri Environ 9: 41-49.
- Day JF, Curtis GA (1989) Influence of rainfall on Culex nigripalpus(Diptera: Culicidae) blood-feeding behavior in Indian River County,Florida. Annals of the Entomological Society of America 82: 32-37.
- El-Badry A, Al-Ali K (2010) Prevalence and seasonal distribution of dengue mosquito, Aedes aegypti (Diptera: Culicidae) in Al-Madinah Al-Munawwarah, Saudi Arabia. J Entomol 7: 80-88.
- Altman D, Bland J (1983) Measurement in medicine: the analysis of method comparison studies. J Royal Stat Soc 32: 307-317.
- Azil AH, Long SA, Ritchie SA, Williams CR (2010) The development of predictive tools for pre-emptive dengue vector control: A study of Aedes aegypti abundance and meteorological variables in North Queensland, Australia. Trop Med Int Health 15: 1190-1197.
- Estallo EL, Lamfri MA, Scavuzzo CM, Almeida FFL, Introini MV, et al. (2008) Models for predicting Aedes aegypti larval indices based on satellite images and climatic variables. J Am Mosq Control Assoc 24: 368-376.
- Tun-Lin W, Burkot TR, Kay BH (2000) Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegyptiin north Queensland, Australia. Med Vet Entomol 14: 31-37.
- Williams CR, Johnson PH, Long SA, Rapley LP, Ritchie SA (2008) Rapid estimation of Aedes aegypti population size using simulation modeling, with a novel approach to calibration and field validation. J Med Entomol 45: 1173-1179.
- Aljawi A, Mariappan T, Abo-Khatwa A, Al-Ghamdi K, Aburas H (2008) Fibre-glass drums as the key containers of Aedes aegypti breeding in apartments occupied by expatriates in Jeddah, Saudi Arabia. Dengue Bulletin 32: 228-231.
- Bisset JA, Marquetti MC, Suarez S, Rodriguez MM, Padmanabha H (2006) Application of the pupal/demographic-survey methodology in an area of Havana, Cuba, with low densities of Aedes aegypti (L.). Ann Trop Med Parasitol 100: S45-S51.
- Yang GJ, Brook BW, Whelan PI, Cleland S, Bradshaw CJA (2008) Endogenous and exogenous factors controlling temporal abundance patterns of tropical mosquitoes. Ecol Appl 18: 2028-2040.
- Otero M, Solari H, Schweigmann N (2006) A stochastic population dynamics model for Aedes Aegypti: Formulation and application to a city with a temperate climate. Bull Math Biol 68: 1945-1974.
Citation: Khormi HM, Kumar L, Elzahrany RA (2013) Regression Model for Predicting Adult Female Aedes aegypti Based on Meteorological Variables: A Case Study of Jeddah, Saudi Arabia. J Earth Sci Clim Change 5: 168. DOI: 10.4172/2157-7617.1000168
Copyright: ©2013 Khormi HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15954
- [From(publication date): 1-2014 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 11170
- PDF downloads: 4784